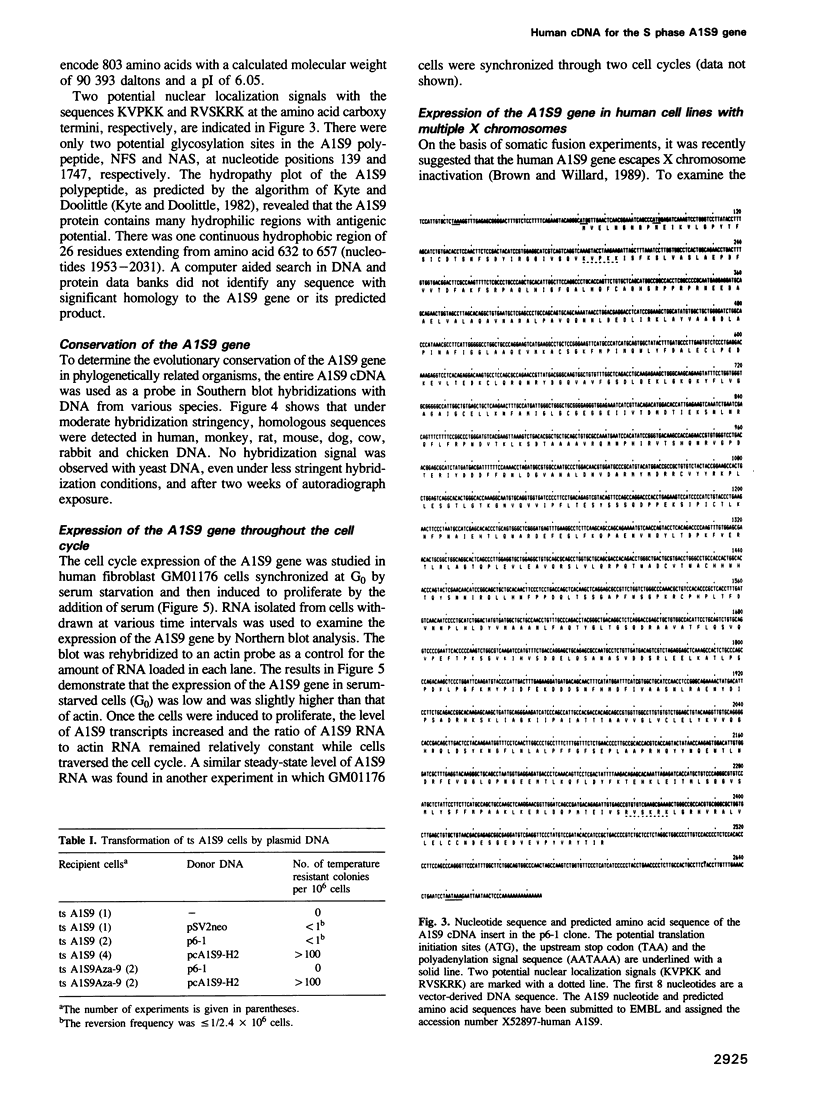
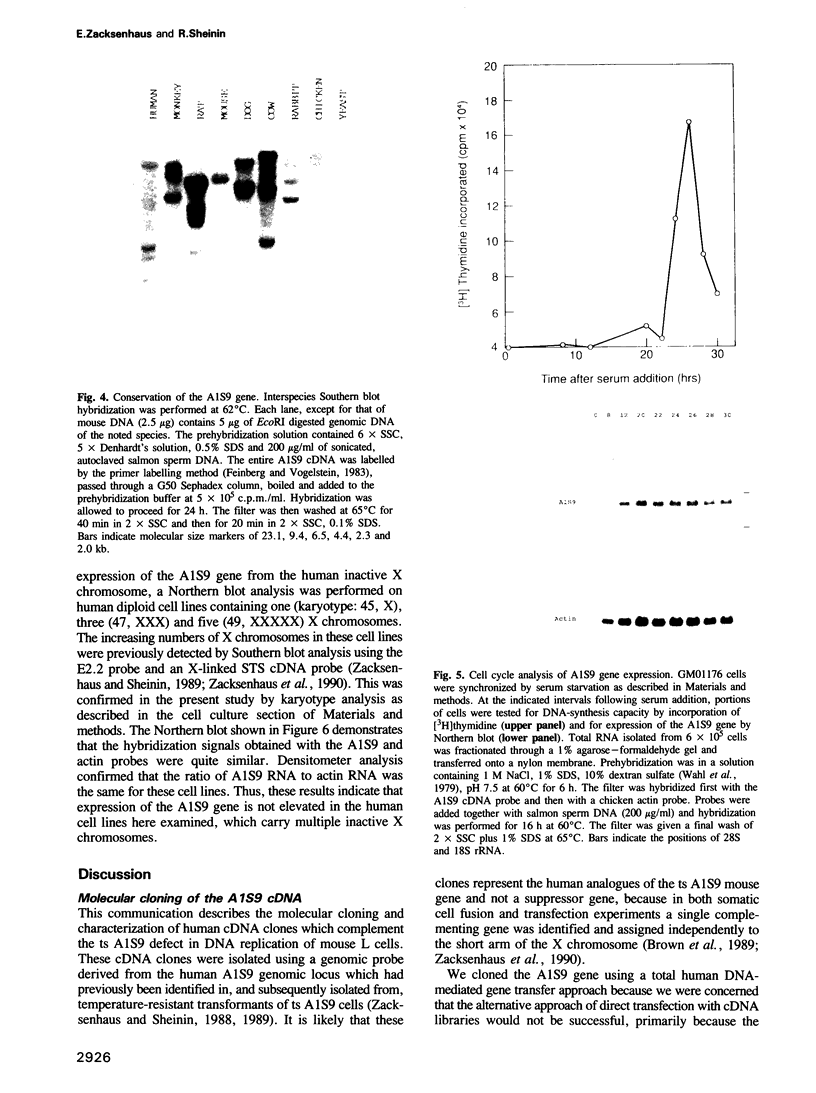
Abstract
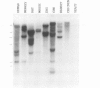
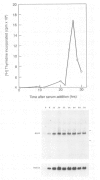
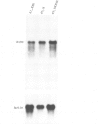
The temperature-sensitive ts A1S9 mutation of mouse L cells was previously shown to affect nuclear DNA replication and to be complemented by active and inactive human X chromosomes in human-ts A1S9 somatic cell hybrids. We report the isolation of cDNA clones which correct the ts A1S9 lesion, using as a probe a genomic fragment derived from the human A1S9 locus. The nucleotide sequence of the A1S9 cDNA encompasses a single open reading frame of 2409 bp which could encode a heretofore unreported protein of 90 393 daltons. Southern blot hybridization of the A1S9 cDNA probe with DNA from various species revealed homologous sequences in vertebrates but not in yeast. Northern blot analysis of serum-starved, synchronized cells demonstrated that the A1S9 gene was expressed at a relatively low level in quiescent cells and at a higher and constant level throughout the cell cycle. Human cell lines harbouring increasing numbers of inactive X chromosomes (47, XXX, 49, XXXXX) were found to express the A1S9 gene at the same level as control cells (45, X), suggesting that the gene does not escape X chromosome inactivation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Shimizu K., Koyama H., Kaneda S., Takeishi K., Seno T. Cell-cycle-directed regulation of thymidylate synthase messenger RNA in human diploid fibroblasts stimulated to proliferate. J Mol Biol. 1986 Aug 20;190(4):559–567. doi: 10.1016/0022-2836(86)90241-x. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Powers V. E., Munroe D. L., Sheinin R., Willard H. F. Gene on short arm of human X chromosome complements murine tsA1S9 DNA synthesis mutation. Somat Cell Mol Genet. 1989 Mar;15(2):173–178. doi: 10.1007/BF01535079. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Willard H. F. Noninactivation of a selectable human X-linked gene that complements a murine temperature-sensitive cell cycle defect. Am J Hum Genet. 1989 Oct;45(4):592–598. [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L. Eukaryotic DNA replication. Annu Rev Biochem. 1986;55:733–771. doi: 10.1146/annurev.bi.55.070186.003505. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coppock D. L., Pardee A. B. Control of thymidine kinase mRNA during the cell cycle. Mol Cell Biol. 1987 Aug;7(8):2925–2932. doi: 10.1128/mcb.7.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Lee W. M. Identification of the human c-myc protein nuclear translocation signal. Mol Cell Biol. 1988 Oct;8(10):4048–4054. doi: 10.1128/mcb.8.10.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Eki T., Enomoto T., Miyajima A., Miyazawa H., Murakami Y., Hanaoka F., Yamada M., Ui M. Isolation of temperature-sensitive cell cycle mutants from mouse FM3A cells. Characterization of mutants with special reference to DNA replication. J Biol Chem. 1990 Jan 5;265(1):26–33. [PubMed] [Google Scholar]
