Abstract
Rationale: The standard approach to diagnosis of primary ciliary dyskinesia (PCD) in the United Kingdom consists of assessing ciliary function by high-speed microscopy and ultrastructure by election microscopy, but equipment and expertise is not widely available internationally. The identification of biallelic disease-causing mutations is also diagnostic, but many disease-causing genes are unknown, and testing is not widely available outside the United States. Fluorescent antibodies to ciliary proteins are used to validate research genetic studies, but diagnostic utility in this disease has not been systematically evaluated.
Objectives: To determine utility of a panel of six fluorescent labeled antibodies as a diagnostic tool for PCD.
Methods: The study used immunofluorescent labeling of nasal brushings from a discovery cohort of 35 patients diagnosed with PCD by ciliary ultrastructure, and a diagnostic accuracy cohort of 386 patients referred with symptoms suggestive of disease. The results were compared with diagnostic outcome.
Measurements and Main Results: Immunofluorescence correctly identified mislocalized or absent staining in 100% of the discovery cohort. In the diagnostic cohort immunofluorescence successfully identified 22 of 25 patients with PCD and normal staining in all 252 in whom PCD was considered highly unlikely. In addition, immunofluorescence provided a result in 55% (39) of cases that were previously inconclusive. Immunofluorescence results were available within 14 days, costing $187 per sample compared with electron microscopy (27 days; cost $1,452).
Conclusions: Immunofluorescence is a highly specific diagnostic test for PCD, and it improves the speed and availability of diagnostic testing. However, sensitivity is limited and immunofluorescence is not suitable as a stand-alone test.
Keywords: cilia, electron microscopy, antibody
At a Glance Commentary
Scientific Knowledge on the Subject
Primary ciliary dyskinesia is a genetically heterogeneous chronic condition. Early diagnosis is key to attenuating disease progression by implementation of appropriate medical management. Currently diagnosis requires expensive and complex equipment.
What This Study Adds to the Field
This study validates the clinical use of a panel of commercially available antibodies to diagnose primary ciliary dyskinesia by immunofluorescence—a simpler, more widely available, cost-effective alternative to current confirmatory diagnostic tests. Immunofluorescence is a useful diagnostic test for primary ciliary dyskinesia, reduces the need for repeat biopsies, and improves turnaround time without compromising diagnostic accuracy.
Primary ciliary dyskinesia (PCD) affects approximately 1 in 15,000 of the population. Its manifestations are caused by defective ciliary beating and reduced mucociliary clearance. Diagnosis is frequently delayed, and delay is associated with significant impairment of lung function. Diagnostic delay is related to two factors: the nonspecificity of symptoms (cough, rhinitis) and the lack of an easy and widely available diagnostic test for the condition (1).
The diagnostic pathway for PCD typically includes measurement of nasal nitric oxide and nasal brush biopsy for light and electron microscopy. Light microscopic assessment of ciliary function on cells ex vivo is by high-speed video analysis of the frequency and pattern (waveform) of cilia movement. Electron microscopy allows visualization of the ultrastructure of cilia and can often provide a definitive diagnosis (1). All these tests require sophisticated equipment and considerable expertise, and in consequence are only available in very few centers. Genetic testing for PCD is also increasingly used, but there are at least 200 potential motile cilia genes that are widely scattered through the human genome. To date, more than 30 disease-associated mutations have been identified, which are estimated to account for 60–65% of known cases (1–3).
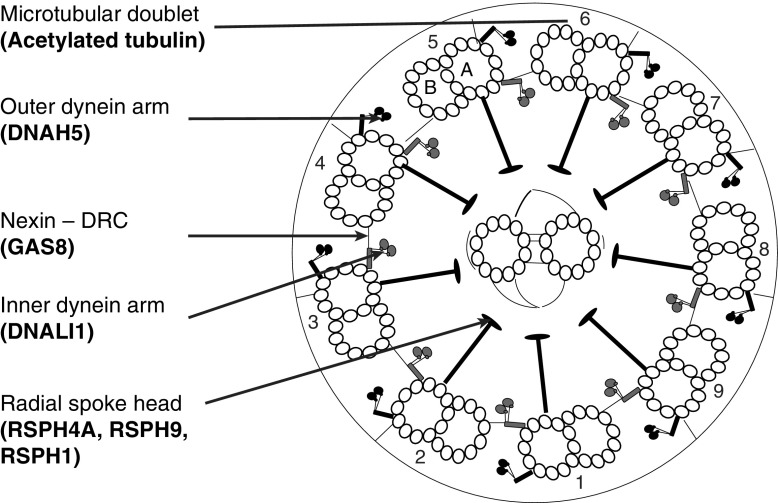
Immunofluorescence allows indirect imaging of target proteins by fluorescent or confocal microscopy using specific antibodies with fluorescent tags. The use of different tags for double labeling allows the colocalization of proteins to be determined. Immunofluorescence for the diagnosis of PCD was first described in 2005 and was subsequently recommended in the European Respiratory Society expert consensus statement for diagnosis and treatment of PCD (4, 5). Despite this recommendation limited availability of validated antibodies and lack of evidence for the diagnostic accuracy of the technique has limited its use. Immunofluorescence has been used extensively in PCD research in confirming protein absence caused by genetic mutations. Several antibodies to proteins defective in PCD have been developed and validated. These include DNAH5 (an outer dynein arm heavy chain) (4, 6); DNALI1 (an inner dynein arm light chain) (6, 7); GAS8 (a component of the nexin-dynein regulatory complex) (7); and RSPH4A, RSPH9, and RSPH1 (components of the radial spoke) (8). These antibodies represent markers of the four key ultrastructural abnormalities in PCD. Detected by electron microscopy these defects are the end products of multiple gene defects (Table 1, Figure 1). By immunofluorescence the absence or mislocation of a single protein can allow the effects of mutations in multiple genes to be detected (Table 1). We hypothesized that immunofluorescence using a panel of antibodies would be a useful diagnostic test for PCD, and aimed to assess this in the clinic in a large cohort of patients with possible PCD referred for diagnostic testing.
