Abstract
Rationale: The prevalence of frailty (diminished physiologic reserve) and its effect on outcomes for those aged 18 years and older with critical illness is unclear.
Objectives: We hypothesized greater frailty would be associated with subsequent mortality, disability, and cognitive impairment, regardless of age.
Methods: At enrollment, we measured frailty using the Clinical Frailty Scale (range, 1 [very fit] to 7 [severely frail]). At 3 and 12 months post-discharge, we assessed vital status, instrumental activities of daily living, basic activities of daily living, and cognition. We used multivariable regression to analyze associations between Clinical Frailty Scale scores and outcomes, adjusting for age, sex, education, comorbidities, baseline disability, baseline cognition, severity of illness, delirium, coma, sepsis, mechanical ventilation, and sedatives/opiates.
Measurements and Main Results: We enrolled 1,040 patients who were a median (interquartile range) of 62 (53–72) years old and who had a median Clinical Frailty Scale score of 3 (3–5). Half of those with clinical frailty (i.e., Clinical Frailty Scale score ≥5) were younger than 65 years old. Greater Clinical Frailty Scale scores were independently associated with greater mortality (P = 0.01 at 3 mo and P < 0.001 at 12 mo) and with greater odds of disability in instrumental activities of daily living (P = 0.04 at 3 mo and P = 0.002 at 12 mo). Clinical Frailty Scale scores were not associated with disability in basic activities of daily living or with cognition.
Conclusions: Frailty is common in critically ill adults aged 18 years and older and is independently associated with increased mortality and greater disability. Future studies should explore routine screening for clinical frailty in critically ill patients of all ages. Interventions to reduce mortality and disability among patients with heightened vulnerability should be developed and tested.
Clinical trial registered with www.clinicaltrials.gov (NCT 00392795 and NCT 00400062).
Keywords: frailty, activities of daily living, critical illness, survivors
At a Glance Commentary
Scientific Knowledge on the Subject
Reduced long-term survival, disability in activities of daily living, cognitive impairment, and poor health-related quality of life complicate survivorship from critical illness. The mechanisms underlying these sequelae of critical illness are incompletely understood. Frailty is a multidimensional syndrome characterized by the loss of physiologic reserve that reduces the ability to recover from acute stress. In noncritically ill older adults, frailty is predictive of subsequent mortality, disability, and cognitive impairment. Although frailty before critical illness has been evaluated as a risk factor for short- and long-term mortality and health-related quality of life, the effect of preexisting frailty on disability in activities of daily living and long-term cognitive impairment after critical illness among young and old patients is unclear.
What This Study Adds to the Field
This multicenter, prospective cohort study demonstrated that the clinical syndrome frailty was present in one out of three patients aged 18 years and older with medical and/or surgical critical illness. Half of all patients with frailty were younger than 65 years of age. After accounting for several potential confounders of the association between frailty and long-term outcomes, increasing clinical frailty was independently associated with greater mortality, greater odds of disability in instrumental activities of daily living, and poorer physical health-related quality of life 3 and 12 months after discharge. These associations were not affected by age.
Survivorship from a critical illness is frequently complicated by reduced long-term survival, newly acquired disabilities in activities of daily living, cognitive impairment, and poor health-related quality of life (1–5). The mechanisms underlying these sequelae of critical illness are incompletely understood. Nevertheless, they are generally thought to result from the complex relationship between the severity of the critical illness, intensive care unit (ICU) care, and a patient’s underlying vulnerability (6).
Clinically, increased vulnerability is recognized by a reduced ability to maintain or restore homeostasis in the setting of acute stress, a phenomenon that has been termed “frailty” (7–9). In community-dwelling older persons, frailty is independently associated with subsequent mortality, disability in activities of daily living (basic self-care activities necessary for independent living), and cognitive impairment (7, 8, 10–16). Although frailty before critical illness has been evaluated as a risk factor for short- and long-term mortality and health-related quality of life (17–21) in older adults with critical illness, the effect of preexisting frailty on mortality, disability, cognitive impairment, and health-related quality of life after critical illness among adult patients of all ages is unclear.
We therefore sought to describe the prevalence and severity of frailty in adults age 18 years of age and older and to determine the independent association between preexisting frailty (i.e., frailty present before critical illness) and long-term outcomes 3 and 12 months after critical illness. We hypothesized that more severe preexisting frailty, as assessed with the Clinical Frailty Scale, would be independently associated with increased mortality, worse disability in instrumental and basic activities of daily living, impaired cognition, and poorer health-related quality of life, regardless of age.
Methods
We tested our hypotheses in a combined cohort of patients enrolled in the identical BRAIN-ICU (Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors) (NCT00392795) and MIND-ICU (Delirium and Dementia in Veterans Surviving ICU Care) (NCT00400062) studies (2). Some of these original data have been reported in the form of an abstract (22).
Setting and Participants
The study protocol including inclusion/exclusion criteria have been published elsewhere and may be found in the online supplement (2). We enrolled patients 18 years of age and older treated for respiratory failure or shock from the medical and/or surgical ICUs at five U.S. centers. We excluded those with organ dysfunction for greater than 72 hours, recent ICU exposure, severe cognitive impairment, inability to communicate in English, substance abuse, homelessness, or residence greater than 200 miles from the enrollment site. Patients and proxies provided consent. Each center’s institutional review board approved the study.
Measuring Frailty
At enrollment (i.e., within 72 hours of ICU admission), study personnel, trained by a geriatrician with expertise in frailty assessments (A.M.), used patient/proxy interviews and medical records to determine preexisting frailty with the Clinical Frailty Scale (8). The Clinical Frailty Scale is a well-validated, seven-point scale that classifies patients along the continuum from very fit to severely frail, with a score of 5 or greater representing clinical frailty (see online supplement). Enrollment Clinical Frailty Scale scores were the primary exposure for all analyses.