- Farnham P. J., Schimke R. T. Transcriptional regulation of mouse dihydrofolate reductase in the cell cycle. J Biol Chem. 1985 Jun 25;260(12):7675–7680. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Riggs A. D. Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- Goodfellow P., Pym B., Mohandas T., Shapiro L. J. The cell surface antigen locus, MIC2X, escapes X-inactivation. Am J Hum Genet. 1984 Jul;36(4):777–782. [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grant S. G., Chapman V. M. Mechanisms of X-chromosome regulation. Annu Rev Genet. 1988;22:199–233. doi: 10.1146/annurev.ge.22.120188.001215. [DOI] [PubMed] [Google Scholar]
- Greco A., Ittmann M., Basilico C. Molecular cloning of a gene that is necessary for G1 progression in mammalian cells. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1565–1569. doi: 10.1073/pnas.84.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittmann M., Greco A., Basilico C. Isolation of the human gene that complements a temperature-sensitive cell cycle mutation in BHK cells. Mol Cell Biol. 1987 Oct;7(10):3386–3393. doi: 10.1128/mcb.7.10.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Keith D. H., Singer-Sam J., Riggs A. D. Active X chromosome DNA is unmethylated at eight CCGG sites clustered in a guanine-plus-cytosine-rich island at the 5' end of the gene for phosphoglycerate kinase. Mol Cell Biol. 1986 Nov;6(11):4122–4125. doi: 10.1128/mcb.6.11.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Fairman M. P., Blow J. J. S phase of the cell cycle. Science. 1989 Nov 3;246(4930):609–614. doi: 10.1126/science.2683076. [DOI] [PubMed] [Google Scholar]
- Lester S. C., Korn N. J., DeMars R. Derepression of genes on the human inactive X chromosome: evidence for differences in locus-specific rates of derepression and rates of transfer of active and inactive genes after DNA-mediated transformation. Somatic Cell Genet. 1982 Mar;8(2):265–284. doi: 10.1007/BF01538681. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. The William Allan memorial award address: X-chromosome inactivation and the location and expression of X-linked genes. Am J Hum Genet. 1988 Jan;42(1):8–16. [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Fainsod A., Diamond G. The genetic analysis of mammalian cell-cycle mutants. Annu Rev Genet. 1985;19:389–421. doi: 10.1146/annurev.ge.19.120185.002133. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Shapiro L. J., Norum R. A., Mohandas T., Axelman J., Dabora R. L. Differential expression of steroid sulphatase locus on active and inactive human X chromosome. Nature. 1982 Oct 28;299(5886):838–840. doi: 10.1038/299838a0. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Kai R., Furuno N., Sekiguchi T., Sekiguchi M., Hayashida H., Kuma K., Miyata T., Fukushige S., Murotsu T. Isolation and characterization of the active cDNA of the human cell cycle gene (RCC1) involved in the regulation of onset of chromosome condensation. Genes Dev. 1987 Aug;1(6):585–593. doi: 10.1101/gad.1.6.585. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Beer-Romero P., Brown L. G., Nussbaum R., Page D. C. ZFX has a gene structure similar to ZFY, the putative human sex determinant, and escapes X inactivation. Cell. 1989 Jun 30;57(7):1247–1258. doi: 10.1016/0092-8674(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Seabright M. The use of proteolytic enzymes for the mapping of structural rearrangements in the chromosomes of man. Chromosoma. 1972;36(2):204–210. doi: 10.1007/BF00285214. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Sekiguchi T., Miyata T., Nishimoto T. Molecular cloning of the cDNA of human X chromosomal gene (CCG1) which complements the temperature-sensitive G1 mutants, tsBN462 and ts13, of the BHK cell line. EMBO J. 1988 Jun;7(6):1683–1687. doi: 10.1002/j.1460-2075.1988.tb02996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. J., Mohandas T., Weiss R., Romeo G. Non-inactivation of an x-chromosome locus in man. Science. 1979 Jun 15;204(4398):1224–1226. doi: 10.1126/science.156396. [DOI] [PubMed] [Google Scholar]
- Sheinin Polyoma and cell DNA synthesis in mouse L cells temperature sensitive for the replication of cell DNA. J Virol. 1976 Mar;17(3):692–704. doi: 10.1128/jvi.17.3.692-704.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin R., Fabbro J., Dubsky M. Mouse polyoma virus and adenovirus replication in mouse cells temperature-sensitive in DNA synthesis. Intervirology. 1985;24(3):174–180. doi: 10.1159/000149638. [DOI] [PubMed] [Google Scholar]
- Sheinin R., Guttman S. Semi-conservative and non-conservative replication of DNA in temperature-sensitive mouse L-cells. Biochim Biophys Acta. 1977 Nov 2;479(1):105–118. doi: 10.1016/0005-2787(77)90130-7. [DOI] [PubMed] [Google Scholar]
- Sheinin R. Preliminary characterization of the temperature-sensitive defect in DNA replication in a mutant mouse L cell. Cell. 1976 Jan;7(1):49–57. doi: 10.1016/0092-8674(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Shenoy S., Choi J. K., Bagrodia S., Copeland T. D., Maller J. L., Shalloway D. Purified maturation promoting factor phosphorylates pp60c-src at the sites phosphorylated during fibroblast mitosis. Cell. 1989 Jun 2;57(5):763–774. doi: 10.1016/0092-8674(89)90791-5. [DOI] [PubMed] [Google Scholar]