Table 1.
Major Classes of Ultrastructural Defects Seen in Primary Ciliary Dyskinesia and the Gene Associated with Each Defect
| Ultrastructural Defect | Gene | Immunofluorescence |
|---|---|---|
| Outer dynein arm defect | DNAH5 | DNAH5 absent |
| DNAI1 | ||
| ARMC4 | ||
| CCDC114 | ||
| TXNDC3 (NME8) | ||
| DNAI2 | ||
| DNAL1 | ||
| CCDC151 | ||
| Inner and outer dynein arm defect | C21orf59 | DNAH5 absent |
| ZYMND10 | DNALI1 absent | |
| CCDC103 | ||
| DNAFF2 (KTU) | ||
| DNAFF1 (LRRC50) | ||
| LRRC6 | ||
| DNAFF3 (C19orf31) | ||
| HEATR2 | ||
| DYX1C1 | ||
| SPAG1 | ||
| Central complex/transposition defect | RSPH4A | RSPH9, RSPH4A, and RSPH1 absent |
| RSPH9 | RSPH9 absent | |
| RSPH1 | RSPH9 and RSPH1 absent | |
| RSPH3 | All present | |
| Microtubular disorganization with loss of inner dynein arm | CCDC39 | DNALI1 absent |
| CCDC40 | GAS8 absent | |
| Microtubular disorganization with present inner dynein arms | CCDC65 | GAS8 absent |
| CCDC164 | ||
| GAS8 | ||
| Normal ciliary ultrastructure | HYDIN | All present |
| DNAH11 | ||
| OFD1 | ||
| RPGR | ||
| Ciliary “aplasia” | CCNO | All present |
| MCIDAS | DNAH5 and LI1 absent |
Definition of abbreviations: Absent = complete or partial absence from the axoneme.
Former gene name is shown in parentheses. Column three “Immunofluorescence” shows the assumed coverage of the six antibodies investigated in this study over the known ultrastructural and gene defects.
Figure 1.
Diagram of the ultrastructure of a motile cilium in transverse section. Labels indicate ultrastructural features targeted by immunofluorescence with corresponding antibodies (bold text). The cilium has a 9+2 arrangement. Nine microtubular doublets (numbered), each consisting of an A and B tubule, surround a central pair of microtubules. DRC = dynein regulatory complex.
Methods
Subjects
In the discovery cohort, nasal brushings were analyzed from a cohort of 35 patients with a known PCD ultrastructural defect (thus excluding 30% of patients with PCD with normal ultrastructure) (9).
In the diagnostic accuracy cohort, nasal brushings were analyzed from 386 patients sequentially referred to the Royal Brompton Hospital for PCD diagnosis. Referrals were because of symptoms suggestive of PCD, such as situs inversus, neonatal respiratory distress, bronchiectasis, recurrent chest infections, rhinosinusitis, and otitis media (see Table E1 in the online supplement).
Diagnosis of PCD
All patients underwent a standardized diagnostic protocol regularly audited across three U.K. PCD centers that form a national specialized service (Leicester Royal Infirmary, Southampton Hospital, and Royal Brompton Hospital) (1). This consisted of six assessments as follows:
-
1.
Assessment for symptoms suggestive of PCD (n = 378 of 386; eight external samples had a limited clinical history).
-
2.
Nasal nitric oxide measurement in children older than 4 years. Two readings from each nostril were taken during a breath hold maneuver using a chemiluminescent analyzer LR2000 (Logan Research, Rochester, UK) (n = 129 of 386) (10).
-
3.
High-speed videomicroscopy for cilia beat frequency and ciliary beat pattern measured at 37°C using a ×100 objective (n = 386 of 386) (11).
-
4.
Quantitative transmission electron microscopy of ciliary ultrastructure (n = 208 of 386) (12).
-
5.
Air–liquid interface culture of difficult samples and repeat light and electron microscopy (n = 115 of 386) (13).
-
6.
Genotyping from blood sample in patients with likely PCD based on positive results from at least two of the other investigations (n = 16 of 386).
