Abstract
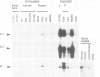
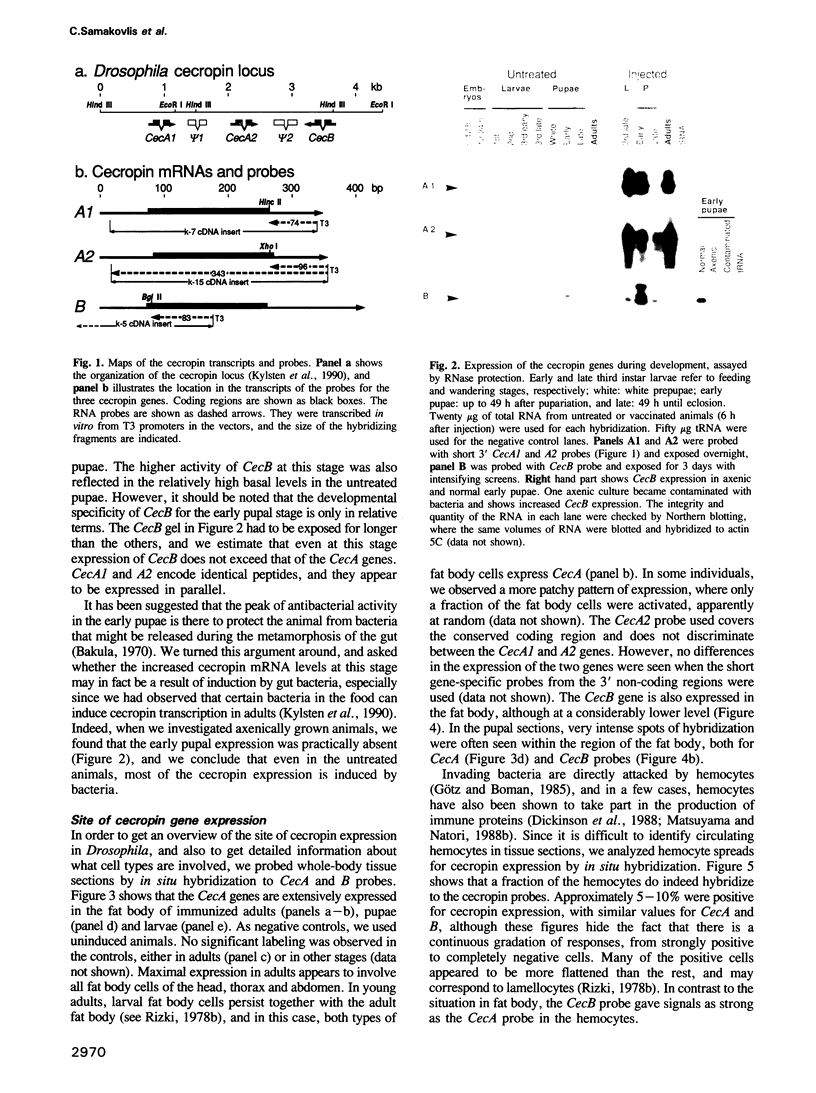
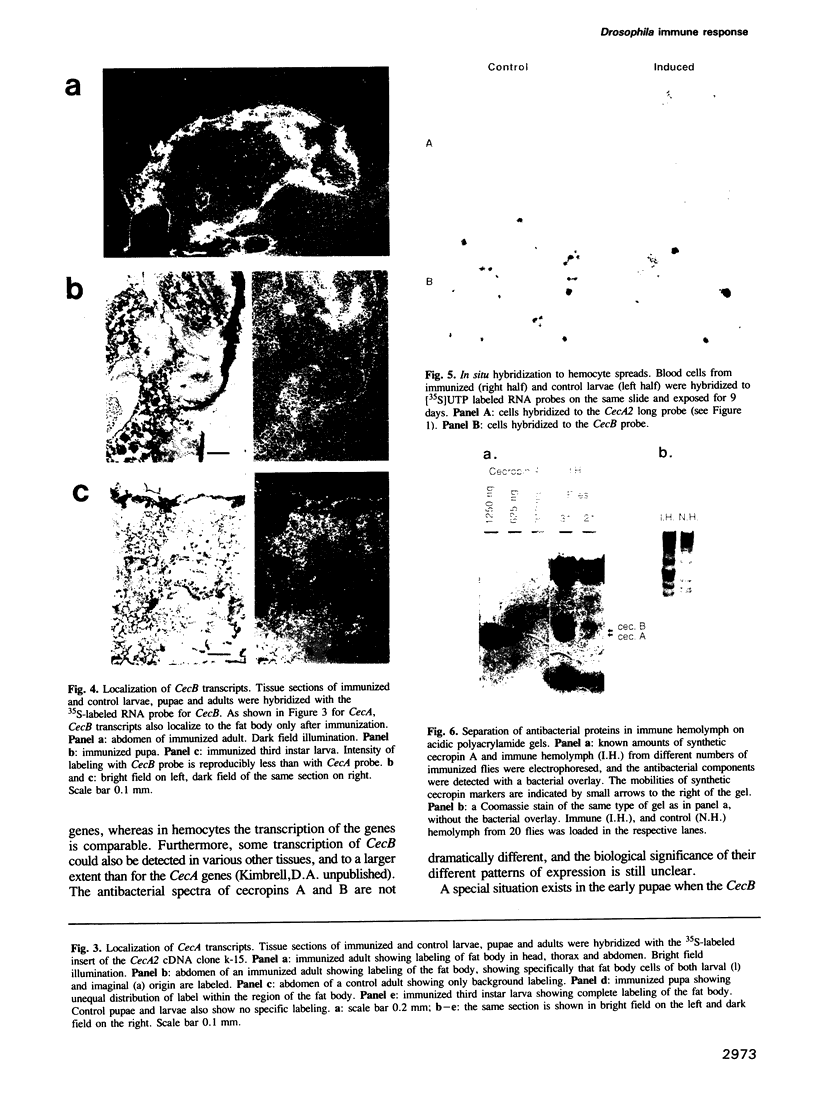
Cecropins are antibacterial peptides, induced in Drosophila as part of the humoral immune response to a bacterial invasion. We have used the cloned Drosophila cecropin genes CecA1, A2 and B as probes to study the developmental and tissue specific regulation of this response. The genes are strongly expressed in fat body and hemocytes after injection of bacteria, the CecA genes being much more active than CecB in the fat body. All parts of the fat body and 5-10% of the hemocytes are involved in this response. CecA1 and A2 are most active in larvae and adults; CecB is preferentially active in early pupae. A small peak of constitutive cecropin expression in early pupae appears to be caused by bacteria in the food. Cecropin A, the common product of the CecA1 and A2 genes, was identified in the hemolymph of immunized flies at a concentration of 25-50 microM, enough to kill all tested bacteria except Serratia, a Drosophila pathogen. A useful in vitro system to study the immune response has been found in Schneider's line 2 cells which respond to lipopolysaccharide and laminarin by cecropin expression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. E., Martinez-Arias A. The distribution of Ultrabithorax transcripts in Drosophila embryos. EMBO J. 1985 Jul;4(7):1689–1700. doi: 10.1002/j.1460-2075.1985.tb03838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akam M. E. The location of Ultrabithorax transcripts in Drosophila tissue sections. EMBO J. 1983;2(11):2075–2084. doi: 10.1002/j.1460-2075.1983.tb01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakula M. Antibacterial compounds in the cell-free haemolymph of Drosophila melanogaster. J Insect Physiol. 1970 Jan;16(1):185–197. doi: 10.1016/0022-1910(70)90125-3. [DOI] [PubMed] [Google Scholar]
- Boman H. C., Boman I. A., Andreu D., Li Z. Q., Merrifield R. B., Schlenstedt G., Zimmermann R. Chemical synthesis and enzymic processing of precursor forms of cecropins A and B. J Biol Chem. 1989 Apr 5;264(10):5852–5860. [PubMed] [Google Scholar]