Outcomes
At 3- and 12-month follow-up, study personnel blinded to Clinical Frailty Scale scores assessed vital status using patients/proxies, medical records, and the Social Security Death Index. Among survivors, study personnel assessed instrumental activities of daily living using the Functional Activities Questionnaire (23), basic activities of daily living using the Katz ADL (24), cognition using the Repeatable Battery for the Assessment of Neuropsychological Status (25), and health-related quality of life using the Short Form (SF)-36 (26).
Missing Data
We used multiple imputation to account for missing covariate and incomplete outcome data (27). Mortality, instrumental activities of daily living, basic activities of daily living, and health-related quality of life outcomes data were greater than 96% complete. Cognitive outcome data were 84% complete. We excluded patients who died or withdrew before follow-up from the disability, cognitive, and health-related quality-of-life analyses.
Statistical Analysis
We constructed box plots to determine the distributions of Clinical Frailty Scale scores by age and Kaplan-Meier survival curves to describe the probability of survival according to Clinical Frailty Scale score. We used multivariable regression, adjusting for covariates, to determine the association between Clinical Frailty Scale scores and outcomes. We modeled the relationship with mortality using Cox proportional hazards regression, with instrumental activities of daily living and basic activities of daily living outcomes using proportional odds logistic regression, and with cognitive and health-related quality-of-life outcomes using linear regression.
We included the full Clinical Frailty Scale score as the exposure in each model rather than dichotomizing the score into frail versus nonfrail. We chose this approach because using the full ordinal variable (rather than dichotomizing) provides the most statistical power to detect change (reducing the risk of type II error) and increases precision of the estimates.
As covariates, we a priori selected age, sex, years of education, Charlson comorbidity index score (28), baseline Functional Activities Questionnaire and Katz ADL scores, baseline Informant Questionnaire on Cognitive Decline in the Elderly score (29), daily mean modified Sequential Organ Failure Assessment score (30), days the Confusion Assessment Method-ICU was positive (31), days the Richmond Agitation-Sedation Scale (32) was −4 or −5, days of severe sepsis, days of mechanical ventilation, and mean daily sedative and opiate doses. For detailed covariate descriptions, see the online supplement.
Associations with continuous covariates were allowed to be nonlinear using restricted cubic splines. We assessed for an interaction between frailty and delirium duration and, in a sensitivity analysis, between Clinical Frailty Scale score and age. Nonlinear and interaction terms were excluded if the P-value for the global test for nonlinearity or interaction was greater than 0.20. All model assumptions were met (see online supplement). We used R version 3.1.2 (R Project for Statistical Computing, Vienna, Austria) for all analyses. P values less than 0.05 were considered significant.
Results
We enrolled 1,047 patients between January 2007 and December 2010. While in the hospital, seven patients withdrew permission to use their data (see Figure E1 in the online supplement). Therefore, our cohort included 1,040 patients, who were a median age of 62 years old and 60% male (Table 1).
Table 1.
Demographic and Clinical Characteristics of Patients
| In-hospital Cohort (n = 1,040) | Follow-up Cohort (n = 580) | |
|---|---|---|
| Age | 62 (53–72) | 61 (52–70) |
| Male sex, % (n) | 60 (627) | 60 (346) |
| IQCODE score at enrollment | 3 (3–3) | 3 (3–3) |
| Charlson Comorbidity Index score | 2 (1–4) | 2 (1–4) |
| Clinical Frailty Scale score, % (n) | ||
| 1 (very fit) | 3 (31) | 4 (21) |
| 2 (well) | 13 (133) | 17 (96) |
| 3 (well, with treated comorbidities) | 34 (355) | 35 (204) |
| 4 (apparently vulnerable) | 21 (214) | 22 (126) |
| 5 (mildly frail) | 13 (140) | 12 (70) |
| 6 (moderately frail) | 13 (135) | 9 (53) |
| 7 (severely frail) | 3 (32) | 2 (10) |
| Functional Activities Questionnaire score | ||
| Enrollment | 0 (0–3) | 0 (0–2) |
| 3-mo | — | 3 (0–9) |
| 12-mo | — | 2 (0–8) |
| Katz ADL score | ||
| Enrollment | 0 (0–1) | 0 (0–1) |
| 3-mo | — | 0 (0–2) |
| 12-mo | — | 0 (0–1) |
| APACHE II score at admission | 24 (18–30) | 23 (17–29) |
| Mean daily SOFA score | 7 (6–10) | 7 (5–9) |
| Diagnosis at admission, % (n) | ||
| Sepsis | 32 (329) | 31 (178) |
| Acute respiratory failure | 17 (178) | 17 (96) |
| Cardiogenic shock, myocardial infarction, or arrhythmia | 17 (174) | 17 (99) |
| Upper airway obstruction | 10 (109) | 10 (60) |
| Gastric or colonic surgery | 7 (71) | 5 (31) |
| Neurologic disease or seizure | 1 (12) | 1 (7) |
| Other surgical procedure | 9 (97) | 13 (73) |
| Other diagnosis | 7 (70) | 6 (36) |
| Mechanical ventilation | ||
| Patients, % (n) | 89 (923) | 88 (510) |
| Days of mechanical ventilation among those who were ever mechanically ventilated | 2 (1–6) | 2 (1–5) |
| Severe sepsis | ||
| Patients, % (n) | 71 (727) | 64 (369) |
| Days of severe sepsis among those who were ever septic | 4 (2–9) | 4 (2–8) |
| Delirium | ||
| Patients, % (n) | 71 (740) | 71 (453) |
| Days of delirium among those who were ever delirious | 4 (2–7) | 3 (2–7) |
| Coma | ||
| Patients, % (n) | 60 (627) | 53 (305) |
| Days of coma among those who were ever comatose | 3 (1–6) | 2 (1–5) |
| Duration of ICU stay | 5 (3–11) | 5 (3–10) |
| Duration of hospital stay | 10 (6–17) | 10 (6–18) |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation, version II; ICU = intensive care unit; IQCODE = Informant Questionnaire on Cognitive Decline in the Elderly, assessment of preillness cognition; Katz ADL = assessment of basic activities of daily living; Functional Activities Questionnaire = assessment of instrumental activities of daily living; SOFA = Sequential Organ Failure Assessment.
