Abstract
Exosomes, a subgroup of extracellular vesicles (EVs), have been recognized as important mediators of long distance intercellular communication and are involved in a diverse range of biological processes. Because of their ideal native structure and characteristics, exosomes are promising nanocarriers for clinical use. Exosomes are engineered at the cellular level under natural conditions, but successful exosome modification requires further exploration. The focus of this paper is to summarize passive and active loading approaches, as well as specific exosome modifications and examples of the delivery of therapeutic and imaging molecules. Examples of exosomes derived from a variety of biological origins are also provided. The biocompatible characteristics of exosomes, with suitable modifications, can increase the stability and efficacy of imaging probes and therapeutics while enhancing cellular uptake. Challenges in clinical translation of exosome-based platforms from different cell sources and the advantages of each are also reviewed and discussed.
Keywords: exosome, nanoparticles, drug delivery systems, imaging probes, therapeutics
Introduction
In the past decade, nanoscale drug delivery systems (DDS) have attained considerable prominence1. Various nanobased drug formulations have been used to improve the therapeutic efficacy of chemical and biomolecular drugs2,3,4. However, clinical translation of these systems faces two distinct issues: cytotoxicity of materials, and rapid clearance by the reticuloendothelial system (RES) or the mononuclear phagocyte system (MPS)5. Consequently, only a small number of nanobased DDS have been approved by the FDA for use in humans1,6. Although PEGylation can extend the circulation time of these nanoparticles, it can also hinder the interaction between the nanobased DDS and target cells, thus decreasing the drug biodistribution in diseased tissues7,8,9. Endogenous DDS, as compared with synthetic nanoformulations, have shown promising results in enhancing drug delivery and therapeutic efficacy because of their native biocompatibility in vivo10,11,12,13.
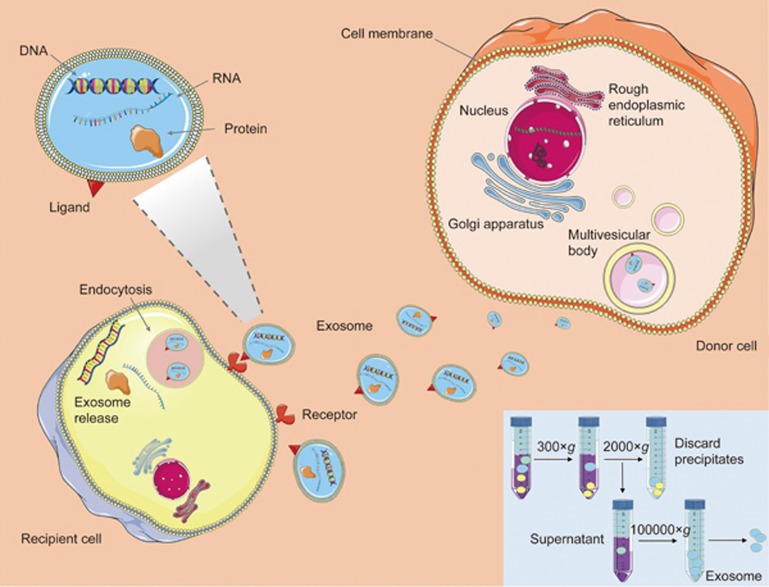
Exosomes are nanosized (30–120 nm) membrane vesicles secreted by various types of cells13. Since the discovery that exosomes function as intercellular communication tools that transfer their cargo to recipient cells, they have garnered considerable research attention14. Exosomes originate in multivesicular bodies (MVBs) and are released into the extracellular environment after fusion with the plasma membrane15. They relay information by transferring their contents from donor cells to recipient cells (Figure 1), thus resulting in functional changes and/or differentiation of target cells16. Exosomes contain and protect a specific mRNAs, regulatory microRNAs, lipids and proteins11,12. Thus, exosomes are important mediators that facilitate intercellular communication without direct cell-to-cell contact17. Several pioneering reports have shown the advantages of using exosomes as nanocarriers12. These advantages include their small size for penetration into deep tissues12, slightly negative zeta potential for long circulation18,19, and deformable cytoskeleton, as well as their similarity to cell membranes20. In addition, some exosomes also exhibit an increased capacity to escape degradation or clearance by the immune system11. Overall, exosomes are ideal natural nanocarriers for clinical application because of their naturally biocompatible characteristics.
Figure 1.
