All eukaryotes, from yeast to man, harbour the molecular machinery to modify cellular proteins with the 76-amino acid polypeptide ubiquitin, through a three-step process termed ubiquitination. Protein ubiquitination is among the most widely used post-translational modifications and it controls a wide range of essential cellular processes including cell cycle, genome integrity, cell death, inflammatory signalling, and defence against pathogens. A key feature of ubiquitin is that it can be assembled into polymeric chains through linking the C-terminal carboxyl group to the ε-amino group of any of the seven lysine residues (Lys6, Lys11, Lys27, Lys29, Lys33, Lys48, and Lys63) or the α-amino group of the N-terminal methionine (Met1) within ubiquitin itself.1 Through this process, eight types of homotypic polymeric ubiquitin chains and an almost infinite variety of heterotypic or branched ubiquitin chains can be generated. Recent advances in our understanding of the ubiquitin system has uncovered that different linkages serve distinct cellular functions, and that even heterotypic and branched ubiquitin chains may be considered as unique cellular signals.1, 2, 3
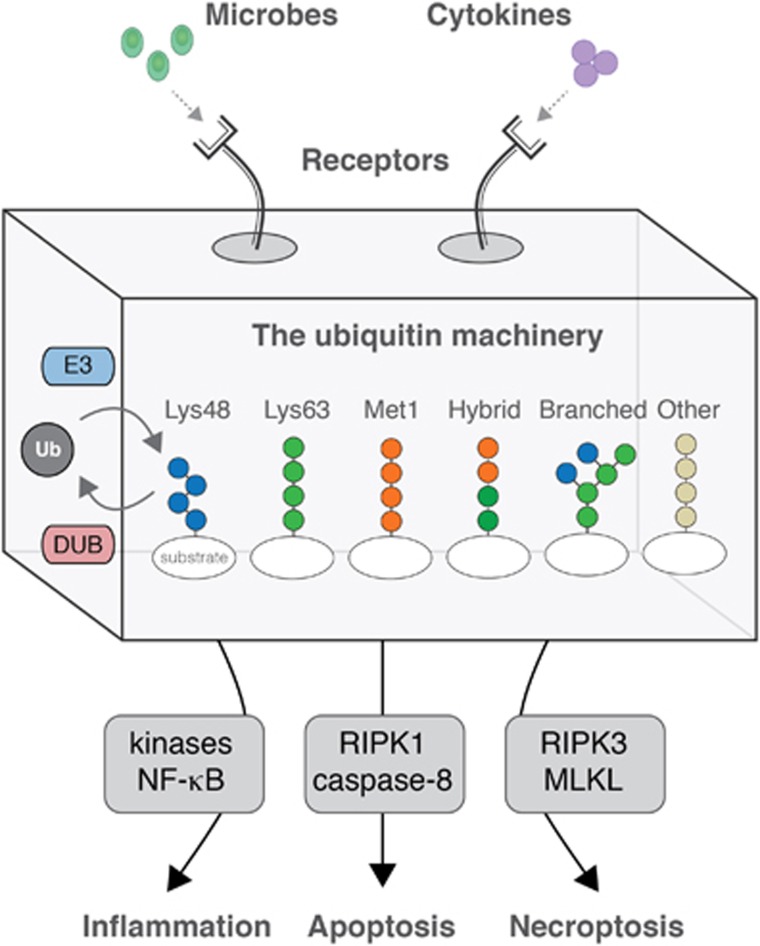
Inflammatory signalling triggered by immune receptors is an excellent example for how different ubiquitin chain types are used to control cellular processes (Figure 1). At least three ubiquitin linkages – Lys48, Lys63 and Met1 – coordinate the transmission of signals from cytokine receptors, antigen receptors, and pattern recognition receptors to the activation of transcriptional programmes mediated by transcription factors such as nuclear factor-kappa B (NF-κB).4 Lys63- and Met1-linked ubiquitin chains are generated at receptor-induced signalling complexes and facilitate the activation of ubiquitin-dependent kinase complexes, whereas Lys48-linked ubiquitin facilitates proteasomal degradation of the NF-κB inhibitor, IκBα. These ubiquitin signals are generated by E3 ubiquitin ligases such as inhibitor of apoptosis proteins, tumour necrosis factor receptor-associated factors, and the linear ubiquitin chain assembly complex (LUBAC), and are regulated by deubiquitinases such as A20, CYLD, and OTULIN.5, 6
Figure 1.