Data are median (interquartile range) unless otherwise indicated.
Between enrollment and 3-month follow-up, 329 (32%) patients died. At 3 months after ICU discharge, we assessed 546 of the 711 (77%) surviving patients. Between 3- and 12-month follow-up, an additional 80 (8%) patients died. We assessed 467 of the 631 (74%) surviving patients at 12 months after discharge (see Figure E1).
Prevalence of Frailty and Age
The median Clinical Frailty Scale score at study enrollment was 3 (interquartile range, 3–5). Clinical frailty (i.e., a Clinical Frailty Scale score ≥5) was present in 307 patients (30%). Among the 580 patients who completed at least one follow-up assessment, 133 (23%) were clinically frail at baseline (Table 1). Those with clinical frailty had higher baseline scores on the Functional Activities Questionnaire and Katz ADL than those without clinical frailty (see Tables E1 and E2).
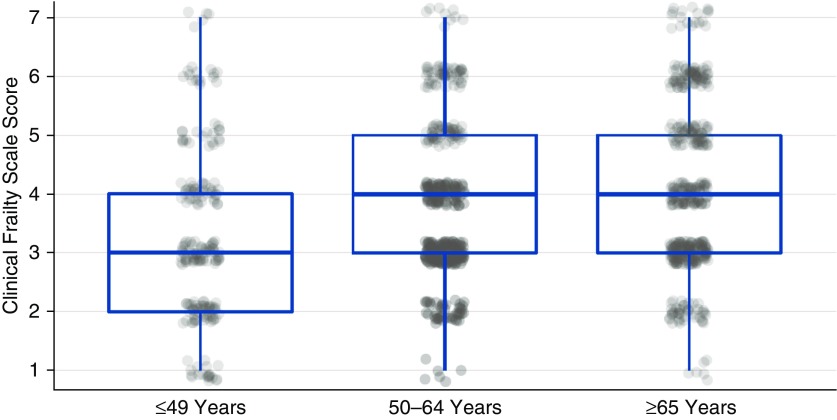
Patients of all ages demonstrated a range of Clinical Frailty Scale scores (Figure 1). On average, patients who were 49 years old and younger had lower median scores compared with those who were 50 years of age and older. Clinical frailty was present in 153 of 444 (34%) of patients aged 65 years and older. Nevertheless, clinical frailty was not limited to older patients. Among even the youngest patients (i.e., ≤49 years old), clinical frailty was present in 40 of 203 (20%) and among middle-aged patients (i.e., those 50–64 years old), clinical frailty was present in 114 of 393 (29%). Half of all patients with Clinical Frailty Scale scores greater than or equal to 5 were younger than 65 years old.
Figure 1.
Distribution of Clinical Frailty Scale (CFS) scores according to age. Box plots represent the median (horizontal line) and interquartile range (top and bottom of box represent the 75th percentile and 25th percentile, respectively) of CFS scores for each age group. Scatter plots represent the CFS score for individual patients.
A range of ages was present at each level of the Clinical Frailty Scale score (see Figure E2). Those with scores of 1 or 2 were younger than those with higher Clinical Frailty Scale scores. The median ages of those with scores greater than or equal to 3, however, were similar, indicating no age-related increase in the severity of frailty.
Frailty and Long-Term Outcomes
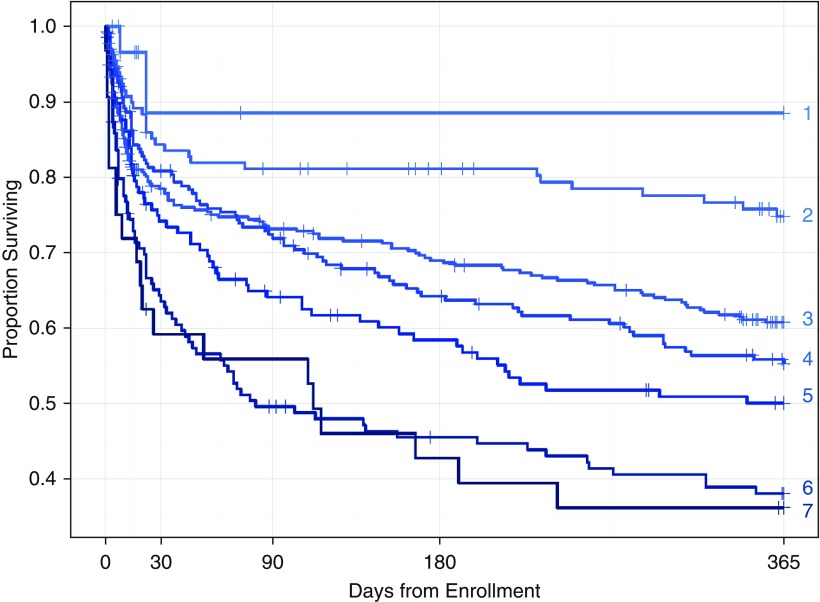
Kaplan-Meier survival curves showed an increased risk of death at each level of Clinical Frailty Scale score throughout the first year after enrollment (Figure 2). After adjusting for potential confounders of the association enrollment Clinical Frailty Scale score and mortality, Clinical Frailty Scale score was associated with increased hazard of death at both 3 and 12 months (P = 0.01 and P < 0.001, respectively) (Table 2). Clinical Frailty Scale score, however, was not associated with an increased hazard of death during the index hospitalization (hazard ratio, 1.1; 95% confidence interval, 0.9–1.5; P = 0.56). Thus, at 12 months, two patients, alike in all other ways (i.e., with all covariates adjusted to their median or mode value), a patient with a Clinical Frailty Scale score of 5 (the 75th percentile of frailty scores) had a 50% increase in the risk of death compared with a patient with a Clinical Frailty Scale score of 3 (the 25th percentile of frailty scores). We present the hazard ratios for mortality at each level of the Clinical Frailty Scale score during the index hospitalization, at 3 months, and at 12 months in Figure E3.
