Abstract
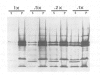
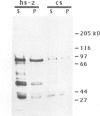
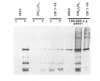
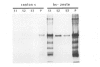
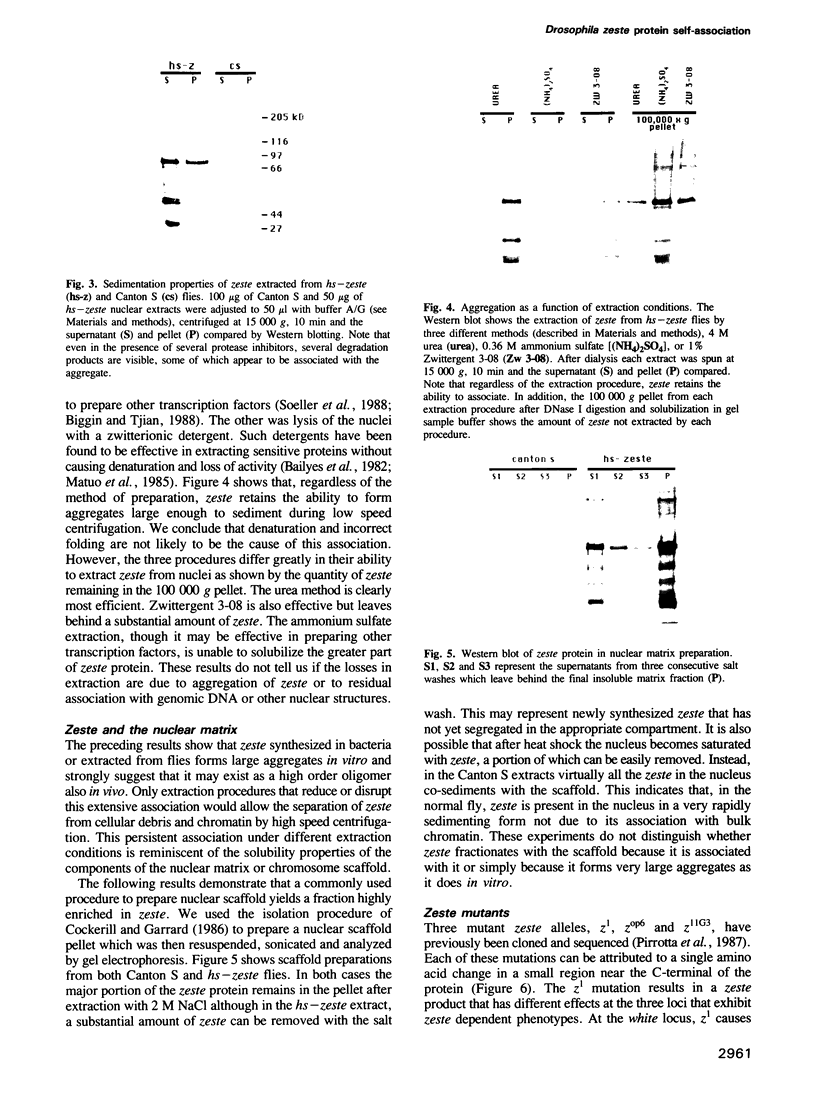
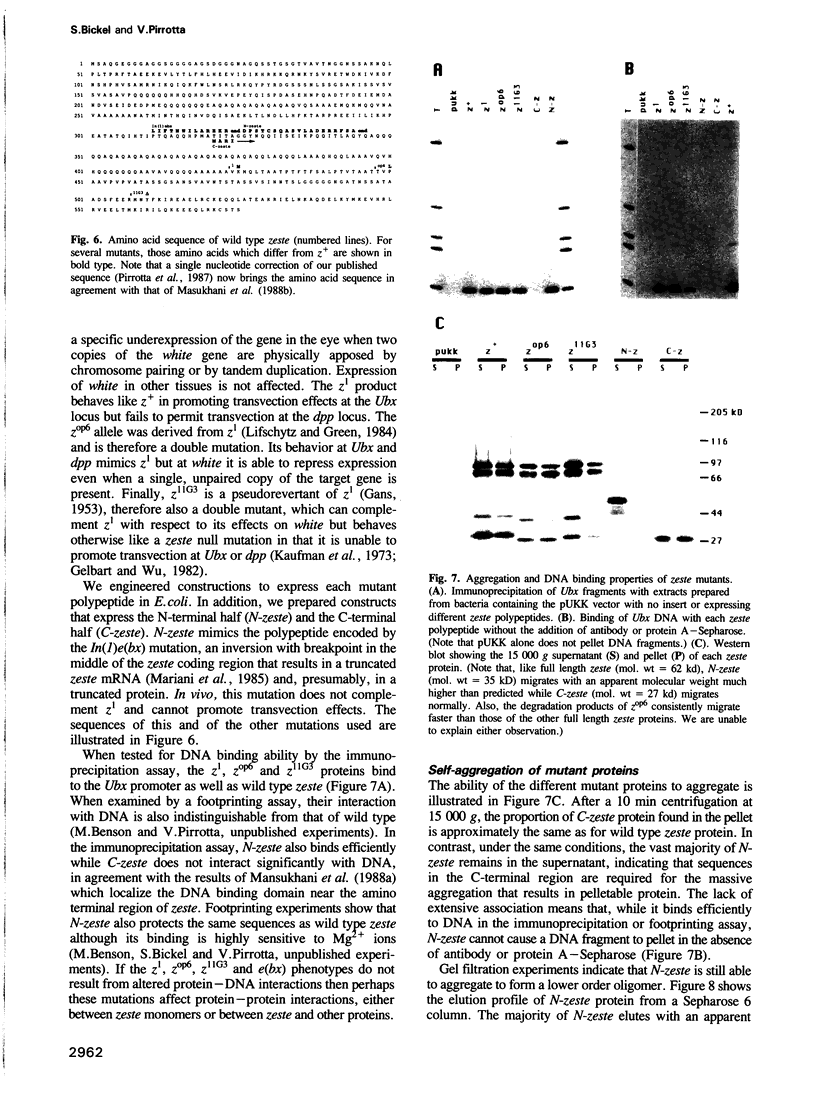
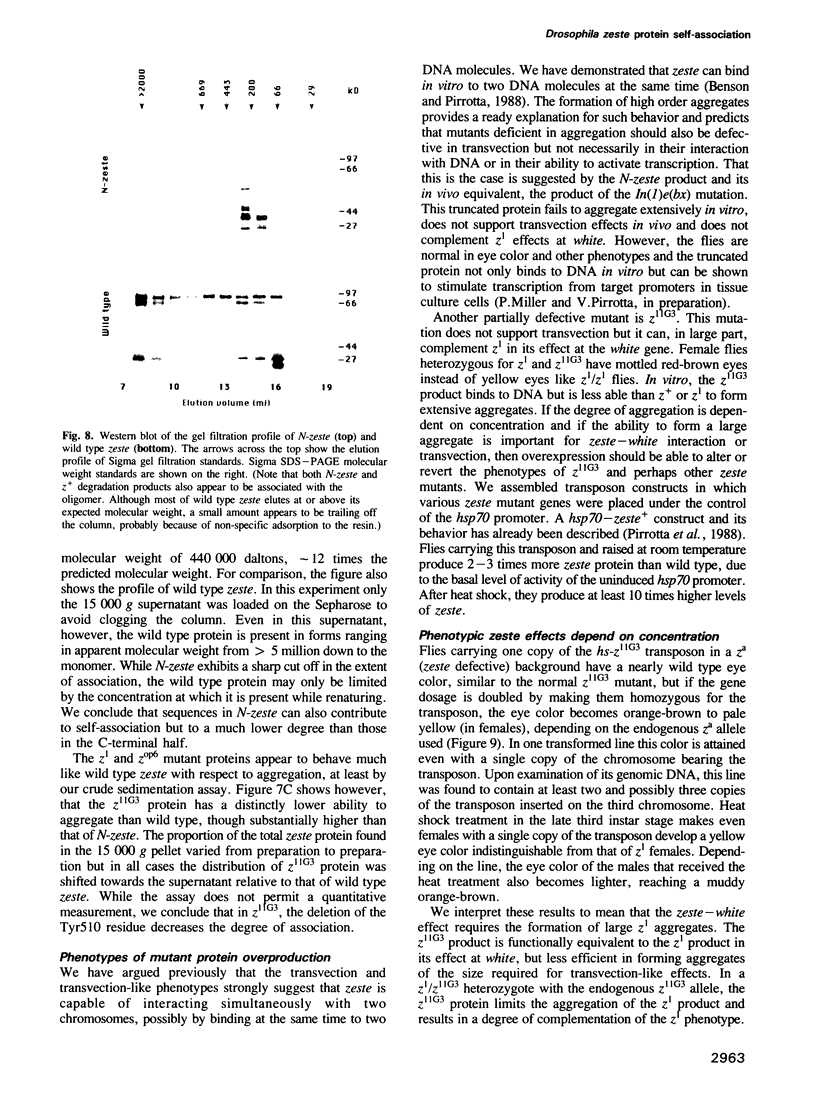
The zeste gene product is required for transvection effects that imply the ability of regulatory elements on one chromosome to affect the expression of the homologous gene in a somatically paired chromosome. The z1 mutation causes a pairing dependent inhibition of the expression of the white gene. Both of these phenomena can be explained by the tendency of zeste protein, expressed in bacteria or in flies, to self-associate, forming complexes of several hundred monomers. These large aggregates bind to DNA and are found in nuclear matrix preparations, probably because they co-sediment with the matrix. The principal determinants of this self-association are located in the C-terminal half of the protein but some limited aggregation is obtained also with the N-terminal half, which contains the DNA binding domain. The z1 and zop2 mutant proteins aggregate to the same degree as the wild type but the z11G3 product, a pseudorevertant of z1, has a reduced tendency to aggregate. This mutation, which in vivo is antagonistic to z1 and does not support transvection effects, can be made to revert its phenotype when the mutant protein is over-produced under the control of the heat shock promoter. These results indicate that both the zeste-white interaction and transvection effects require the formation of high order aggregates. When the z1 protein is over-produced in vivo, it reduces the expression of an unpaired copy of white, indicating that the normal requirement for chromosome pairing is simply a device to increase the size of the aggregate bound to the white regulatory region.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M. The genetic analysis of puffing in polytene chromosomes of Drosophila. Proc R Soc Lond B Biol Sci. 1970 Dec 1;176(1044):319–327. doi: 10.1098/rspb.1970.0052. [DOI] [PubMed] [Google Scholar]
- Bailyes E. M., Newby A. C., Siddle K., Luzio J. P. Solubilization and purification of rat liver 5'-nucleotidase by use of a zwitterionic detergent and a monoclonal-antibody immunoadsorbent. Biochem J. 1982 Apr 1;203(1):245–251. doi: 10.1042/bj2030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M., Pirrotta V. The Drosophila zeste protein binds cooperatively to sites in many gene regulatory regions: implications for transvection and gene regulation. EMBO J. 1988 