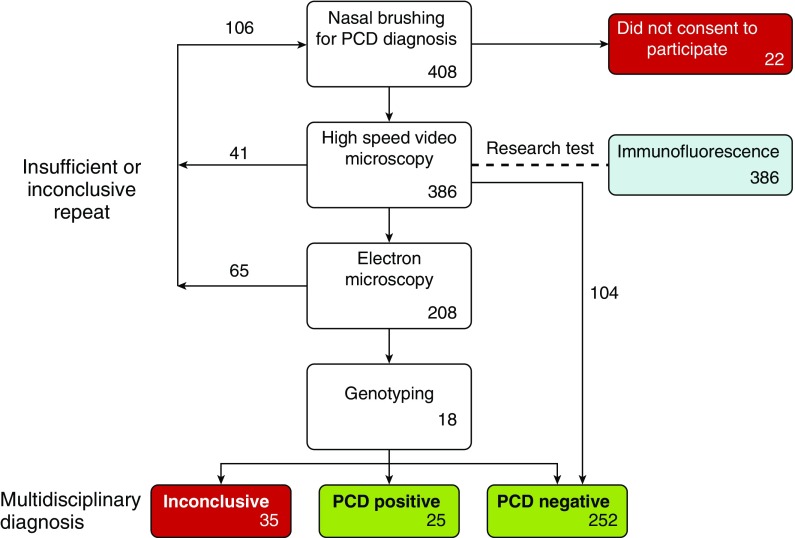
In the absence of an established gold standard, a diagnosis of PCD was made after a review of clinical and laboratory findings in a monthly multidisciplinary meeting led by a consultant clinician with expertise in PCD. Study design and recruitment are shown in Figure 2. Further details of diagnostic decision making pathways are described in the online supplement.
Figure 2.
Workflow diagram indicating the pathway of patients from referral to diagnosis during the study period. Numbers indicate the number of patients at each stage of the study (not the number of samples). Excluded groups are indicated in red. PCD = primary ciliary dyskinesia.
Experimental Methods
Methodologic details are provided in the online supplement (4). All slides were double labeled with acetylated α tubulin (T7451, Sigma Aldrich, St. Louis, MO) to visualize cilia. Antibodies of interest were used in a two-step protocol. All nasal brushings were assessed for (Panel 1) DNAH5 (HPA037470), DNALI1 (HPA053129), and RSPH4A (HPA031196). A second round of antibodies was used in selected cases (Panel 2): RSPH9 (HPA031703), RSPH1 (HPA017382), or GAS8 (HPA041311).
Slides were scanned under a fluorescent microscope for ciliated cells at ×40 magnification to identify acetylated tubulin. In each ciliated cell, the colocalized ciliary protein of interest was assessed in a second channel. If there was visual colocalization of the antibody label with acetylated tubulin, the target protein was considered present. If more than 7 of 10 ciliated cells were clearly labeled with the target protein antibody the sample was considered normal for that protein. PCD was defined as diagnosed when on duplicate slides all ciliated cells observed had absent staining of the target protein from the axoneme or staining consistently isolated to the distal or proximal portion of the cilia. Insufficient (<10 cells observed) and inconclusive slides were repeated. If the first slide was insufficient or inconclusive but the second assessment was normal the result was considered normal (n = 71).
Statistical Methods
The number of patient results reviewed was based on a 10% prevalence of PCD in the population tested. Power calculations predicted 271 patients with a confirmed diagnosis of “PCD” or “PCD highly unlikely” would be required for 95% confidence. Results were analyzed in GraphPad Prism version 5 (La Jolla, CA) and a P less than 0.05 was considered significant.
Ethics
This study was conducted according to the recommendations of the Declaration of Helsinki. The protocol was approved by ethical review committee and written consent was obtained from subjects or their parent or guardian.
Results
Immunofluorescence Can Be Used to Confirm a Diagnosis of PCD
Antibodies were tested on nasal brushings from a discovery cohort of 35 patients with PCD with a confirmed ultrastructural ciliary defect. Results shown in Table 2 demonstrated a complete agreement between the absence of structure by electron microscopy and absence of associated protein by immunofluorescence.
Table 2.
Immunofluorescence Antibody Results from 35 Patients with PCD Caused by a Known Ultrastructural Defect
| Electron Microscopy Defect | Absent or Mislocalized Antibody | Number of Patients with PCD Tested (N = 35) |
|---|---|---|
| Outer dynein arm defect | DNAH5 | 14 |
| Outer and inner dynein arm defect | DNAH5, DNALI1 | 10 |
| Inner dynein arm and microtubular disorganization defect | DNALI1, GAS8 | 7 |
| Transposition defect/central pair absence | RSPH4A, RSPH9, RSPH1 | 4 |
Definition of abbreviation: PCD = primary ciliary dyskinesia.
Diagnostic Accuracy of Immunofluorescence in the Diagnostic Accuracy Cohort
The immunofluorescence technique successfully demonstrated an absence of target proteins from the axoneme in 22 of 25 patients with a diagnosis of PCD as defined previously. Normal results were obtained in all 252 patients who were considered highly unlikely to have PCD.