Exosome generation, secretion and cargo transfer from the donor cells to the recipient cells. Exosome are small membrane vesicles secreted by most cell types. Internal vesicles form by the inward budding of the cellular compartments known as multivesicular body (MVB). When MVB fuse with the plasma membrane, these internal vesicles are released as exosomes, which can transfer the DNA, RNA and proteins to the distant recipient cells, and influent various aspects of cell behavior and physiology. The inset shows a typical ultracentrifugation protocol. In consecutive rounds of centrifugation and pouring off, the RCF (g) and the centrifugation time are increased to pellet smaller particles. After first 200×g and 2000×g centrifugations, pellets that contain dead cells and cell debris are discarded, and the supernatant is kept for the next step. In contrast, after the 100 000×g centrifugations, pellets (containing EVs) are kept, and supernatants are discarded. The pellets are resuspended in phosphate buffered saline (PBS) for further analysis.
Exosomes are engineered at the cellular level under natural conditions, but the successful modification of exosomes requires further exploration21. As compared with the cellular level endogenous method, which relies on biological approaches, exogenous methods, after production in cell culture, make exosome application more reliable when exosome source cells are not amenable to customized modification12. In addition, exogenous methods expand the possible sources for exosomes, thereby greatly facilitating mass production. Successful post-isolation modification of exosomes relies on a complete understanding of their structural characteristics and underlying cellular biology and is necessary to facilitate future diagnostic and therapeutic interventions. The yield of exosomes and the physicochemical properties that affect their pharmacokinetics may vary with the type of donor cell22. Because these factors are expected to greatly influence the therapeutic efficacy of exosomes, it is necessary to select the appropriate type of donor cells for the development of exosome-based DDS11,12. Here, we will mainly focus on different exogenous modification strategies for exosome-based therapeutic and diagnostic applications.
Appropriate source cells for engineered exosomes
Exosomes from a vast variety of cells have been investigated for use in clinical therapeutic applications12,22,23. Exploring different cell sources for therapeutic exosomes is of interest, because the lipid and surface protein composition of exosomes may be crucial to their function, and preservation of these characteristics is very important24. Additionally, surface markers, depending on the cell of origin, may have functions that jeopardize the intended effect of the exosomal therapy and even endanger the recipient. It is therefore crucial to carefully study and consider the biological characteristics of exosomes derived from different cell types and to weigh their benefits and drawbacks for therapeutic purposes.
A number of groups have investigated the use of tumor cell-derived exosomes for delivery of chemotherapeutic agents or other anticancer agents12,25. There are appealing aspects to the use of tumor-derived exosomes for delivery of therapeutic agents and vaccines for immunotherapy. For example, tumor cells and tumor exosomes can be found in high numbers in malignant effusions, and it has been shown that tumor exosomes carry tumor-associated antigens specific to the tumors from which they are derived, as well as MHC class I molecules. Tumor exosomes can deliver antigens to dendritic cells and consequently induce a T-cell-mediated immune response against tumor cells26. In fact, a phase I clinical trial has been completed on the release of tumor exosomes, which were presumed to bear tumor-specific antigens ready for presentation to immune cells and to stimulate the immune systems of glioma patients to clear remaining tumor cells after resection27. In addition, tumor targeting and selective drug delivery using tumor-derived exosomes has been proposed as a possibility because of their specific expression of tetraspanins, which preferentially interact with ligands in different tissues28. On the one hand, tumor-derived exosomes demonstrate specific targeting capabilities25; on the other hand, they have been shown to signal an increase in metastatic behavior in less metastatic cancer cell types29 and to contain tetraspanins, which mediate tumor growth. Proteases, such as urokinase plasminogen activator, which promotes tumor cell invasion, and cathepsin D, and adhesion modulators, such as vimentin, galectin 3-binding protein, and annexin A1, have also been found in tumor-derived exosomes30. miRNAs and other nucleic acids, which can lead to malignant changes in target cells, have been found in tumor cell exosomes31. In addition, exosomes from melanoma patients have been shown to enhance the production of myeloid-derived suppressor cells (MDSCs), in an important mechanism by which tumors avoid immune recognition32,33. Most importantly, tumor exosomes can inhibit tumor-reactive effector T cells through the expression of apoptosis-inducing ligands, such as FasL and TRAIL or PDL-234,35. Tumor exosomes exhibit potential drawbacks, including induction of apoptosis in activated cytotoxic T cells, impairment of monocyte differentiation, induction of myeloid-suppressive cells and T regulatory cells, suppression of lymphoid activation signaling molecules, and induction of a pro-inflammatory microenvironment33. The potential risk of tumor exosomes aggravating a patient's malignancy, instead of improving it, makes choosing the right exosomes crucial for therapy.
