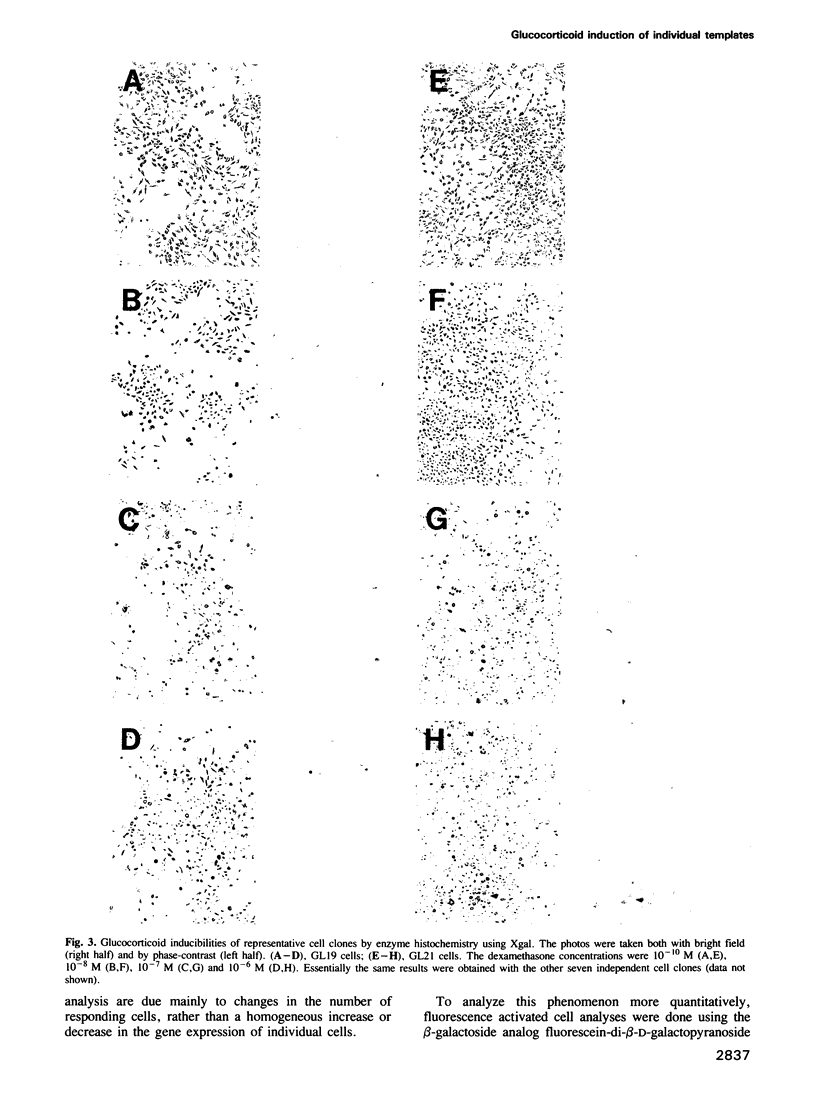
Abstract
Glucocorticoid hormones induce the transcription of genes having glucocorticoid response elements in a dose dependent manner. To determine whether this dose dependence represents a response of individual templates or of the mass of templates, we introduced a bacterial beta-galactosidase gene linked to the glucocorticoid-inducible enhancer/promoter of the mouse mammary tumor virus (MTV) into Ltk- cells and obtained stable transformants containing a single or a few templates per cell. Visual inspection and flow cytometry analysis by enzyme histochemistry assay for beta-galactosidase revealed that individual cells showed very heterogeneous beta-galactosidase activity after 48 h induction with dexamethasone. When the glucocorticoid concentration was increased, an increasing cell population producing beta-galactosidase was observed. These phenomena were probably not due to heterogeneity of template copy number or to a predetermined cellular state among individual cells, since cells forming a single small colony gave similar results. This was also supported by data showing that recloned cells retained both their responsiveness to the glucocorticoid hormone and their digestion pattern in Southern blotting analyses. These results indicate that the dose dependent increase of glucocorticoid-inducible gene expression is caused by an increase in the number of transcriptionally active templates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Brunet L. J., Berk A. J. Concentration dependence of transcriptional transactivation in inducible E1A-containing human cells. Mol Cell Biol. 1988 Nov;8(11):4799–4807. doi: 10.1128/mcb.8.11.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen M., Northrop J. P., Ringold G. M. The mouse glucocorticoid receptor: mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 1986 Oct;5(10):2513–2522. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P., Hage W. J., Hendriks H. F., de Vries N. J., Heideveld M., Nieuwkoop P. D. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989 Jul 13;340(6229):140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Eichele G. Retinoids and vertebrate limb pattern formation. Trends Genet. 1989 Aug;5(8):246–251. doi: 10.1016/0168-9525(89)90096-6. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone G. L., Payvar F., Yamamoto K. R. Glucocorticoid regulation of protein processing and compartmentalization. Nature. 1982 Nov 18;300(5889):221–225. doi: 10.1038/300221a0. [DOI] [PubMed] [Google Scholar]