The ubiquitin machinery generates a variety of ubiquitin chain types that determine the cellular response to immune receptor activation by environmental factors. DUB, deubiquitinase; E3, E3 ubiquitin ligase; Ub, ubiquitin
The engagement of cytokine receptors and pattern recognition receptors that trigger inflammatory or antiviral responses also stimulate cell death programmes under certain circumstances. Recent insights indicate that these processes are interconnected, and that ubiquitin chains are at the centre of the decision-making between inflammatory signalling, caspase-mediated apoptosis, and receptor-interacting protein kinase (RIPK)-mediated necroptosis7, 8, 9, 10 (Figure 1). Conversely, proteolysis of E3 ubiquitin ligases and deubiquitinases by caspases and the paracaspase MALT1 may regulate the signalling outcomes of cytokine receptors and pattern recognition receptors.11, 12
Accompanying the molecular characterisation of how ubiquitin regulates inflammatory signalling and cell death has been the realisation that defects in the ubiquitin machinery involved in these processes underlies several immune-related disorders and contributes to cancer, in particular, haematological malignancies.13
The reviews that follow this introduction focus on specific aspects of how the ubiquitin system impinges on inflammatory signalling and cell death. Cohen and Strickson3 review the existing literature on the role and regulation of ubiquitin in MyD88-signalling, and discuss the recently discovered heterotypic ubiquitin chains composed of Lys63 and Met1 linkages. Witt and Vucic10 discuss how ubiquitination of RIPKs regulate their function in inflammatory signalling and cell death. Feltham and Silke8 discuss how ubiquitin modifications regulate the assembly and activity of RIPK1-FADD-caspase 8 containing complexes, and how this impacts on cell death outcomes. Grootjans et al.9 discuss the current knowledge about the (patho)physiological role and molecular regulation of RIPK3-MLKL-mediated necroptosis. The review by Lork et al.12 focusses on the disassembly and editing of ubiquitin modification by the deubiquitinases A20, CYLD, and OTULIN, and the implications this has on inflammatory signalling and cell death. In a review centred on four recent articles describing SPATA2 as a bridge linking the deubiquitinase CYLD to LUBAC, Schlicher et al.14 discuss the role of SPATA2 in inflammatory signalling and cell death.
Together, these excellent reviews by leading experts bring our readership a broad and comprehensive overview of the fascinating and still emerging ways by which ubiquitin intertwines with cellular processes that control inflammation and cell death in health and disease.
Acknowledgments
MG-H is supported by the Ludwig Institute for Cancer Research Ltd, a Wellcome Trust Senior Research Fellowship (102894/Z/13/Z), a Sapere Aude: Danish Council for Independent Research Starting Grant, the EMBO Young Investigator Programme.
Footnotes
The authors declare no conflict of interest.
References
- Kulathu Y, Komander D. Nat Rev Mol Cell Biol 2012; 13: 508–523. [DOI] [PubMed]
- Yau R, Rape M. Nat Cell Biol 2016; 18: 579–586. [DOI] [PubMed]
- Cohen P, Strickson S. Cell Death Differ 2017. (In press, this issue). [DOI] [PMC free article] [PubMed]
- Fiil BK, Gyrd-Hansen M. FEBS J 2014; 281: 4337–4350. [DOI] [PMC free article] [PubMed]
- Sasaki K, Iwai K. Immunol Rev 2015; 266: 175–189. [DOI] [PubMed]
- Harhaj EW, Dixit VM. Immunol Rev 2012; 246: 107–124. [DOI] [PMC free article] [PubMed]
- Shimizu Y, Taraborrelli L, Walczak H. Immunol Rev 2015; 266: 190–207. [DOI] [PMC free article] [PubMed]
- Feltham R, Silke J. Cell Death Differ 2017. (In press, this issue). [DOI] [PMC free article] [PubMed]
- Grootjans S, Vanden Berghe T, Vandenabeele P. Cell Death Differ 2017. (In press, this issue). [DOI] [PMC free article] [PubMed]
- Witt A, Vucic D. Cell Death Differ 2017. (In press, this issue). [DOI] [PMC free article] [PubMed]
- Jaworski M, Thome M. Cell Mol Life Sci 2016; 73: 459–473. [DOI] [PMC free article] [PubMed]
- Lork M, Verhelst K, Beyaert R. Cell Death Differ 2017. [DOI] [PMC free article] [PubMed]
- Popovic D, Vucic D, Dikic I. Nat Med 2014; 20: 1242–1253. [DOI] [PubMed]
- Schlicher L et al. Cell Death Differ 2017. [DOI] [PMC free article] [PubMed]