For the reasons mentioned above, exosomes isolated from fruits and plants have been explored as alternative options for clinical use because they come from reliable sources and have better safety profiles36. Exosomes derived from food have garnered attention, owing to the obvious conclusion that these exosomes are known to be commonly ingested and thus are generally considered safe37. In addition, agricultural products such as fruits and milk are relatively economically practical and scalable sources from which to isolate exosomes. A phase I clinical trial has been undertaken for the use of exosomes derived from fruit to deliver curcumin to the colon for the treatment of colon cancer38. Other groups are also isolating exosomes from plants for use in therapeutic applications. For example, Ju et al have isolated exosome-like nanoparticles from grapes and have found that oral administration of these vesicles induces growth and differentiation of intestinal stem cells and protects mice from intestinal damage initiated by dextran sulfate sodium36. Another group, led by Wang et al, has modified exosomes from grapefruit to improve their tumor targeting capability and loaded them with the anticancer agents doxorubicin and curcumin. These modified exosomes have been found to target inflammatory tumors and to have efficacy against inflammation in a mouse model39. Another agricultural source of reliable, scalable, and safe exosomes for therapeutic delivery is bovine milk. Munagala et al have isolated exosomes naturally present in milk and loaded them with different therapeutic cargos, including both hydrophilic and hydrophobic small molecules and chemotherapeutic agents. They then tested these drug-loaded exosomes on lung tumor cells in culture and in xenograft models and have found enhanced biological efficacy of exosome-encapsulated formulations over free drug, especially when the tumor targeting ligand folic acid is added to the exosomes37. Regardless of the high yield and superior safety profiles of exosomes isolated from food, these exosomes are incapable of boosting host immune systems and lack immunotherapy benefits.
Immune cell-derived exosomes, as a consequence, are receiving a great deal of attention regarding drug delivery and therapeutic vaccine applications40,41,42. Monocytes and macrophages in particular have been investigated as sources of exosomes for immunotherapy43. These exosomes have been shown to be especially good at evading immune phagocytosis, a clearing mechanism that is a drawback associated with other exosome types, thus allowing these exosomes to circulate longer and therefore extend their efficacy44. However, the future looks brightest for dendritic cell (DC)-derived exosomes for vaccine delivery, because they have been proven safe in multiple phase I trials in different types of cancers40. Phase II clinical efficacy studies have recently been completed using these exosomes loaded with tumor antigen as a vaccine against non-small cell lung cancer in combination with cyclophosphamide45. It has been shown that DC-derived exosomes can facilitate tumor rejection in vivo by transferring peptide-MHC complexes from DCs that have been exposed to an antigen to other DCs that have not been in contact with the same antigen41,42,43,46.
Engineering methods for encapsulation of therapeutic agents and imaging probes
Exosomes are composed of a lipid membrane bilayer structure that expresses the surface ligands and receptors from source cells24. The exosome lipid membrane surrounds and contains a hydrophilic core24. A thorough understanding of exosome structure is useful for manipulating the function and cargo packaging of modified exosomes. Therapeutic agents are incorporated into exosomes or exosome mimetics using two major approaches: (I) active or (II) passive encapsulation. These approaches result in different loading efficiencies and stabilities of the drugs in the exosome vesicles.
Passive cargo-loading methods
These methods are relatively simple and do not require the addition of active substances into the system.
Incubation with exosomes
Exosomes are simply incubated with drugs, and the drugs diffuse into the exosomes along the concentration gradient. The loading efficiency depends on the hydrophobicity of the drug molecules. Hydrophobic drugs can interact with the lipid layers of the vesicle membrane47. For instance, in one study, mouse lymphoma-derived exosomes have been incubated with curcumin in phosphate-buffered saline (PBS) at 22 °C for 5 min before purification by gradient centrifugation47. In another study, Haney et al have successfully loaded the enzyme catalase, a tetramer protein of 250 kDa, into exosomes extracted from RAW264.7 cells in PBS buffer at room temperature for 18 h44. The main drawback to this method may be its low loading capacity.