- Firzlaff J. M., Diggelmann H. Dexamethasone increases the number of RNA polymerase II molecules transcribing integrated mouse mammary tumor virus DNA and flanking mouse sequences. Mol Cell Biol. 1984 Jun;4(6):1057–1062. doi: 10.1128/mcb.4.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. P., Yoshinaga S. K., Vanderbilt J. N., Yamamoto K. R. In vitro transcription enhancement by purified derivatives of the glucocorticoid receptor. Science. 1989 Jul 21;245(4915):298–301. doi: 10.1126/science.2473529. [DOI] [PubMed] [Google Scholar]
- Govindan M. V., Devic M., Green S., Gronemeyer H., Chambon P. Cloning of the human glucocorticoid receptor cDNA. Nucleic Acids Res. 1985 Dec 9;13(23):8293–8304. doi: 10.1093/nar/13.23.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Groner B., Hynes N. E., Rahmsdorf U., Ponta H. Transcription initiation of transfected mouse mammary tumor virus LTR DNA is regulated by glucocorticoid hormones. Nucleic Acids Res. 1983 Jul 25;11(14):4713–4725. doi: 10.1093/nar/11.14.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Functional steps in transcription initiation and reinitiation from the major late promoter in a HeLa nuclear extract. J Biol Chem. 1987 Mar 15;262(8):3452–3461. [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Weigel N. L., Allan G. F., Riley D., Rodriguez R., Schrader W. T., Tsai M. J., O'Malley B. W. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell. 1990 Jan 26;60(2):247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- Ko M. S., Takano T. A highly inducible system of gene expression by positive feedback production of glucocorticoid receptors. DNA. 1989 Mar;8(2):127–133. doi: 10.1089/dna.1.1989.8.127. [DOI] [PubMed] [Google Scholar]
- Ladiges W. C., Raff R. F., Brown S., Deeg H. J., Storb R. The canine major histocompatibility complex. Supertypic specificities defined by the primed lymphocyte test (PLT). Immunogenetics. 1984;19(4):359–365. doi: 10.1007/BF00345410. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Tsou A. P., Chan H., Thomas J., Petrie K., Eugui E. M., Allison A. C. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Gene transcription: activator's target in sight. Nature. 1989 Sep 28;341(6240):279–280. doi: 10.1038/341279a0. [DOI] [PubMed] [Google Scholar]
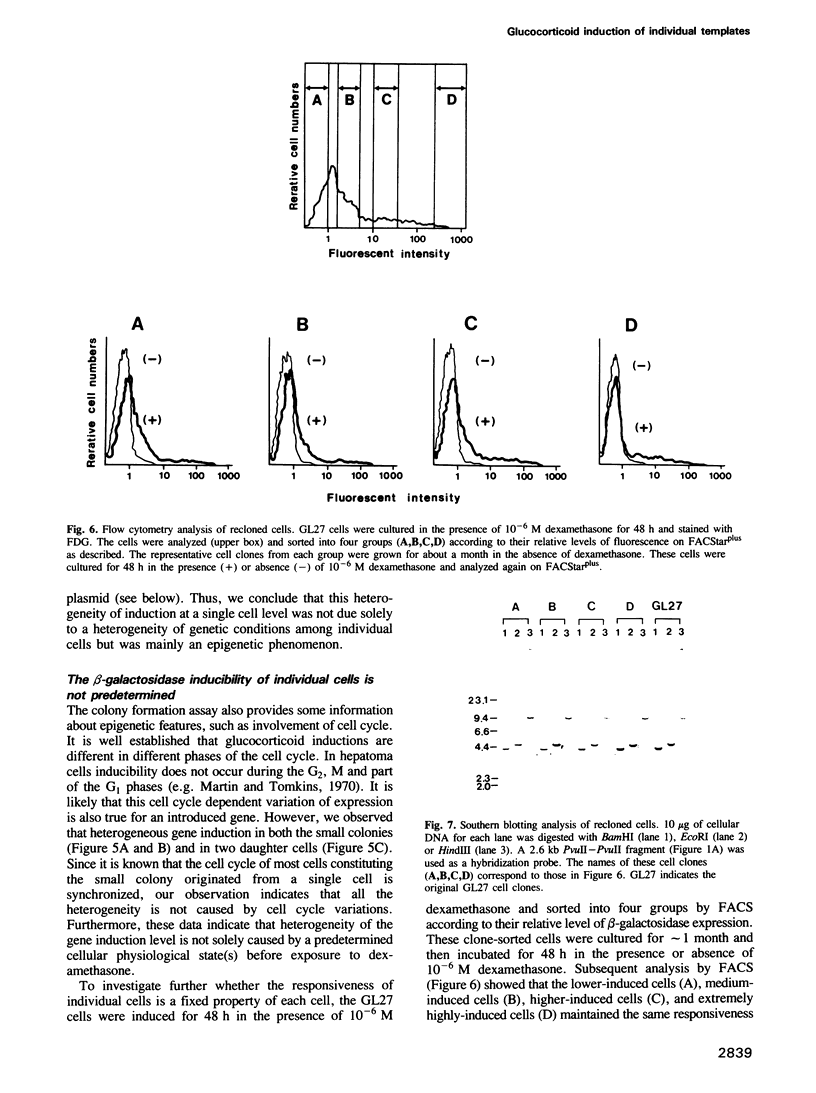