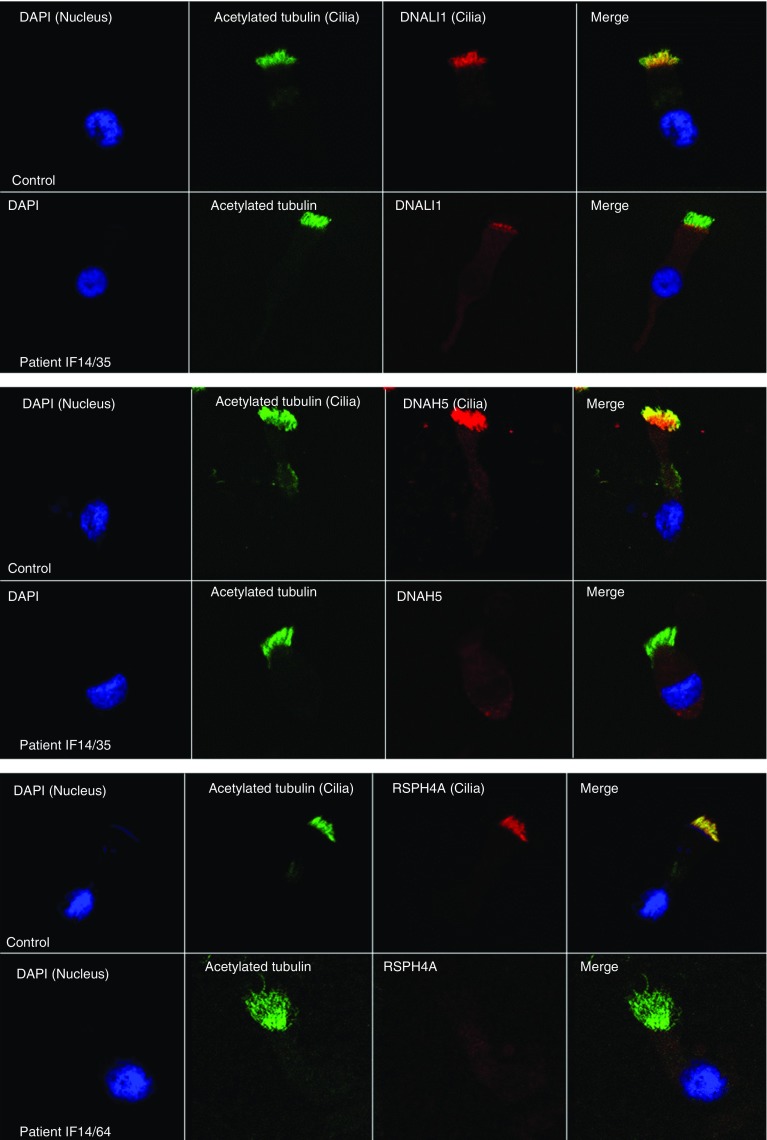
Figure 3 shows an example of immunofluorescent staining of ciliated epithelial cells from two patients who were PCD-positive and a patient who was PCD highly unlikely.
Figure 3.
Example results in primary ciliary dyskinesia and “primary ciliary dyskinesia highly unlikely” (control) samples for three antibodies used in the immunofluorescence panel. The left column shows the cell nucleus in blue by 4’,6-diamidino-2-phenylindole (DAPI); the next column shows presence of cilia on the cell in green by acetylated tubulin. The third column shows the protein of interest in red, and the final column a merged image of the three channels. In the merged image yellow suggests colocalization and presence of the protein of interest, and green suggests absence of the protein of interest. The top images show DNALI1 (an inner dynein arm component), the middle images DNAH5 (an outer dynein arm component), and the bottom images RSPH4A (a radial spoke head component).
Results of the diagnostic tests and immunofluorescence tests are shown in Tables 3 and 4. Immunofluorescence identified PCD protein defects in all patients who had an identifiable ultrastructural defect by electron microscopy. The number of patients in which PCD was confirmed by immunofluorescence was the same as that of electron microscopy.
Table 3.
Diagnostic Outcome Table Comparing Immunofluorescence Technique with the Standard Diagnostic Approach in 271 Patients Referred to a National Referral Center for Investigation into Symptoms Suggestive of PCD
| Immunofluorescence Diagnosis | Standard Diagnosis |
|
|---|---|---|
| PCD Positive | PCD Negative | |
| PCD positive | 22 | 0 |
| PCD negative | 3 | 252 |
Definition of abbreviation: PCD = primary ciliary dyskinesia.
Table 4.