- Boman H. G., Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Nilsson I., Rasmuson B. Inducible antibacterial defence system in Drosophila. Nature. 1972 May 26;237(5352):232–235. doi: 10.1038/237232a0. [DOI] [PubMed] [Google Scholar]
- De Verno P. J., Chadwick J. S., Aston W. P., Dunphy G. B. The in vitro generation of an antibacterial activity from the fat body and hemolymph of non-immunized larvae of Galleria mellonella. Dev Comp Immunol. 1984 Summer;8(3):537–546. doi: 10.1016/0145-305x(84)90086-7. [DOI] [PubMed] [Google Scholar]
- Dickinson L., Russell V., Dunn P. E. A family of bacteria-regulated, cecropin D-like peptides from Manduca sexta. J Biol Chem. 1988 Dec 25;263(36):19424–19429. [PubMed] [Google Scholar]
- Dunn P. E., Dai W., Kanost M. R., Geng C. X. Soluble peptidoglycan fragments stimulate antibacterial protein synthesis by fat body from larvae of Manduca sexta. Dev Comp Immunol. 1985 Summer;9(3):559–568. doi: 10.1016/0145-305x(85)90019-9. [DOI] [PubMed] [Google Scholar]
- Faye I., Wyatt G. R. The synthesis of antibacterial proteins in isolated fat body from Cecropia silkmoth pupae. Experientia. 1980 Nov 15;36(11):1325–1326. doi: 10.1007/BF01969615. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Engström A., Bennich H., Kapur R., Boman H. G. Insect immunity: isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur J Biochem. 1982 Sep;127(1):207–217. doi: 10.1111/j.1432-1033.1982.tb06857.x. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H. G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980 May;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Kylsten P., Samakovlis C., Hultmark D. The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection. EMBO J. 1990 Jan;9(1):217–224. doi: 10.1002/j.1460-2075.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landureau J. C., Jollès P. Lytic enzyme produced in vitro by insect cells: lysozyme or chitinase? Nature. 1970 Mar 7;225(5236):968–969. doi: 10.1038/225968a0. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Boman A., Sun C. X., Andersson M., Jörnvall H., Mutt V., Boman H. G. Antibacterial peptides from pig intestine: isolation of a mammalian cecropin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9159–9162. doi: 10.1073/pnas.86.23.