Figure 2.
Kaplan-Meier survival curves indicate an incremental worsening of survival with increasing Clinical Frailty Scale (CFS) scores. Numbers to the right of each curve indicate CFS score.
Table 2.
Effect of Baseline Frailty on Mortality, Disability in Activities of Daily Living, Cognition, and Health-related Quality of Life at Follow-Up
| 3 Months |
12 Months |
|||
|---|---|---|---|---|
| Point Estimate* (95% CI) | P Value | Point Estimate* (95% CI) | P Value | |
| Mortality† | 1.4 (1.1 to 1.8) | 0.01 | 1.5 (1.2 to 1.8) | <0.001 |
| IADL disability‡ | 1.2 (1.0 to 1.4) | 0.04 | 1.3 (1.1 to 1.6) | 0.002 |
| BADL disability§ | 1.1 (0.9 to 1.3) | 0.23 | 1.1 (0.9 to 1.4) | 0.10 |
| RBANS score|| | −0.6 (−1.7 to 0.4) | 0.42 | −0.2 (−1.6 to 1.2) | 0.12 |
| SF-36 Physical Component¶ | −2.1 (−3.0 to −1.1) | <0.001 | −1.9 (−2.9 to −0.8) | <0.001 |
| SF-36 Mental Component¶ | 0.5 (−0.9 to 2.0) | 0.08 | −0.5 (−2.0 to 1.0) | 0.16 |
Definition of abbreviations: BADL = basic activities of daily living; CI = confidence interval; IADL = instrumental activities of daily living; Katz ADL = assessment of basic activities of daily living; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; SF-36 = Medical Outcomes Survey Short Form-36.
Comparisons are between patients with a Clinical Frailty Scale score at the 75th percentile and those with a Clinical Frailty Scale score at the 25th percentile. Mortality models include all enrolled patients. Thus, the comparison for these models is between those with a score of 5 and those with a score of 3. All other outcome models included those who survived and participated in follow-up. Therefore, they compare those with a Clinical Frailty Scale score of 4 with those with a Clinical Frailty Scale score of 3. All covariates are adjusted to their respective median or mode value.
Via Cox proportional hazards model.
Via proportional odds logistic regression. Point estimate represents the adjusted odds ratio for a higher score on the Functional Activities Questionnaire at follow-up, in which a higher score indicates new or worsened disability in instrumental activities of daily living.
Via proportional odds logistic regression. Point estimate represents the adjusted odds ratio for a higher score on the Katz ADL at follow-up, in which higher scores indicate new or worsened disability in basic activities of daily living.
Via linear regression. Point estimate represents the difference in Global Cognition Score on the Repeatable Battery for the Assessment of Neuropsychological Status, where lower scores indicate worse cognition.
Via linear regression. Point estimate represents the difference in SF-36 score.
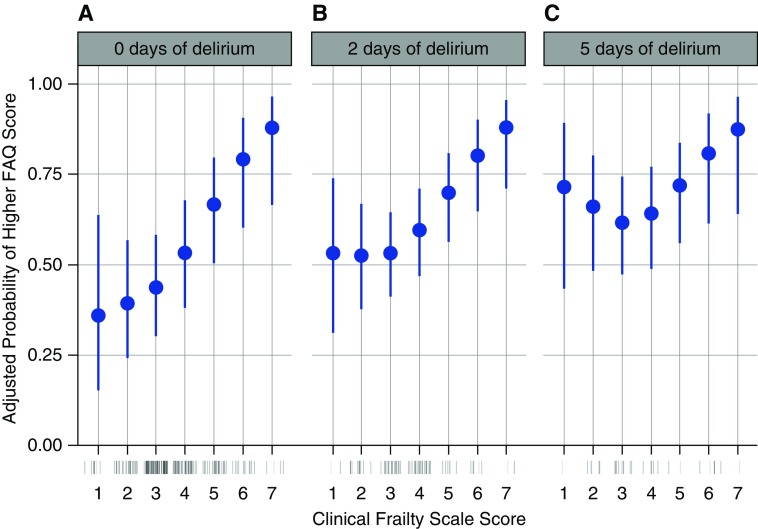
Clinical Frailty Scale score was also independently associated with greater odds of new or worsened disability in instrumental activities of daily living at the 3- and 12-month follow-up assessments (P = 0.04 and P = 0.002, respectively) (Table 2). The association between enrollment Clinical Frailty Scale score and disability in instrumental activities of daily living at 3 months may be found in Figure E4. The 12-month model suggested a statistical interaction between enrollment Clinical Frailty Scale score and the duration of delirium (P for interaction = 0.14). Therefore, we present the associations between enrollment Clinical Frailty Scale score and probability of new or worsened disability in instrumental activities of daily living at 12 months according to different durations of delirium (Figures 3A–3C). Although the association seems to be strongest among those patients who suffered the least amounts of delirium (Figure 3A), because of greater variability at lower Clinical Frailty Scale scores (Figures 3B and 3C), the possibility that the association is similar across all durations of delirium cannot be excluded. Nevertheless, the association was consistent at higher Clinical Frailty Scale scores (i.e., 4–7), regardless of the duration of delirium (Figures 3A–3C).
Figure 3.