Comparison of Results from Electron Microscopy and Immunofluorescence Testing of Nasal Brushings from Patients Referred because of Clinical Symptoms Suggestive of PCD
| Multidisciplinary Diagnosis | Electron Microscopy | Immunofluorescence |
|||||
|---|---|---|---|---|---|---|---|
| Panel 1 |
Panel 2 |
||||||
| DNAH5 | DNALI1 | RSPH4A | GAS8 | RSPH9 | RSPH1 | ||
| Normal (n = 208) | Normal ultrastructure (97% both dynein arms present [±7%] 89% normal microtubular arrangement [±6%]) | Present | Present | Present | ND | ND | ND |
| PCD (n = 25) | IDA and ODA defect (n = 8) | Absent | Absent | Present | Present | ND | ND |
| ODA defect (n = 8) | Absent | Present | Present | ND | ND | ND | |
| IDA and MTD defect (n = 2) | Present | Absent | Present | Absent | ND | ND | |
| Transposition defect (n = 1) | Present | Present | Absent | Present | Absent | Absent | |
| MTD defect (n = 3) | Present | Present | Present | Absent | Present | Present | |
| Normal ultrastructure (n = 3) | Present | Present | Present | Present | Present | Present | |
Definition of abbreviations: IDA = inner dynein arm; MTD = microtubular disorganization; ND = test not performed; ODA = outer dynein arm; PCD = primary ciliary dyskinesia.
Absent or mislocalized proteins are shown as Absent in bold. Results from all diagnostic tests are shown in Table E1.
Three patients who were diagnosed as PCD-positive were not identified using the immunofluorescence protocol and were examined in closer detail. Genetic tests in two patients showed mutations in DNAH11. Both patients had a beat pattern consistent with this defect (hyperfrequent, stiff, and static) on more than one biopsy and nasal nitric oxide less than 77 nl/min. One patient had consanguineous parents and was homozygous for a nonsense mutation (c.3380G > A, p.Trp1127*), and the other had heterozygous nonsense changes (c.5506C > T, p.Arg1836* and c.5636T > A, p.Leu1879*). The third individual was also from consanguineous parentage and was homozygous for a frame shift mutation in the HYDIN gene (c.2196dupT, p.Y372fs). The cilia ultrastructure in these three patients was considered normal by standard electron microscopy. Electron tomography from the patient with the HYDIN mutations showed absence of the c2b central pair projection (14).
Insufficient and Inconclusive Samples
Immunofluorescence as part of the diagnostic pathway could reduce the requirement for repeat nasal brushing
In 71 patients a conclusive multidisciplinary diagnosis was not made on first sampling because of insufficient tissue for light and/or electron microscopy. The immunofluorescence protocol required fewer ciliated cells and was able to produce a definitive result for 55% (39 of 71) of these cases. Two of these cases showed an absence of DNAH5 by immunofluorescence and this was supported subsequently by electron microscopy on a repeat biopsy as an outer dynein arm defect. The remaining 37 samples, in which immunofluorescence testing was normal, were considered PCD highly unlikely on second testing either by repeat nasal brushing (n = 31) or by culturing the original sample at air-liquid interface (n = 6). Once a sufficiently ciliated sample had been acquired by repeat brushing or cell culture the high-speed video and electron microscopy were performed as per the original protocol. None of these patients underwent genetic testing.
Insufficient and inconclusive results were sometimes obtained by immunofluorescence when a multidisciplinary result was conclusive
In 42 samples in which a multidisciplinary diagnosis was made there were not enough cells for immunofluorescence. In a further 32 samples in which a multidisciplinary diagnosis was made the immunofluorescence result for one or more antibody was inconclusive on more than one occasion. Sixty nine of these patients (represented by 71 samples) did not have PCD; however, two were diagnosed with PCD at the multidisciplinary meeting. One of these two patients yielded a sample that was insufficient for immunofluorescence, light microscopy, and electron microscopy but was found to have a mutation in the CCNO gene (c.258_262dupGGCCC, p.Gln88Argfs*8). This patient was related to a previously genetically characterized family with reduced generation of multiple motile cilia (15). The second patient with PCD had DNAH5, DNALI1, and GAS8 present; however, inconclusive results were obtained by immunofluorescence for RSPH4A on two separate slides and the other radial spoke head proteins RSPH1 and RSPH9 were absent. This patient had a circular beat pattern on light microscopy and a transposition/central pair complex defect detected by electron microscopy. These insufficient and inconclusive patients were excluded from the diagnostic accuracy analysis. In 35 patients a diagnosis of PCD-positive or PCD highly unlikely could not be reached during the study period.
We further investigated factors relating to insufficient and inconclusive immunofluorescent results. Both blood and mucus in the sample seemed to be confounding factors. In 63% of samples with inconclusive results by immunofluorescence viscous mucus was surrounding the cilia on the high-speed video light microscopy assessment compared with 46% of conclusive samples (P < 0.05). In 25% of samples insufficient for immunofluorescence blood was seen in the sample compared with 8% in sufficient samples (P < 0.01). The cause of this relationship is unknown. We hypothesize that increased blood could prevent cells from attaching to the slides thus reducing available sample for immunofluorescence. Alternatively an increase of blood in a sample could represent damaged mucosa denuded of cilia as a result of a recent infective or inflammatory process.