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Q., Merrifield R. B., Boman I. A., Boman H. G. Effects on electrophoretic mobility and antibacterial spectrum of removal of two residues from synthetic sarcotoxin IA and addition of the same residues to cecropin B. FEBS Lett. 1988 Apr 25;231(2):299–302. doi: 10.1016/0014-5793(88)80837-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Okada M., Takahashi H., Ming Q. X., Nakajima Y., Nakanishi Y., Komano H., Natori S. Molecular cloning of a cDNA and assignment of the C-terminal of sarcotoxin IA, a potent antibacterial protein of Sarcophaga peregrina. Biochem J. 1986 Nov 1;239(3):717–722. doi: 10.1042/bj2390717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K., Natori S. Molecular cloning of cDNA for sapecin and unique expression of the sapecin gene during the development of Sarcophaga peregrina. J Biol Chem. 1988 Nov 15;263(32):17117–17121. [PubMed] [Google Scholar]
- Matsuyama K., Natori S. Purification of three antibacterial proteins from the culture medium of NIH-Sape-4, an embryonic cell line of Sarcophaga peregrina. J Biol Chem. 1988 Nov 15;263(32):17112–17116. [PubMed] [Google Scholar]
- Nanbu R., Nakajima Y., Ando K., Natori S. Novel feature of expression of the sarcotoxin IA gene in development of Sarcophaga peregrina. Biochem Biophys Res Commun. 1988 Jan 29;150(2):540–544. doi: 10.1016/0006-291x(88)90427-5. [DOI] [PubMed] [Google Scholar]
- Okada M., Natori S. Primary structure of sarcotoxin I, an antibacterial protein induced in the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J Biol Chem. 1985 Jun 25;260(12):7174–7177. [PubMed] [Google Scholar]
- Robertson M., Postlethwait J. H. The humoral antibacterial response of Drosophila adults. Dev Comp Immunol. 1986 Spring;10(2):167–179. doi: 10.1016/0145-305x(86)90001-7. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Steiner H., Andreu D., Merrifield R. B. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim Biophys Acta. 1988 Apr 7;939(2):260–266. doi: 10.1016/0005-2736(88)90069-7. [DOI] [PubMed] [Google Scholar]
- Steiner H., Hultmark D., Engström A., Bennich H., Boman H. G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981 Jul 16;292(5820):246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]