- Martin D. W., Jr, Tomkins G. M. The appearance and disappearance of the post-transcriptional repressor of tyrosine aminotransferase synthesis during the HTC cell cycle. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1064–1068. doi: 10.1073/pnas.65.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Okret S., Wikström A. C., Wrange O., Gustafsson J. A., Yamamoto K. R. Characterization of a steroid hormone receptor gene and mRNA in wild-type and mutant cells. Nature. 1984 Dec 20;312(5996):779–781. doi: 10.1038/312779a0. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Raptis L., Lamfrom H., Benjamin T. L. Regulation of cellular phenotype and expression of polyomavirus middle T antigen in rat fibroblasts. Mol Cell Biol. 1985 Sep;5(9):2476–2486. doi: 10.1128/mcb.5.9.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Dobson D. E., Grove J. R., Hall C. V., Lee F., Vannice J. L. Glucocorticoid regulation of gene expression: mouse mammary tumor virus as a model system. Recent Prog Horm Res. 1983;39:387–424. doi: 10.1016/b978-0-12-571139-5.50014-8. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Ucker D. S., Firestone G. L., Yamamoto K. R. Glucocorticoids and chromosomal position modulate murine mammary tumor virus transcription by affecting efficiency of promoter utilization. Mol Cell Biol. 1983 Apr;3(4):551–561. doi: 10.1128/mcb.3.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Ross S. R., Yamamoto K. R. Mammary tumor virus DNA contains sequences required for its hormone-regulated transcription. Cell. 1981 Dec;27(2 Pt 1):257–266. doi: 10.1016/0092-8674(81)90409-8. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Sawadogo M., Roeder R. G. Stability of transcription complexes on class II genes. Mol Cell Biol. 1989 Jan;9(1):342–344. doi: 10.1128/mcb.9.1.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S. M., Ong E. S., Harmon J. M., Brower S. T., Cidlowski J., Thompson E. B., Rosenfeld M. G., Evans R. M. Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Science. 1985 May 10;228(4700):740–742. doi: 10.1126/science.2581314. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Formation of stable transcription complexes as assayed by analysis of individual templates. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5819–5823. doi: 10.1073/pnas.85.16.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969 Oct;25(1):1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Young H. A., Shih T. Y., Scolnick E. M., Parks W. P. Steroid induction of mouse mammary tumor virus: effect upon synthesis and degradation of viral RNA. J Virol. 1977 Jan;21(1):139–146. doi: 10.1128/jvi.21.1.139-146.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]