Samples were deemed insufficient or inconclusive if a result could not be obtained for one or more antibodies. Thirty percent were inconclusive or insufficient for just one antibody, 16% for two antibodies, and 54% for three or more antibodies. Couriered and cultured samples showed similar results compared with nasal brushings taken on site.
Time for Results to be Available and Cost of Investigation
The effectiveness of immunofluorescence for diagnosis was compared with electron microscopy. The turnaround time, defined as time from the sample being taken to the results being reported was median 14 days (range, 1–40) for immunofluorescence compared with 27 days (range, 9–61) for electron microscopy (P < 0.05). In addition, a cost assessment exercise that included staff hours, equipment running costs, and consumables showed that the cost per sample was $187 for immunofluorescence and $1,452 for electron microscopy. The assessment did not include the purchase and set up of equipment required or brushes for obtaining cells, because surplus cells from videomicroscopy are used for both techniques.
Discussion
Using immunofluorescence all cilia structural defects were correctly identified in an initial discovery cohort of patients with a known electron microscopy–based ultrastructural diagnosis of PCD. In a cohort subsequently tested for diagnostic accuracy consisting of 386 consecutively referred cases immunofluorescence successfully identified 22 of 25 patients with a multidisciplinary diagnosis of PCD and 252 of 252 in whom the diagnosis was considered highly unlikely. The accuracy of immunofluorescence was the same as that of electron microscopy. Immunofluorescence failed to identify 12% PCD cases in the present study, and may well miss more cases in populations with a different genetic makeup, and is therefore not suitable for use as a standalone test. This report does, however, provide strong evidence for introducing this test into clinical practice as part of the diagnostic armamentarium for PCD.
The main strength of the immunofluorescence technique is the cost reduction and improved turnaround time relative to electron microscopy to confirm a diagnosis of PCD. The cost of the test and the basic equipment and the simplicity of the test may allow improved accessibility to a wider population of patients. The immunofluorescence technique also works on small samples where there are too few cells to process for electron microscopy analysis. In the diagnostic cohort an additional 34 cases could have been diagnosed with the inclusion of immunofluorescence before electron microscopy in the diagnostic pathway.
The strengths of this study include that it has been conducted in the United Kingdom clinical setting, which follows a nationally audited and standardized algorithm for PCD diagnosis giving a realistic indication of how this test performs. Other strengths include the use of discovery and validation cohorts, with large numbers in this latter group in particular.
The foundation for pursuing this study, and an acknowledged problem in PCD diagnostics, is the lack of gold standard for the diagnosis of PCD. It is difficult to exclude a diagnosis of PCD due to poor sensitivity of electron microscopy and genetic testing and the poor specificity of nasal nitric oxide (16). Despite use of a multidisciplinary diagnosis to maximize diagnostic capability, 15 patients had an indeterminate diagnosis (a situation also seen in cystic fibrosis, for example).
Our approach to ruling in or ruling out a diagnosis of PCD is certainly not perfect. There are multiple variables that could lead to false negatives. (1) The lack of standardized clinical phenotyping, whereby diagnostic decisions are made by a monthly multidisciplinary meeting (an approach that is used in interstitial lung disease for example) (17), using clinical judgment. There are two recently published approaches to define specific criteria for a clinical phenotype that have significant predictive power for a subject having PCD, which could be used in future studies (18, 19), although both also have imperfect sensitivity and specificity. (2) The lack of ciliary electron microscopy defects in approximately 30% of patients with PCD (9), and many of these cannot be diagnosed by immunofluorescence. (3) The limitations of high-speed videomicroscopy. (4) Only a minority of patients (129 of 386) were tested by nasal nitric oxide, although normal nitric oxide does also not rule out PCD (10). (5) There is limited genetic testing (16 of 386), and in any event, many PCD genes are currently unknown. Thus, it seems logical to conclude that an unknown number with indeterminate diagnosis will have PCD, and exclusion of these cases from this study will have exaggerated the perceived accuracy of the immunofluorescence test. Nonetheless, our results suggest that immunofluorescence is a useful part of diagnostic testing for PCD.
Immunofluorescence was unsuccessful in several cases. These cases were excluded from the analysis. This resulted in a case with reduced generation of motile cilia (CCNO) being excluded from this study because there were insufficient cilia to perform the immunofluorescence test. Exclusion of unsuccessful immunofluorescence analysis is another factor that could lead to an overestimation of the test performance. Test failure again highlights that immunofluorescence should not be used as a stand-alone test, because several patients would need further repeat investigations. We identified blood and mucus to be associated with failed testing and suggest further methodology improvements could focus on these factors.
The major limitation of the immunofluorescence technique is that the antibodies used are directed to specific proteins of interest and therefore defects in unrelated proteins are missed. The selected six-antibody panel represents the major ultrastructural defects and end products of multiple gene defects; however, three cases were still missed. The three cases missed in this study had biallelic mutations in DNAH11 and HYDIN. This is expected because previous publications have demonstrated the outer and inner dynein arms, nexin links, and radial spokes are present in patients with these defects (14, 20). Normal ultrastructure cases such as these are also the most likely to be missed using our diagnostic protocol because of limited genetic studies. Despite patients with DNAH11 mutations making up 8% of PCD-positive patients in the validation cohort, which is in keeping with predicted numbers of 6–9% from genetic screening programs, it is possible that additional genetic testing may lead to the diagnosis of further PCD cases (21).