Incubation with donor cells
The donor cells are treated with a drug, and these cells then secrete exosomes loaded with the drug. Pascucci et al have treated SR4987 mesenchymal stroma cells with a low dose of paclitaxel for 24 h, then washed the cells and reseeded them in a new flask with fresh medium48. After 48 h of culture, the cell conditioned medium was collected, and exosomes were isolated. The paclitaxel-loaded exosomes from the treated cells had significant, strong anti-proliferative activities against CFPAC-1 human pancreatic cells in vitro, as compared with the exosomes from untreated cells. Interestingly, the exosomes from the untreated SR4987 cells also showed some anti-proliferative effect, possibly as a result of the presence of certain proteins or nucleic acids within the exosomes that can change the tumor microenvironment48.
Active cargo-loading methods
Sonication
Exosomes from donor cells are mixed with drugs or proteins and subsequently sonicated by using a homogenizer probe. The mechanical shear force from the sonicator probe compromises the membrane integrity of the exosomes and allows the drug to diffuse into the exosomes during this membrane deformation. Kim et al have demonstrated that exosome membrane microviscosity significantly decreases after sonication49. Nevertheless, this membrane deformation process does not significantly affect the membrane-bound proteins or the lipid contents of the exosomes. The membrane integrity of the exosomes has been found to be restored within an hour when the exosomes are incubated at 37 °C49. However, in some cases, drugs are not only encapsulated inside the exosomes but also attached to the outer layer of the membrane; as a result, two phases of drug release are observed. The first burst release phase results from the release of the drug attached to the outer layer of the exosomes, and this is followed by the slow release of the drug encapsulated inside the exosome vesicles49.
Extrusion
Exosomes from donor cells are mixed with a drug, and the mixture is loaded into a syringe-based lipid extruder with 100–400 nm porous membranes under a controlled temperature. During the extrusion, the exosome membrane is disrupted and vigorously mixed with the drug. Whether the harsh mechanical force used in this method changes the membrane properties, such as zeta potential, and membrane protein structures is still unclear. Fuhrmann et al have reported that loading exosomes extracted from MDA-MB231 breast cancer cells with porphyrin using the extrusion method alters the zeta potential of the original exosomes and causes cytotoxicity, whereas porphyrin-loaded exosomes prepared using other methods do not show significant cytotoxicity50. These observations might have resulted from the intensive extrusion process (the exosomes were extruded 31 times) transformed the vesicle constitution50. However, catalase loaded into RAW264.7 macrophage-derived exosomes by using 10 extrusions has been found to have no cytotoxicity and to demonstrate greater neuroprotective activity than that of the exosomes prepared by freeze/thaw or simple incubation methods44. Therefore, further detailed studies into this method are required.
Freeze and thaw cycles
In this procedure, drugs are incubated with exosomes at room temperature for a fixed amount of time, and then, the mixture is rapidly frozen at -80 °C or in liquid nitrogen and thawed at room temperature. This process is repeated for at least 3 cycles to ensure drug encapsulation51. However, the method can induce aggregation of the exosomes, thus resulting in a broad size distribution of the drug-loaded exosomes. The drug loading capacity of the freeze/thaw method is generally lower than that of the sonication or extrusion methods. Interestingly, this method can be used for membrane fusion between exosomes and liposomes and has been used to create exosome-mimetic particles, as reported by Sato et al51, who have isolated exosomes from RAW264.7 macrophages and fused the exosomes with several types of phospholipid-based liposomes. The fused exosome-liposomes were characterized using a fluorescence resonance energy transfer (FRET) assay51, and the number of freeze/thaw cycles has been found to affect the lipid dilution ratios, thus resulting in changes in the fluorescence intensity.
Electroporation
This technique creates small pores in the exosome membrane through application of an electrical field to exosomes suspended in a conductive solution. The electrical current disturbs the phospholipid bilayer of the exosomes, thus resulting in the formation of temporary pores. Drugs or nucleotides can subsequently diffuse into the interior of the exosomes via the pores. The integrity of the exosome membrane is then recovered after the drug loading process. This method is widely used for loading siRNA or miRNA into exosomes, because these nucleotides are relatively large and cannot diffuse into the exosome spontaneously, as do small hydrophobic molecules. Electroporation leads to superior loading of siRNA over chemical transfection52. However, electroporation may cause RNA aggregation and exosome instability, thereby resulting in a low loading capacity. Johnsen et al have reported that electroporation in an optimized buffer such as trehalose disaccharide can aid in maintaining the structural integrity and can inhibit the aggregation of exosomes extracted from adipose-derived stem cells53. Electroporation not only enhances RNA loading into exosomes but also increases hydrophilic small molecule loading into exosomes, for example, 5,10,15,20-tetrakis (1-methyl-4-pyridinio) porphyrin tetra (p-toluenesulfonate) or TMP, which is used for photodynamic effects50.