These panels display Clinical Frailty Scale scores on the x-axis versus the adjusted probability of a higher Functional Activities Questionnaire score on the y-axis. Point estimates are indicated with blue dots, with 95% confidence intervals indicated by blue bars. The rug plot (just below the x-axis) shows the distribution of Clinical Frailty Scale scores. Overall, higher baseline Clinical Frailty Scale scores were independently associated with greater probability of higher Functional Activities Questionnaire scores at 12-month follow-up (P = 0.002), indicating new or worsened disability in instrumental activities of daily living. This association was modified by the duration of delirium (P = 0.14). Therefore, we present the association between Clinical Frailty Scale score and Functional Activities Questionnaire score for patients who had 0 days of delirium (A), 2 days of delirium (the 50th percentile of delirium duration, B), and those with 5 days of delirium (the 75th percentile of delirium duration, C). FAQ = Functional Activities Questionnaire.
Clinical Frailty Scale score at enrollment was not associated with greater odds of new or worsened disability in basic activities of daily living (P = 0.23 and P = 0.10, respectively) (Table 2). Enrollment Clinical Frailty Scale score was not associated with worse global cognition scores on the Repeatable Battery for the Assessment of Neuropsychological Status at either 3- or 12-month follow-up (P = 0.42 and P = 0.12, respectively) (Table 2). The finding of a lack of association between Clinical Frailty Scale score and long-term cognition was robust to several sensitivity analyses, including the models where duration of delirium was excluded as a covariate in the models (see Table E3).
Higher Clinical Frailty Scale score at enrollment, however, was associated with lower SF-36 Physical Component Scores at 3 and 12 months (P < 0.001 for both time points) (Table 2). Clinical Frailty Scale score was not associated with SF-36 Mental Component Scores at either follow-up assessment (P = 0.08 and P = 0.16, respectively) (Table 2).
To determine the effect of age on the independent associations between enrollment Clinical Frailty Scale score and long-term outcomes, we allowed for an interaction between frailty score and age in each of our models. In no model did the interaction term reach the a priori defined level of significance of P less than or equal to 0.20 (see Table E4). This lack of significant interaction between Clinical Frailty Scale score and age indicates that the associations between frailty and long-term outcomes reported herein were not modified by age.
Complete Case Analyses
The results of the complete case analyses were consistent with the primary analyses except for the association between Clinical Frailty Scale score at enrollment and global cognition at 12 months (see Table E5). This analysis found that Clinical Frailty Scale score was associated with a statistically, but nonclinically significant (i.e., less than one point), lower global cognition score on the Repeatable Battery for the Assessment of Neuropsychological Status (P = 0.03).
Discussion
In this large, multicenter prospective cohort study of patients age 18 years old and older who were treated for a range of medical and surgical critical illnesses, preexisting clinical frailty was present in nearly one out of three. Half of the patients with clinical frailty were younger than 65 years old. Although less common among younger patients, frailty was present in one out of five patients age 49 years or younger. After adjusting for a large number of confounders, those with a higher Clinical Frailty Scale score at enrollment (i.e., at the 75th percentile) suffered a 50% greater risk of death in the 12 months after a critical illness compared with those with a lower score (i.e., at the 25th percentile). Likewise, those with a higher Clinical Frailty Scale score had a 30% independent increase in the odds of disability in instrumental activities of daily living at 12-month follow-up. Patients with a higher Clinical Frailty Scale score also had worse physical health-related quality of life. The strength of these independent associations was not affected by age, indicating that patients of all ages along the fitness to frailty continuum are at risk for poor outcomes after critical illness.
Our findings build on and extend those of the prior multicenter Canadian and French cohorts that also found an association between baseline Clinical Frailty Scale score and mortality among older adults (17, 18). A recent subgroup analysis also reported greater mortality associated with frailty among patients age 50–65 years old (33). Unlike these prior studies, however, we considered the effect of frailty on adult patients of all ages, including those age 50 years and younger. Because frailty is classically considered to be an age-related condition, additional studies are needed to understand better the clinical conditions and biological mechanisms resulting in frailty among younger adults with critical illness.
We evaluated the independent association of each level of the Clinical Frailty Scale score with outcomes rather than stratifying patients into categories or considering patients as frail or not frail. We found the risk of poor outcomes increased with the Clinical Frailty Scale score. For example, the adjusted hazard of death increased with the Clinical Frailty Scale score even at the lowest levels, such that those with a score of 3 had twice the risk of death at 12 months as those with a score of 1, after considering a robust set of potential confounders of this association not evaluated in previous studies of frailty in the critically ill. These covariates included baseline cognitive status, granular details about exposure to a critical illness including daily severity of illness scores and days of severe sepsis, delirium, coma, and mechanical ventilation. Our findings suggest that future studies of frailty in patients with critical illness should consider the full continuum of fitness to frailty in patients of all ages.
We also report an independent association between baseline Clinical Frailty Scale scores and disability in instrumental activities of daily living. Patients with worse clinical frailty before developing critical illness were 20% and 30% less likely to be able to carry out instrumental activities of daily living at 3 and 12 months, respectively. Instrumental activities of daily living include such activities as handling finances, managing medications, using the telephone, and preparing meals. Thus, our findings advance the understanding of the types of disabilities suffered by survivors of critical illness across the continuum of frailty. The prior Canadian study reported a loss of functional independence after hospital discharge, but did not describe the underlying nature of disabilities (17, 19). The present study was underpowered to report data on specific instrumental activities of daily living disability phenotypes associated with frailty. This should be evaluated in future studies. Nevertheless, our findings suggest that future work to promote functional independence after critical illness should emphasize rehabilitation in instrumental activities of daily living.