New reliable antibodies to DNAH11 have recently been reported and will benefit diagnosis and research in this area (20). Antibodies to HYDIN proteins are commercially available; however, we have not been able to validate the use sufficiently for use in PCD diagnosis, nor is there any report in the literature of the successful use of these antibodies to identify PCD. Furthermore, the cilium consists of more than 200 proteins and patients with partial defects or missense mutations have been shown to have normal immunofluorescence results. It is therefore likely that other cases of PCD and reduced ciliation will be missed by the current immunofluorescence technique and the panel of antibodies will need to be increased in the future as the field of PCD genetics expands (20).
Given the similar diagnostic rate as electron microscopy we envisage that immunofluorescence could be useful where transmission electron microscopy equipment or expertise is not available. In specialist diagnostic centers where microscopy facilities are available the technique could be added to the diagnostic protocol to improve diagnostic success and reduce the number of electron microscopy tests required. Application of the immunofluorescence technique in this study was incorporated into our PCD diagnostic pathway after light microscopy assessment of cilia beat frequency and waveform. First, a core panel of three antibodies was applied, and then a second panel based on the primary panel and the light microscopy findings. This two-step protocol allows cost, time, and tissue savings but introduces a selection bias. In this study three patients were diagnosed using the second panel by the GAS8 antibody. Because absence of DNALI1 always coexisted with absence of GAS8 or DNAH5 we suggest DNALI1 might be substituted for GAS8 in the first panel. Recent data show that use of a RSPH9 antibody may detect a broader range of central pair complex defects than RSPH4A (22). Therefore, GAS8, DNAH5, and RSPH9 might be a more appropriate selection for the first panel.
In conclusion immunofluorescence is a useful diagnostic test for PCD, reduces the need for repeat biopsies, and improves turnaround time without compromising diagnostic accuracy. We suggest it should be included in the routine PCD diagnostic pathway.
Acknowledgments
Acknowledgment
The authors thank National Health Service England for their continued support of the UK PCD specialized service; Winston Banyan from the Royal Brompton Hospital statistical services and Rachael Joynes from the research and development office for their help and support; Faye Boswell and Adrian Morgan Long for their contribution to immunofluorescence staining; and Thomas Cullup, Christopher Boustred, Bethan Hoskins, and Lucy Jenkins from the North East Thames Regional Genetics Service at Great Ormond Street Hospital for Children NHS Foundation Trust for genotyping and bioinformatics analysis.
Footnotes
Supported by a postdoctoral research fellowship from the National Institute of Health Research and Health Education England (A.S., mentored by C.H., H.M.M., and A.B.), the National Institute of Health Research Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield National Health Service (NHS) Foundation Trust and Imperial College London (A.B.), the Great Ormond Street Hospital Children’s Charity (H.M.M.), and grants from the Milena Carvajal Pro-Kartagener Foundation and Action Medical Research (GN2101, H.M.M.). This report is independent research arising from a postdoctoral research fellowship supported by the National Institute of Health Research and Health Education England. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute of Health Research, or the Department of Health.
Author Contributions: A.S., C.H., and A.B. designed the study. E.F., K.K., S.O., and A.S. obtained patient consent, conducted light microscopy, collected nasal brushings, and prepared slides. E.F. and A.S. conducted immunofluorescent staining and analysis. M.D. conducted light and electron microscopy. H.M.M., M.P., and J.S. provided genotyping. A.S. and E.F. analyzed the data. A.S., C.H., and A.B. drafted the manuscript. All authors contributed to manuscript drafts and preparation. A.S. is custodian of the data and takes responsibility for its accuracy.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201607-1351OC on February 15, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lucas JS, Burgess A, Mitchison HM, Moya E, Williamson M, Hogg C National PCD Service, UK. Diagnosis and management of primary ciliary dyskinesia. Arch Dis Child. 2014;99:850–856. doi: 10.1136/archdischild-2013-304831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horani A, Brody SL, Ferkol TW. Picking up speed: advances in the genetics of primary ciliary dyskinesia. Pediatr Res. 2014;75:158–164. doi: 10.1038/pr.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fliegauf M, Olbrich H, Horvath J, Wildhaber JH, Zariwala MA, Kennedy M, Knowles MR, Omran H. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am J Respir Crit Care Med. 2005;171:1343–1349. doi: 10.1164/rccm.200411-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, Bartoloni L, Eber E, Escribano A, Haarman E, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–1276. doi: 10.1183/09031936.00176608. [DOI] [PubMed] [Google Scholar]