Incubation with membrane permeabilizers
Saponin is a surfactant molecule that can form complexes with cholesterol in cell membranes and generate pores, thus leading to an increase in membrane permeabilization54. Saponin enhances the loading capacity of catalase into exosomes, as compared with the simple incubation method. Although saponin is a surface-active agent, it does not degrade the catalase, whose activity is preserved44. Saponin can also assist in loading hydrophilic molecules into exosomes. A study has shown that incubation of a small hydrophilic molecule with saponin increases drug loading into exosomes 11-fold compared with passive loading without saponin50. However, there are concerns regarding the in vivo hemolytic activity of saponin when this compound is used54. Therefore, the concentration of saponin used for drug loading should be limited, and the exosomes should be purified after incubation with saponin.
Click chemistry method for direct conjugation
Chemistry methods can also be used to directly attach molecules to the surfaces of exosomes via covalent bonds. Copper-catalyzed azide alkyne cycloaddition, known as click chemistry, is ideal for bioconjugation of small molecules and macromolecules to the surfaces of exosomes11. An alkyne chemical group and an azide chemical group react to form a triazole linkage. The reaction is rapid and efficient, as compared with traditional cross-linking reactions, such as maleimide-thiol coupling, and provides better control over the conjugation site. It is also suitable for modification of biological macromolecules, because it can occur in aqueous media55. Smyth et al have reported that an exosome cross-linked with alkyne groups using carbodiimide chemistry can be conjugated to a model azide, azide-fluor 54556. This conjugation has no effect on the size and internalization of the exosomes, thus suggesting that the reaction is mild and does not affect exosome structure or function.
Antibody against exosomal proteins
Recent studies have indicated that exosomes carry the genetic and proteomic contents of their parent cells. Fluorophores and microbeads conjugated to highly specific antibodies can bind a particular antigen on the cell surface. Higginbotham et al have demonstrated the feasibility of using fluorescence-activated vesicle sorting to analyze and sort individual exosomes isolated from DiFi cells57. EGFR and the exosomal marker CD9 has been used to conjugate Alexa-647 to the surfaces of exosomes for detection. Because exosomes carry the antigens of their parent cells, it is logical to assume that specific antigen-conjugated microbeads can be used for exosome isolation and tracking in vivo.
The various approaches used to load cargos into exosomes each have different loading capacities, which depend upon the properties of the cargo such as hydrophilicity, hydrophobicity, and molecular weight. Haney et al have reported that sonication and extrusion provide the highest catalase loading into exosomes, as compared with freeze/thaw cycles and passive incubation, which yield the lowest drug loading efficiency44. However, loading efficiency is not the only factor that must be considered. Exosome membrane integrity and stability are also important in drug delivery. As mentioned earlier, although catalase loading into exosomes using sonication and extrusion results in good enzymatic activity, other studies have found that membrane integrity is compromised, and the membrane-bound protein structure may change, thus affecting the therapeutic effect of the drug-loaded exosomes. Therefore, further investigation is necessary.
Engineering exosomes for delivery of therapeutic and diagnostic molecules
The previously described strategies for engineering exosomes further highlight the unique advantages of exosome-based nanoplatforms for cargo delivery. A series of good examples of these strategies that have been successfully applied for exosome-mediated delivery of functional molecules are presented in Table 1.
Table 1. Examples of engineering exosomes for cargo delivery.