We did not find an association between enrollment Clinical Frailty Scale score and disability in basic activities of daily living at follow-up. This finding contrasts with those from studies of frailty in both community-dwelling older adults (7, 11) and hospitalized, but noncritically ill, older adults (14). Several factors may explain these differences. First, because both baseline basic activities of daily living disability and Clinical Frailty Scale score are associated with greater mortality in the critically ill, the association between frailty and disability may be diminished by the competing risk of death (17, 18, 34, 35). Second, we adjusted for several factors relating to critical illness and ICU management that may be stronger predictors of disability in basic activities of daily living among patients with frailty. Third, we enrolled a younger cohort than these studies of community-dwelling older adults, and the causes of disability in older adults may differ from the younger, critically ill patients in the current study. Fourth, they may represent a type II error. Finally, we assessed disability at 3 and 12 months after ICU discharge. Because disability is a dynamic phenomenon, we may have missed short episodes of disability that resolved before 3-month follow-up (36–38).
Enrollment Clinical Frailty Scale score was not associated with worse cognition at follow-up. This finding is also in contrast to studies of community-dwelling older adults without critical illness, where frailty is an independent predictor of dementia (13, 15, 16). Our findings in critically ill patients differ from those in prior studies for several reasons. First, the follow-up time points in earlier studies were measured in years, in comparison with our 3- and 12-month assessments. The shorter duration of follow-up in the current study may be one reason for our divergent findings. Second, unlike prior studies (2, 39), we adjusted for factors that are known predictors of long-term cognitive impairment after critical illness, including duration of delirium (2). Nevertheless, even after removing delirium duration from our models, frailty was not associated with long-term cognitive impairment, indicating that delirium duration was not confounding a possible association of frailty with cognition. Third, the BRAIN-ICU and MIND-ICU cohort studies were designed to explore risk factors for new long-term cognitive impairment after critical illness. We used validated surrogate measures to exclude patients with dementia and other forms of severe cognitive impairment. Therefore, because cognitive impairment may be one way by which patients could become frail, our findings may not be generalizable to those who are frail as the result of cognitive impairment and dementia.
Finally, although frailty was not associated with worse cognition, it was associated with disability in instrumental activities of daily living. Three large cohort studies in noncritically ill older adults found disability in instrumental activities of daily living to be a predictor of cognitive impairment and dementia years later (40–43). Instrumental activities of daily living include such activities as handling finances, shopping, managing medications, preparing a balanced meal, and traveling outside one’s neighborhood and therefore place greater demands on cognitive function rather than on physical function (44). Thus, our findings may represent a preclinical form of cognitive impairment among survivors of critical illness (2, 39). Alternatively, a loss of independence in instrumental activities of daily living may represent a subjective form of cognitive impairment whereby patients alter their behavior in response to subtle cognitive changes. These possibilities should be explored further in future studies with longer follow-up periods than were available in the present study.
We also found that higher Clinical Frailty Scale scores at study enrollment were independently associated with worse physical health-related quality of life, but not mental health-related quality of life, using the SF-36. These findings seem to contradict those described by Bagshaw and colleagues (19), who found that frailty was independently associated with worse mental, but not physical, health-related quality of life measured using the SF-12. When health-related quality of life was measured in their cohort using the Euro-QoL 5D (EuroQol-5 Dimension), however, frailty was independently associated with worse health-related quality of life in the domains of mobility, self-care, carrying out usual activities, bodily pain, and anxiety/depression. Taken together, our data and those of Bagshaw suggest worse health-related quality of life in those with clinical frailty who survive a critical illness may be related to poor physical function. Nevertheless, because health-related quality of life reflects the way patients perceive and react to their health status or functional status (rather than being a direct measure of them) (45), work is needed to objectively assess long-term physical function in patients with preexisting clinical frailty who survive a critical illness.
Future studies are needed to evaluate and refine frailty assessment tools for patients with critical illness. Although we and others have demonstrated the association between the well-validated Clinical Frailty Scale and poor outcomes after critical illness, expert consensus recommendations (46) state that the Clinical Frailty Scale and other frailty screening tools (i.e., the FRAIL [Fatigue, Resistance, Ambulation, Illnesses, and Loss of Weight] questionnaire [47], the Cardiovascular Health Study Frailty Screening Measure [7], and the Gérontopôle Frailty Screening Tool [48]) should be used to identify patients who are in need of more in-depth assessment. These in-depth assessments include evaluation with a multidimensional tool, such as the Edmonton Frailty Scale (49) or via a comprehensive geriatric assessment. Nevertheless, the applicability of these reference standards for diagnosing frailty in patients with acute critical illness may be limited because they require performance-based assessments of physical and cognitive function and few in the ICU have formal training in geriatrics or gerontology. Therefore, work is needed to adapt these reference standards to the unique needs of patients with critical illness. A well-validated, ICU-specific, frailty reference standard could then be used to better understand the key elements and domains contributing to frailty in this population and to facilitate the study of the underlying mechanisms of frailty in the context of critical illness.
Frailty is believed to be a manageable and potentially reversible condition (46). Therefore, given the poor outcomes among patients with frailty who become critically ill, therapies shown to reduce frailty and improve function in noncritical illness settings, such as physical activity programs (50, 51), and multicomponent strategies (i.e., physical activity, nutrition, and cognitive training) (52) should be tested further in patients for whom critical illness can be anticipated (i.e., those undergoing large surgical procedures).
Strengths of the current investigation include a large, multicenter cohort of patients 18 years of age and older enrolled from a geographically diverse set of medical and surgical ICUs across the United States including academic, community, and Veterans Affairs hospitals treated for a wide range of critical illness diagnoses, thereby enhancing the generalizability of our findings. We also prospectively collected a range of detailed clinical, physiologic, and pharmacologic parameters daily throughout the hospitalization, along with measures of preillness disabilities, cognition, comorbidities, and sociodemographics, which allowed us to adjust for these potential confounders in our multivariable analyses. Finally, we were able to achieve excellent long-term follow-up that were performed by study staff blinded to Clinical Frailty Scale scores at enrollment and to details of the ICU course.