- Soriano P., Keitges E. A., Schorderet D. F., Harbers K., Gartler S. M., Jaenisch R. High rate of recombination and double crossovers in the mouse pseudoautosomal region during male meiosis. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7218–7220. doi: 10.1073/pnas.84.20.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Mankovitz R., Baker R. M., Till J. E., Siminovitch L., Whitmore G. F. Isolation of temperature-sensitive mutants of L-cells. Proc Natl Acad Sci U S A. 1970 Jun;66(2):377–384. doi: 10.1073/pnas.66.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Multiple replication factors augment DNA synthesis by the two eukaryotic DNA polymerases, alpha and delta. EMBO J. 1989 Dec 1;8(12):3883–3889. doi: 10.1002/j.1460-2075.1989.tb08567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup D. M., Kauffman M. G., Kelly T. J. Activation of SV40 DNA replication in vitro by cellular protein phosphatase 2A. EMBO J. 1989 Dec 1;8(12):3891–3898. doi: 10.1002/j.1460-2075.1989.tb08568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A. F., Geis A. M., Spain B. H., Wong S. W., Korn D., Wang T. S. Gene expression of human DNA polymerase alpha during cell proliferation and the cell cycle. Mol Cell Biol. 1988 Nov;8(11):5016–5025. doi: 10.1128/mcb.8.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareham K. A., Lyon M. F., Glenister P. H., Williams E. D. Age related reactivation of an X-linked gene. 1987 Jun 25-Jul 1Nature. 327(6124):725–727. doi: 10.1038/327725a0. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Breg W. R. Human X chromosomes: synchrony of DNA replication in diploid and triploid fibroblasts with multiple active or inactive X chromosomes. Somatic Cell Genet. 1980 Mar;6(2):187–198. doi: 10.1007/BF01538795. [DOI] [PubMed] [Google Scholar]
- Wychowski C., Benichou D., Girard M. A domain of SV40 capsid polypeptide VP1 that specifies migration into the cell nucleus. EMBO J. 1986 Oct;5(10):2569–2576. doi: 10.1002/j.1460-2075.1986.tb04536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen P. H., Marsh B., Allen E., Tsai S. P., Ellison J., Connolly L., Neiswanger K., Shapiro L. J. The human X-linked steroid sulfatase gene and a Y-encoded pseudogene: evidence for an inversion of the Y chromosome during primate evolution. Cell. 1988 Dec 23;55(6):1123–1135. doi: 10.1016/0092-8674(88)90257-7. [DOI] [PubMed] [Google Scholar]
- Zacksenhaus E., Sheinin R. Identification of human gene complementing ts AlS9 mouse L-cell defect in DNA replication following DNA-mediated gene transfer. Somat Cell Mol Genet. 1988 Jul;14(4):371–379. doi: 10.1007/BF01534645. [DOI] [PubMed] [Google Scholar]
- Zacksenhaus E., Sheinin R. Molecular cloning of human A1S9 locus: an X-linked gene essential for progression through S phase of the cell cycle. Somat Cell Mol Genet. 1989 Nov;15(6):545–553. doi: 10.1007/BF01534915. [DOI] [PubMed] [Google Scholar]
- Zacksenhaus E., Sheinin R., Wang H. S. Localization of the human A1S9 gene complementing the ts A1S9 mouse L-cell defect in DNA replication and cell cycle progression to Xp11.2----p11.4. Cytogenet Cell Genet. 1990;53(1):20–22. doi: 10.1159/000132887. [DOI] [PubMed] [Google Scholar]