| Advantages | Disadvantages | Model drugs | ||
|---|---|---|---|---|
| I) Passive loading | a) Incubation of exosomes and free drugs | Simple Do not compromise membrane integrity | Low drug loading efficiency | Doxorubicin60 Paclitaxel48 Catalase44 Paclitaxel58 |
| b) Incubation of the donor cells with free drugs | Simple Do not compromise membrane integrity | Low drug loading efficiency Drugs may cause cytotoxicity to the donor cells | ||
| II) Active loading | a) Sonication | High drug loading efficiency | Compromise membrane integrity | Catalase44 |
| b) Extrusion | High drug loading efficiency | Compromise membrane integrity | Porphyrin50 | |
| c) Freeze/thaw | Medium drug loading efficiency Liposome-exosome fusion | Aggregations | Porphyrin | |
| d) Electroporation | Loading with large molecules such as siRNA, miRNA | Aggregations | let-7a miRNA66 MAPK1 siRNA52 | |
| e) Incubation with saponin | Enhanced drug loading | Toxicity | Catalase44 Porphyrin50 | |
| f) Click chemistry | Quick and efficient Better control over the conjugation site | Azide-fluor 545 for in vitro tracking56 | ||
| g) Antibody binding | Specific and easy to operate | CD9 antibody with Alexa-64757 |
Delivery of small therapeutic molecules
Several small molecules, both hydrophobic and hydrophilic, have been incorporated into exosomes by using the different methods mentioned in the above section (Figure 2A, 2B). In most cases, exosomal delivery leads to higher drug accumulation in target cells and improved small molecule stability and blood circulation time, thus improving the potency of small molecule drugs and lowering the IC50. Munagala et al have used exosomes isolated from bovine milk to encapsulate chemotherapeutic and chemopreventive agents, such as paclitaxel, doxorubicin, and withaferin, by passive loading58. These drugs are slowly released from the exosomes in a time-dependent manner. The researchers have demonstrated that withaferin-loaded exosomes and paclitaxel-loaded exosomes have a lower IC50 than those of the free drugs for anti-proliferative activities against A549 lung cancer cells. They have also confirmed the anti-tumor efficacy in vivo by using a tumor-bearing mouse model. Withaferin-loaded exosomes exhibit a significantly greater inhibitory effect on tumors compared with that of controls after intraperitoneal injection at a suboptimal dose58. Sun et al have demonstrated that curcumin-loaded exosomes isolated from the EL-4 mouse lymphoma cell line show better curcumin stability in vitro and higher blood concentration in vivo47. As a result, the level of curcumin increases in the CD11b+Gr-1+ target cells, and significant suppression of inflammatory cytokines such as IL-6 and TNF-α has been achieved in a model of lipopolysaccharide-induced septic shock in immunocompetent mice47. Kim has reported that paclitaxel-loaded macrophage-derived exosomes, compared with paclitaxel-loaded liposomes, significantly increases cellular uptake in the 3LL-M227 murine Lewis lung carcinoma cell line59. Interestingly, paclitaxel-loaded exosomes have been found to overcome the drug resistance problem in MDCK MDR1 cells. The IC50s of paclitaxel-loaded exosomes are significantly lower than those of free paclitaxel and Taxol59. Thus, exosome delivery can bypass the P-glycoprotein (P-gp) drug efflux system. However, the exosomes themselves do not inhibit P-gp, and the mechanism by which they overcome the drug resistance remains unclear.
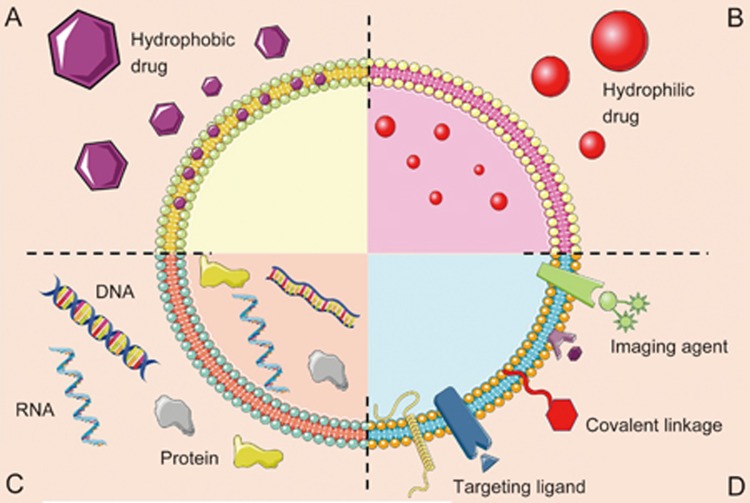
Figure 2.
Schematic representation of the different types of exosomes drug delivery systems. Exosomes consist of a lipid bilayer that can be composed of lipids, which encloses an aqueous core. Both the lipid bilayer and the aqueous space can incorporate hydrophobic (A) or hydrophilic compounds (B), respectively. (C) Exosomes can be used for DNA, RNA and protein delivery. (D) Theranostic or imaging probes, specific targeting ligands and covalent linkage can be attached to exosome surface.