Our findings should be interpreted in the context of several limitations. First, although the Clinical Frailty Scale score is well-validated and widely used in studies of community dwelling older adults and now in critically ill patients, it was designed to have an element of subjectivity (8, 17, 18, 53–55). To minimize this subjectivity, study personnel underwent standardized training and used all information available to them, including patient or proxy interviews, review of the medical records, and study data to determine the baseline Clinical Frailty Scale score. Other well-validated measures of frailty, such as the frailty phenotype assessed using the Cardiovascular Health Study Frailty Screening Measure (7), require patients to perform physical maneuvers, such as handgrip dynamometry and a gait-speed assessment, which are difficult to reliably assess during the emergent phases of a critical illness (7, 35). Preliminary data from a longitudinal cohort study that assessed physical frailty over time, where some participants developed critical illness, however, suggest this complementary concept of frailty is also predictive of death and disability (56). Future studies should compare the predictive validity of these major concepts of frailty in patients with critical illness. Third, this study was conducted contemporaneously with the first modern studies of early mobility. Therefore, it was unlikely that patients received early mobility interventions. The effect of early mobility on disability outcomes among patients of all ages across the fitness to frailty continuum who develop critical illness should be studied in future investigations. Finally, we were unable to assess the trajectories of frailty, physical and cognitive function, and comorbid medical conditions before critical illness, which is a limitation of nearly all studies of emergently critically ill patients (57).
Conclusions
Our results suggest that preexisting frailty, as measured by the Clinical Frailty Scale, is common in critically ill patients, regardless of age. Moreover, the risk of death, disability, and poor health-related quality of life increased along the fitness-frailty continuum, independent of many traditional risk factors, including age. Future studies should explore the utility of the evaluating patients along the fitness-frailty continuum to identify patients with heightened vulnerability in routine ICU practice. Interventions associated with improved outcomes in at-risk community-dwelling persons should be evaluated as a means by which to improve post-critical illness morbidity and mortality.
Footnotes
Supported by the Department of Veterans Affairs Tennessee Valley Health Care System Geriatric Research, Education and Clinical Center and VA-MERIT, the National Institutes of Health (R01AG027472, KL2TR000446, K23AG048347, R03AG040549, R01AG035117, R01HL111111, K07AG043587, and P30AG21342), and the Eisenstein Women's Heart Fund. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The contents of this article are solely the responsibility of the authors and do not necessarily represent those of the Department of Veterans Affairs, the National Institutes of Health, Vanderbilt University, or Yale University.
Author Contributions: N.E.B. and E.W.E. had full access to the study data, and take responsibility for the integrity of the data and the accuracy of the data analysis. Design and conduct of the study, N.E.B., S.P.B., T.D.G., P.P.P., J.C.J., A.M., and E.W.E. Data acquisition, analysis and interpretation of the data, N.E.B., S.P.B., T.D.G., P.P.P., J.C.J., A.M., J.L.T., R.C., G.R.B., R.S.D., T.M.G., and E.W.E. Statistical analysis, J.L.T. and R.C. Drafting of the manuscript, N.E.B., S.P.B., and E.W.E. Critical revision of the article for important intellectual content, all authors. Final approval of the article, all authors. Obtaining funding, E.W.E., N.E.B., R.S.D., and G.R.B.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201605-0939OC on December 6, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, et al. BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 4.Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011;183:1037–1042. doi: 10.1164/rccm.201002-0301OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 6.Brummel NE, Balas MC, Morandi A, Ferrante LE, Gill TM, Ely EW. Understanding and reducing disability in older adults following critical illness. Crit Care Med. 2015;43:1265–1275. doi: 10.1097/CCM.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 8.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hébert R, Hogan DB, Wolfson C, McDowell I. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59:1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 11.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 12.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58:248–255. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med. 2007;69:483–489. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- 14.Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 15.Gray SL, Anderson ML, Hubbard RA, LaCroix A, Crane PK, McCormick W, Bowen JD, McCurry SM, Larson EB. Frailty and incident dementia. J Gerontol A Biol Sci Med Sci. 2013;68:1083–1090. doi: 10.1093/gerona/glt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song X, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict Alzheimer disease and dementia. Neurology. 2011;77:227–234. doi: 10.1212/WNL.0b013e318225c6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, Artiuch B, Ibrahim Q, Stollery DE, Rokosh E, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186:E95–E102. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Maguet P, Roquilly A, Lasocki S, Asehnoune K, Carise E, Saint Martin M, Mimoz O, Le Gac G, Somme D, Cattenoz C, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40:674–682. doi: 10.1007/s00134-014-3253-4. [DOI] [PubMed] [Google Scholar]
- 19.Bagshaw SM, Stelfox HT, Johnson JA, McDermid RC, Rolfson DB, Tsuyuki RT, Ibrahim Q, Majumdar SR. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med. 2015;43:973–982. doi: 10.1097/CCM.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 20.Hope AA, Gong MN, Guerra C, Wunsch H. Frailty before critical illness and mortality for elderly Medicare beneficiaries. J Am Geriatr Soc. 2015;63:1121–1128. doi: 10.1111/jgs.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng A, Song X, Dong J, Mitnitski A, Liu J, Guo Z, Rockwood K. Mortality in relation to frailty in patients admitted to a specialized geriatric intensive care unit. J Gerontol A Biol Sci Med Sci. 2015;70:1586–1594. doi: 10.1093/gerona/glv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brummel NE, Bell SP, Pandharipand PP, Jackson JC, Girard TD, Hughes C, Vasilevskis E, Thompson J, Chandrasekhar R, Ely E. Pre-critical illness frailty and its relationship to long-term clinical outcomes [abstract] Am J Respir Crit Care Med. 2015;191:A2280. [Google Scholar]
