Abstract
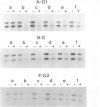
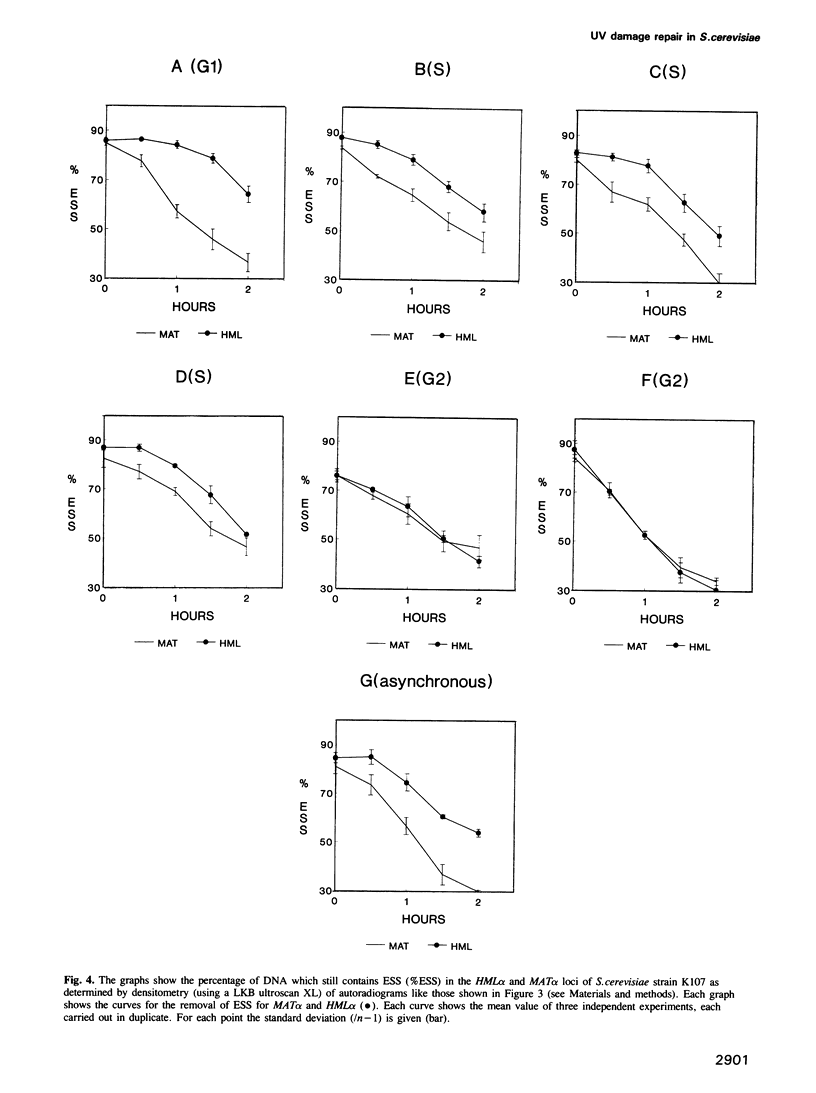
In the yeast Saccharomyces cerevisiae the transcriptionally active MAT alpha locus is repaired preferentially to the inactive HML alpha locus after UV irradiation. Here we analysed the repair of both loci after irradiating yeast cells at different stages of the mitotic cell cycle. In all stages repair of the active MAT alpha locus occurs at a rate of 30% removal of dimers per hour after a UV dose of 60 J/m2. The inactive HML alpha is repaired as efficiently as MAT alpha following irradiation in G2 whereas repair of HML alpha is less efficient in the other stages. Thus differential repair is observed in G1 and S but not in G2. Apparently, in G2 a chromatin structure exists in which repair does not discriminate between transcriptionally active and inactive DNA or, alternatively, an additional repair mechanism might exist which is only operational during G2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohr V. A., Okumoto D. S., Hanawalt P. C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Wassermann K. DNA repair at the level of the gene. Trends Biochem Sci. 1988 Nov;13(11):429–433. doi: 10.1016/0968-0004(88)90216-2. [DOI] [PubMed] [Google Scholar]
- Chanet R., Waters R., Moustacchi E. Letter: The induction of pyrimidine dimers in nuclear DNA after U.V.-irradiation during the synchronous cycle of Saccharomyces cerevisiae. Int J Radiat Biol Relat Stud Phys Chem Med. 1975 May;27(5):481–485. doi: 10.1080/09553007514550471. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. DNA damage and repair in normal, xeroderma pigmentosum and XP revertant cells analyzed by gel electrophoresis: excision of cyclobutane dimers from the whole genome is not necessary for cell survival. Carcinogenesis. 1989 Sep;10(9):1691–1696. doi: 10.1093/carcin/10.9.1691. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C. Preferential DNA repair in expressed genes. Environ Health Perspect. 1987 Dec;76:9–14. doi: 10.1289/ehp.87769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Unger M. W. Unequal division in Saccharomyces cerevisiae and its implications for the control of cell division. J Cell Biol. 1977 Nov;75(2 Pt 1):422–435. doi: 10.1083/jcb.75.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Life cycle of the budding yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Dec;52(4):536–553. doi: 10.1128/mr.52.4.536-553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. J., Elking C. F. Biological significance of domain-oriented DNA repair in xeroderma pigmentosum cells. Cancer Res. 1988 Feb 15;48(4):844–849. [PubMed] [Google Scholar]
- Leadon S. A. Differential repair of DNA damage in specific nucleotide sequences in monkey cells. Nucleic Acids Res. 1986 Nov 25;14(22):8979–8995. doi: 10.1093/nar/14.22.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R., Karran P. DNA repair. Int Rev Cytol. 1981;72:101–146. doi: 10.1016/s0074-7696(08)61195-x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Lu C., Scheuermann R. H., Echols H. Capacity of RecA protein to bind preferentially to UV lesions and inhibit the editing subunit (epsilon) of DNA polymerase III: a possible mechanism for SOS-induced targeted mutagenesis. Proc Natl Acad Sci U S A. 1986 Feb;83(3):619–623. doi: 10.1073/pnas.83.