Delivery of therapeutic RNA
RNA is a large molecule and is difficult to deliver in vivo. Several approaches have been explored, such as use of cationic liposomes60,61, dendrimers62, and cationic polymer-based particles63. However, these carriers are not suitable for use in clinical practice, owing to safety, stability and off-target issues64. The most commonly used technique to incorporate siRNA or miRNA into exosomes is electroporation, because this method destabilizes the vesicle membrane and allows siRNA or miRNA to penetrate the vesicles. However, aggregation of exosomes and RNA during electroporation has previously been reported, as discussed above. Alvarez-Erviti et al have utilized murine self-derived dendritic exosomes targeted with Lamp2b protein to deliver GADPH siRNA and BACE1 siRNA across the blood-brain barrier in mice. The data show that the housekeeping gene, GADPH, and BACE1-loaded exosomes strongly suppress mRNA expression and β-amyloid in the brains of wild-type mice after intravenous injection, as compared with Lipofectamine and siRNA-RVG-9R65. Wahlgren et al have demonstrated that siRNA loaded in exosomes isolated from human plasma successfully target monocytes and lymphocytes and subsequently silence the MAPK1 gene. The data also have shown that siRNA-loaded exosomes co-localize in the cytoplasm of recipient cells. However, only in vitro studies have been undertaken, in which the exosomes were co-cultured with the recipient cells; as a consequence, these recipient cells take up the exosomes more easily than they would in an in vivo situation52. Ohno et al have reported the delivery of GE11-targeted exosomes containing let-7a miRNA to epidermal growth factor (EGFR)-overexpressing breast cancer cells in a mouse xenograft model. GE11-targeted exosomes show higher tumor accumulation than do controls. Moreover, the exosomes isolated from donor cells previously transfected with let-7a miRNA inhibit tumor growth in mice66. These examples suggest that exosomes are a promising method for gene delivery with better safety profiles than those of viral vectors, cationic lipids, or polymer based particles.
Delivery of therapeutic proteins
Utilizing exosomes is one of the most promising methods for delivering macromolecular proteins (Figure 2C). Proteins can be loaded into exosomes through genetic engineering of the donor cells or through direct loaded into the exosomes. In the first method, donor cells are transfected with a plasmid carrying the gene of interest. Consequently, the cells synthesize the protein encoded by the inserted gene, and these proteins are subsequently secreted into the extracellular vesicles. At this stage, the extracellular vesicles can be isolated by collecting the cell culture supernatant and then purified. In the second method, proteins are directly loaded into exosomes as mentioned in the previous section. Catalase, a redox enzyme, loaded in exosomes extracted from macrophages has neuroprotective effects against oxidative stress in mice with acute brain inflammation. Catalase-loaded exosomes significantly decreases microgliosis and astrocytosis in the mouse brain and decreases rotation after apomorphine induction. These data suggest that exosomes are able to preserve catalase function and deliver catalase to the mouse brain after intranasal administration44.
Delivery of imaging molecules
Currently, a number of reports demonstrating post-isolation strategies to modify exosome surface structures have described methods to more effectively track exosomes in vitro or in vivo (Figure 2D). Lai et al have engineered human embryonic kidney 293T exosomes expressing a membrane-bound Gaussian luciferase fused to a biotin receptor domain and then complexed the biotin expressed on the exosomes with fluorescent Alexa Fluor 680-streptavidin67, thus allowing the exosomes to be tracked in vivo either by bioluminescence or fluorescence. In addition, Wilna et al have used fluorophore-conjugated antibodies against the exosomal proteins CD24 and aquaporin 2 (AQP2) to identify a subpopulation of CD24- and AQP2-positive exosomes by using nanoparticle tracking analysis (NTA) in vitro68. Wang et al have used anti-CD63 antibody-conjugated microbeads and secondary antibody-conjugated Q-dots to track endothelial cell exosomes by using NTA69. Exosomes are initially formed during the inward budding of late endosomes and are subsequently stored inside of multi-vesicular bodies (MVBs) before being released into the extracellular space. Thus, it is not surprising that some endosomal forming and sorting proteins (Rab5, Rab27 and Rab35), heat-shock proteins, and tetraspanins (CD9, CD63 and CD81) are enriched in exosomes. Because of this enrichment, and the presence of other donor cell-derived factors within exosomes, the selection of source cells has a great impact on the function and distribution of exosomes and must be considered carefully.