Dec 1;7(12):3907–3915. doi: 10.1002/j.1460-2075.1988.tb03277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M., Pirrotta V. The product of the Drosophila zeste gene binds to specific DNA sequences in white and Ubx. EMBO J. 1987 May;6(5):1387–1392. doi: 10.1002/j.1460-2075.1987.tb02379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Bickel S., Benson M., Pirrotta V., Tjian R. Zeste encodes a sequence-specific transcription factor that activates the Ultrabithorax promoter in vitro. Cell. 1988 Jun 3;53(5):713–722. doi: 10.1016/0092-8674(88)90089-x. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell. 1988 Jun 3;53(5):699–711. doi: 10.1016/0092-8674(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Zachar Z. Evidence that two mutations, wDZL and z1, affecting synapsis-dependent genetic behavior of white are transcriptional regulatory mutations. Cell. 1985 Apr;40(4):819–825. doi: 10.1016/0092-8674(85)90341-1. [DOI] [PubMed] [Google Scholar]
- Brosius J. Plasmid vectors for the selection of promoters. Gene. 1984 Feb;27(2):151–160. doi: 10.1016/0378-1119(84)90136-7. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The Drosophila developmental gene, engrailed, encodes a sequence-specific DNA binding activity. Nature. 1985 Dec 19;318(6047):630–635. doi: 10.1038/318630a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of D. melanogaster. Cell. 1986 Aug 15;46(4):521–530. doi: 10.1016/0092-8674(86)90877-9. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Poole S. J., Kornberg T. B. The Drosophila engrailed protein is phosphorylated by a serine-specific protein kinase. Nucleic Acids Res. 1988 Jul 25;16(14A):6637–6647. doi: 10.1093/nar/16.14.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbart W. M., Wu C. T. Interactions of zeste mutations with loci exhibiting transvection effects in Drosophila melanogaster. Genetics. 1982 Oct;102(2):179–189. doi: 10.1093/genetics/102.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Green M. M., Corces V. G. Tissue-specific transcriptional enhancers may act in trans on the gene located in the homologous chromosome: the molecular basis of transvection in Drosophila. EMBO J. 1990 Jul;9(7):2247–2256. doi: 10.1002/j.1460-2075.1990.tb07395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. L., Colvin R. A., Mellin A. F. The Drosophila zeste locus is nonessential. Genetics. 1989 Sep;123(1):145–155. doi: 10.1093/genetics/123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Dreesen T. D. Trans-inactivation of the Drosophila brown gene: evidence for transcriptional repression and somatic pairing dependence. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6704–6708. doi: 10.1073/pnas.86.17.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. W., Judd B. H. Allelic pairing and gene regulation: A model for the zeste-white interaction in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1368–1372. doi: 10.1073/pnas.76.3.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Kalisch W. E., Rasmuson B. Changes of zeste phenotype induced by autosomal mutations in Drosophila melanogaster. Hereditas. 1974;78(1):97–104. doi: 10.1111/j.1601-5223.1974.tb01432.x. [DOI] [PubMed] [Google Scholar]
- Kaufman T. C., Tasaka S. E., Suzuki D. T. The interaction of two complex loci, zeste and bithorax in Drosophila melanogaster. Genetics. 1973 Oct;75(2):299–321. doi: 10.1093/genetics/75.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge G. Genetic analysis of the larval secretion gene Sgs-4 and its regulatory chromosome sites in Drosophila melanogaster. Chromosoma. 1981;84(3):373–390. doi: 10.1007/BF00286027. [DOI] [PubMed] [Google Scholar]
- Kornher J. S., Brutlag D. Proximity-dependent enhancement of Sgs-4 gene expression in D. melanogaster. Cell. 1986 Mar 28;44(6):879–883. doi: 10.1016/0092-8674(86)90010-3. [DOI] [PubMed] [Google Scholar]
- Krause H. M., Gehring W. J. Stage-specific phosphorylation of the fushi tarazu protein during Drosophila development. EMBO J. 1989 Apr;8(4):1197–1204. doi: 10.1002/j.1460-2075.1989.tb03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschytz E., Green M. M. The zeste-white interaction: induction and genetic analysis of a novel class of zeste alleles. EMBO J. 1984 May;3(5):999–1002. doi: 10.1002/j.1460-2075.1984.tb01919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani A., Crickmore A., Sherwood P. W., Goldberg M. L. DNA-binding properties of the Drosophila melanogaster zeste gene product. Mol Cell Biol. 1988 Feb;8(2):615–623. doi: 10.1128/mcb.8.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani A., Gunaratne P. H., Sherwood P. W., Sneath B. J., Goldberg M. L. Nucleotide sequence and structural analysis of the zeste locus of Drosophila melanogaster. Mol Gen Genet. 1988 Jan;211(1):121–128. doi: 10.1007/BF00338402. [DOI] [PubMed] [Google Scholar]
- Mariani C., Pirrotta V., Manet E. Isolation and characterization of the zeste locus of Drosophila. EMBO J. 1985 Aug;4(8):2045–2052. doi: 10.1002/j.1460-2075.1985.tb03890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuo Y., Matsui S., Nishi N., Wada F., Sandberg A. A. Quantitative solubilization of nonhistone chromosomal proteins without denaturation using zwitterionic detergents. Anal Biochem. 1985 Nov 1;150(2):337–344. doi: 10.1016/0003-2697(85)90520-2. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Murphy C., Levis R., Rubin G. M. DNA sequence of the white locus of Drosophila melanogaster. J Mol Biol. 1984 Dec 15;180(3):437–455. doi: 10.1016/0022-2836(84)90021-4. [DOI] [PubMed] [Google Scholar]