- 6.Loges NT, Olbrich H, Becker-Heck A, Häffner K, Heer A, Reinhard C, Schmidts M, Kispert A, Zariwala MA, Leigh MW, et al. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am J Hum Genet. 2009;85:883–889. doi: 10.1016/j.ajhg.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antony D, Becker-Heck A, Zariwala MA, Schmidts M, Onoufriadis A, Forouhan M, Wilson R, Taylor-Cox T, Dewar A, Jackson C, et al. Uk10k. Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum Mutat. 2013;34:462–472. doi: 10.1002/humu.22261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onoufriadis A, Shoemark A, Schmidts M, Patel M, Jimenez G, Liu H, Thomas B, Dixon M, Hirst RA, Rutman A, et al. UK10K. Targeted NGS gene panel identifies mutations in RSPH1 causing primary ciliary dyskinesia and a common mechanism for ciliary central pair agenesis due to radial spoke defects. Hum Mol Genet. 2014;23:3362–3374. doi: 10.1093/hmg/ddu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boon M, Smits A, Cuppens H, Jaspers M, Proesmans M, Dupont LJ, Vermeulen FL, Van Daele S, Malfroot A, Godding V, et al. Primary ciliary dyskinesia: critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet J Rare Dis. 2014;9:11. doi: 10.1186/1750-1172-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narang I, Ersu R, Wilson NM, Bush A. Nitric oxide in chronic airway inflammation in children: diagnostic use and pathophysiological significance. Thorax. 2002;57:586–589. doi: 10.1136/thorax.57.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chilvers MA, O’Callaghan C. Analysis of ciliary beat pattern and beat frequency using digital high speed imaging: comparison with the photomultiplier and photodiode methods. Thorax. 2000;55:314–317. doi: 10.1136/thorax.55.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoemark A, Dixon M, Corrin B, Dewar A. Twenty-year review of quantitative transmission electron microscopy for the diagnosis of primary ciliary dyskinesia. J Clin Pathol. 2012;65:267–271. doi: 10.1136/jclinpath-2011-200415. [DOI] [PubMed] [Google Scholar]
- 13.Hirst RA, Rutman A, Williams G, O'Callaghan C. Ciliated air-liquid cultures as an aid to diagnostic testing of primary ciliary dyskinesia. Chest. 2010;138:1441–1447. doi: 10.1378/chest.10-0175. [DOI] [PubMed] [Google Scholar]
- 14.Olbrich H, Schmidts M, Werner C, Onoufriadis A, Loges NT, Raidt J, Banki NF, Shoemark A, Burgoyne T, Al Turki S, et al. UK10K Consortium. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am J Hum Genet. 2012;91:672–684. doi: 10.1016/j.ajhg.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey JP, McGettigan PA, Healy F, Hogg C, Reynolds A, Kennedy BN, Ennis S, Slattery D, Lynch SA. Unexpected genetic heterogeneity for primary ciliary dyskinesia in the Irish Traveller population. Eur J Hum Genet. 2015;23:210–217. doi: 10.1038/ejhg.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas JS, Barbato A, Collins SA, Goutaki M, Behan L, Caudri D, Dell S, Eber E, Escudier E, Hirst RA, et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia Eur Respir J 201749:p11:1601090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer KC. Multidisciplinary discussions and interstitial lung disease diagnosis: how useful is a meeting of the minds? Lancet Respir Med. 2016;4:529–531. doi: 10.1016/S2213-2600(16)30065-0. [DOI] [PubMed] [Google Scholar]
- 18.Behan L, Dimitrov BD, Kuehni CE, Hogg C, Carroll M, Evans HJ, Goutaki M, Harris A, Packham S, Walker WT, et al. PICADAR: a diagnostic predictive tool for primary ciliary dyskinesia. Eur Respir J. 2016;47:1103–1112. doi: 10.1183/13993003.01551-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leigh MW, Ferkol TW, Davis SD, Lee HS, Rosenfeld M, Dell SD, Sagel SD, Milla C, Olivier KN, Sullivan KM, et al. Clinical features and associated likelihood of primary ciliary dyskinesia in children and adolescents. Ann Am Thorac Soc. 2016;13:1305–1313. doi: 10.1513/AnnalsATS.201511-748OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dougherty GW, Loges NT, Klinkenbusch JA, Olbrich H, Pennekamp P, Menchen T, Raidt J, Wallmeier J, Werner C, Westermann C, et al. DNAH11 localization in the proximal region of respiratory cilia defines distinct outer dynein arm complexes. Am J Respir Cell Mol Biol. 2016;55:213–224. doi: 10.1165/rcmb.2015-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knowles MR, Leigh MW, Carson JL, Davis SD, Dell SD, Ferkol TW, Olivier KN, Sagel SD, Rosenfeld M, Burns KA, et al. Genetic Disorders of Mucociliary Clearance Consortium. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax. 2012;67:433–441. doi: 10.1136/thoraxjnl-2011-200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frommer A, Hjeij R, Loges NT, Edelbusch C, Jahnke C, Raidt J, Werner C, Wallmeier J, Große-Onnebrink J, Olbrich H, et al. Immunofluorescence analysis and diagnosis of primary ciliary dyskinesia with radial spoke defects. Am J Respir Cell Mol Biol. 2015;53:563–573. doi: 10.1165/rcmb.2014-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]