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Hanawalt P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989 Nov 2;342(6245):95–98. doi: 10.1038/342095a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Meneghini R., Menck C. F., Schumacher R. I. Mechanisms of tolerance to DNA lesions in mammalian cells. Q Rev Biophys. 1981 Aug;14(3):381–432. doi: 10.1017/s0033583500002353. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Yamashita K., Sekiguchi M. Purification and characterization of normal and mutant forms of T4 endonuclease V. J Biol Chem. 1982 Mar 10;257(5):2556–2562. [PubMed] [Google Scholar]
- Nasmyth K., Shore D. Transcriptional regulation in the yeast life cycle. Science. 1987 Sep 4;237(4819):1162–1170. doi: 10.1126/science.3306917. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7315–7317. doi: 10.1073/pnas.77.12.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Game J. C., Stasiewicz S. Genetic effects of UV irradiation on excision-proficient and -deficient yeast during meiosis. Genetics. 1983 Aug;104(4):603–618. doi: 10.1093/genetics/104.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Stasiewicz S., Game J. C. Meiotic DNA metabolism in wild-type and excision-deficient yeast following UV exposure. Genetics. 1983 Aug;104(4):583–601. doi: 10.1093/genetics/104.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R. H., Reynolds P., Prakash S., Prakash L. Cloning and sequence analysis of the Saccharomyces cerevisiae RAD9 gene and further evidence that its product is required for cell cycle arrest induced by DNA damage. Mol Cell Biol. 1989 May;9(5):1882–1896. doi: 10.1128/mcb.9.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleth C., van Sluis C. A., van de Putte P. Differential repair of UV damage in Saccharomyces cerevisiae. Nucleic Acids Res. 1989 Jun 26;17(12):4433–4439. doi: 10.1093/nar/17.12.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey R. A. Different drugs arrest cells at a number of distinct stages in G2. Nature. 1975 Mar 20;254(5497):245–247. doi: 10.1038/254245a0. [DOI] [PubMed] [Google Scholar]
- Tschumper G., Carbon J. Copy number control by a yeast centromere. Gene. 1983 Aug;23(2):221–232. doi: 10.1016/0378-1119(83)90054-9. [DOI] [PubMed] [Google Scholar]
- Waters R. Repair of DNA in replicated and unreplicated portions of the human genome. J Mol Biol. 1979 Jan 5;127(1):117–127. doi: 10.1016/0022-2836(79)90462-5. [DOI] [PubMed] [Google Scholar]
- Weinert T. A., Hartwell L. H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988 Jul 15;241(4863):317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- Wheals A. E. Size control models of Saccharomyces cerevisiae cell proliferation. Mol Cell Biol. 1982 Apr;2(4):361–368. doi: 10.1128/mcb.2.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. H., Barker D. G., Nurse P., Johnston L. H. Periodic transcription as a means of regulating gene expression during the cell cycle: contrasting modes of expression of DNA ligase genes in budding and fission yeast. EMBO J. 1986 Jul;5(7):1705–1709. doi: 10.1002/j.1460-2075.1986.tb04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]