- Phillips M. D., Shearn A. Mutations in polycombeotic, a Drosophila polycomb-group gene, cause a wide range of maternal and zygotic phenotypes. Genetics. 1990 May;125(1):91–101. doi: 10.1093/genetics/125.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
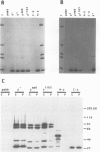
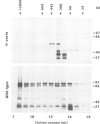
- Pirrotta V., Bickel S., Mariani C. Developmental expression of the Drosophila zeste gene and localization of zeste protein on polytene chromosomes. Genes Dev. 1988 Dec;2(12B):1839–1850. doi: 10.1101/gad.2.12b.1839. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Manet E., Hardon E., Bickel S. E., Benson M. Structure and sequence of the Drosophila zeste gene. EMBO J. 1987 Mar;6(3):791–799. doi: 10.1002/j.1460-2075.1987.tb04821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller W. C., Poole S. J., Kornberg T. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 1988 Jan;2(1):68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanojević D., Hoey T., Levine M. Sequence-specific DNA-binding activities of the gap proteins encoded by hunchback and Krüppel in Drosophila. Nature. 1989 Sep 28;341(6240):331–335. doi: 10.1038/341331a0. [DOI] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. A transposable P vector that confers selectable G418 resistance to Drosophila larvae. EMBO J. 1985 Jan;4(1):167–171. doi: 10.1002/j.1460-2075.1985.tb02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. Expression of the Drosophila white gene under the control of the hsp70 heat shock promoter. EMBO J. 1985 Dec 30;4(13B):3765–3772. doi: 10.1002/j.1460-2075.1985.tb04146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. P transposons controlled by the heat shock promoter. Mol Cell Biol. 1986 May;6(5):1640–1649. doi: 10.1128/mcb.6.5.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C., Heidenthal G. Materials for the Study of the Position Effect of Normal and Mutant Genes. Proc Natl Acad Sci U S A. 1944 Aug 15;30(8):197–205. doi: 10.1073/pnas.30.8.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman J., Desplan C. The products of the Drosophila gap genes hunchback and Krüppel bind to the hunchback promoters. Nature. 1989 Sep 28;341(6240):335–337. doi: 10.1038/341335a0. [DOI] [PubMed] [Google Scholar]