- 23.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 24.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL. A standardized measure of biological and psychological function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 25.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE. SF-36 Physical and Mental Health Summary Scales: a user's manual. Boston: Health Assessment Lab, New England Medical Center; 1994. [Google Scholar]
- 27.Little RJ, D’Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, Frangakis C, Hogan JW, Molenberghs G, Murphy SA, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367:1355–1360. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 30.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 31.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 32.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 33.Bagshaw M, Majumdar SR, Rolfson DB, Ibrahim Q, McDermid RC, Stelfox HT. A prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care. 2016;20:175. doi: 10.1186/s13054-016-1338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175:523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldwin MR, Reid MC, Westlake AA, Rowe JW, Granieri EC, Wunsch H, Dam TT, Rabinowitz D, Goldstein NE, Maurer MS, et al. The feasibility of measuring frailty to predict disability and mortality in older medical intensive care unit survivors. J Crit Care. 2014;29:401–408. doi: 10.1016/j.jcrc.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill TM, Gahbauer EA. Overestimation of chronic disability among elderly persons. Arch Intern Med. 2005;165:2625–2630. doi: 10.1001/archinte.165.22.2625. [DOI] [PubMed] [Google Scholar]
- 37.Gill TM, Gahbauer EA. Evaluating disability over discrete periods of time. J Gerontol A Biol Sci Med Sci. 2008;63:588–594. doi: 10.1093/gerona/63.6.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc. 2002;50:1492–1497. doi: 10.1046/j.1532-5415.2002.50403.x. [DOI] [PubMed] [Google Scholar]
- 39.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sikkes SA, Visser PJ, Knol DL, de Lange-de Klerk ES, Tsolaki M, Frisoni GB, Nobili F, Spiru L, Rigaud AS, Frölich L, et al. Do instrumental activities of daily living predict dementia at 1- and 2-year follow-up? Findings from the Development of Screening guidelines and diagnostic Criteria for Predementia Alzheimer’s disease study. J Am Geriatr Soc. 2011;59:2273–2281. doi: 10.1111/j.1532-5415.2011.03732.x. [DOI] [PubMed] [Google Scholar]
- 41.Luck T, Riedel-Heller SG, Luppa M, Wiese B, Bachmann C, Jessen F, Bickel H, Weyerer S, Pentzek M, König HH, et al. A hierarchy of predictors for dementia-free survival in old-age: results of the AgeCoDe study. Acta Psychiatr Scand. 2014;129:63–72. doi: 10.1111/acps.12129. [DOI] [PubMed] [Google Scholar]
- 42.Marshall GA, Zoller AS, Lorius N, Amariglio RE, Locascio JJ, Johnson KA, Sperling RA, Rentz DM. Functional Activities Questionnaire items that best discriminate and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res. 2015;12:493–502. doi: 10.2174/156720501205150526115003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luck T, Luppa M, Wiese B, Maier W, van den Bussche H, Eisele M, Jessen F, Weeg D, Weyerer S, Pentzek M, et al. AgeCoDe Study Group. Prediction of incident dementia: impact of impairment in instrumental activities of daily living and mild cognitive impairment-results from the German study on ageing, cognition, and dementia in primary care patients. Am J Geriatr Psychiatry. 2012;20:943–954. doi: 10.1097/JGP.0b013e31825c09bc. [DOI] [PubMed] [Google Scholar]
- 44.Fong TG, Gleason LJ, Wong B, Habtemariam D, Jones RN, Schmitt EM, de Rooij SE, Saczynski JS, Gross AL, Bean JF, et al. Successful Aging after Elective Surgery Functional Measures Working Group. Cognitive and physical demands of activities of daily living in older adults: validation of expert panel ratings. PM R. 2015;7:727–735. doi: 10.1016/j.pmrj.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA. 1994;272:619–626. [PubMed] [Google Scholar]
- 46.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16:601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subra J, Gillette-Guyonnet S, Cesari M, Oustric S, Vellas B, Platform T Platform Team. The integration of frailty into clinical practice: preliminary results from the Gérontopôle. J Nutr Health Aging. 2012;16:714–720. doi: 10.1007/s12603-012-0391-7. [DOI] [PubMed] [Google Scholar]
- 49.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cesari M, Vellas B, Hsu FC, Newman AB, Doss H, King AC, Manini TM, Church T, Gill TM, Miller ME, et al. LIFE Study Group. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci. 2015;70:216–222. doi: 10.1093/gerona/glu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gill TM, Baker DI, Gottschalk M, Gahbauer EA, Charpentier PA, de Regt PT, Wallace SJ. A prehabilitation program for physically frail community-living older persons. Arch Phys Med Rehabil. 2003;84:394–404. doi: 10.1053/apmr.2003.50020. [DOI] [PubMed] [Google Scholar]
- 52.Ng TP, Feng L, Nyunt MS, Feng L, Niti M, Tan BY, Chan G, Khoo SA, Chan SM, Yap P, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. Am J Med. 2015;128:1225–1236. doi: 10.1016/j.amjmed.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 53.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 54.Malmstrom TK, Miller DK, Morley JE. A comparison of four frailty models. J Am Geriatr Soc. 2014;62:721–726. doi: 10.1111/jgs.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ravindrarajah R, Lee DM, Pye SR, Gielen E, Boonen S, Vanderschueren D, Pendleton N, Finn JD, Tajar A, O’Connell MD, et al. European Male Aging Study Group. The ability of three different models of frailty to predict all-cause mortality: results from the European Male Aging Study (EMAS) Arch Gerontol Geriatr. 2013;57:360–368. doi: 10.1016/j.archger.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Ferrante L, Pisani M, Murphy T, Gehbauer E, Leo-Summers L, Gill TM. Frailty is associated with new or worsening disability after critical illness [abstract] Am J Respir Crit Care Med. 2016;193:A2614. [Google Scholar]
- 57.Iwashyna TJ, Netzer G, Langa KM, Cigolle C. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]