Engineering targeted molecules on exosomes
Because exosomes are extracellular vesicles secreted from cells, they intrinsically express some lipids and cell adhesion molecules and ligands that naturally target certain types of recipient cells. Several studies have shown that exosomes have natural targeting ability based on donor cells. For instance, exosomes isolated from neuroblastoma intrinsically express glycosphingolipid glycan groups that can bind to the aggregates of amyloid-β in the brain, and therefore may provide an effective treatment for Alzheimer's disease11. Targeting ligands on the surfaces of exosomes can also be engineered. The most commonly used technique is to insert the gene encoding the targeting proteins into the donor cells. The donor cells then secrete this protein in the exosomes. For example, plasmids encoding Lamp2b have been constructed and transfected into dendritic cells. The exosomes harvested after the donor cells have been transfected and found to fuse strongly to the neuron-specific rabies viral glycoprotein (RVG) peptide through Lamp2b on the exosomal membrane. The expression of Lamp2b on the exosomes has been confirmed by western blotting. These targeted exosomes can effectively deliver siRNA to the brain in a mouse model65. Another study has used exosomes to deliver let-7a miRNA in a targeted manner to EGFR-overexpressing breast cancer cells in mice. GE11 or EGF was cloned into a pDisplay vector and transfected into HEK299 cells. The data suggest that intravenous injection of the let-7a-loaded GE11-targeting exosomes can deliver the gene to the EGFR-expressing tumor in a mouse xenograft model66.
Click chemistry is rapidly becoming a popular tool for modification of biomacromolecules. Smyth et al have reported a novel technique that can be used to functionalize the surfaces of exosomes with small molecules, large biomacromolecules and polymers and to monitor the exosome biodistribution in vivo56. It is expected that use of click chemistry may affect exosome biodistribution through conjugation of targeting moieties, such as RGD derivatives, with azide-terminated groups, such as those that have been used in other nanocarriers56,70. In addition, some noncovalent strategies have been used to provide stable modification of the exosome surface. Amstrong et al and Nakase et al have used electrostatic interactions to bind cationic lipids on the surfaces of exosomes, which enhance the exosome uptake71,72. In another study, Qi et al have successfully bound the surfaces of blood exosomes with transferrin-conjugated superparamagnetic nanoparticles targeting the native transferrin receptors present on the exosome membrane73. Despite these successes, achieving efficient exosome targeting via surface modification is nontrivial. The reaction conditions must be strictly limited to avoid exosome disruption and aggregation due to inappropriate temperature, pressure and osmotic stress71.
Future directions and concluding remarks
The advent of nanotechnology for use in medicine has heralded a new chapter in drug delivery. Exosomes are promising for use as vectors for clinical application, owing to their strong biocompatibility. However, there are risks associated with use of exosomes as well, such as immunosuppression and reversion to tumorigenesis74,75,76. Therefore, the relentless search for safe and effective exosome-based nanoformulations has led to exogenous modification of exosomes as a viable path. Because exosomes can be derived from various types of cells, the issues associated with the use of tumor exosomes can be circumvented. It is highly probable that modified exosomes will be engineered for clinical use, the exosome source will be immune cells43, and the exosomes will be artificially optimized by incorporation of specific payloads.
Despite the advances outlined in this article, there are still many challenges ahead. One challenge is achieving large-scale production of exosomes for clinical use77. It has previously been reported that large-scale manufacturing of therapeutic exosomes can be achieved via rapid purification78. However, this technique still requires further testing with different types of cells. Furthermore, the question of which cell type to use for exosome derivation still remains to be answered. Nevertheless, the concept of utilizing exosomes as delivery vehicles is attractive and promising. Hence, exosomes may be the answer to the ongoing search for a clinically suitable nanoplatform. More systematic in vivo studies regarding the potency and toxicology of exosomes are imperative for bringing this exciting development a step closer to clinical reality.
References
- Liu D, Yang F, Xiong F, Gu N. The smart drug delivery system and its clinical potential. Theranostics 2016; 6: 1306–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde E, Thammasiraphop K, Duong HT, Yeow J, Karagoz B, Boyer C, et al. Pair correlation microscopy reveals the role of nanoparticle shape in intracellular transport and site of drug release. Nat Nanotechnol 2017; 12: 81–9. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Gothwal A, Kesharwani P, Alsaab H, Iyer AK, Gupta U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov Today 2016; doi: 10.1016/j.drudis.2016.09.013. [DOI] [PubMed]
- Luan X, Guan YY, Lovell JF, Zhao M, Lu Q, Liu YR, et al. Tumor priming using metronomic chemotherapy with neovasculature-targeted, nanoparticulate paclitaxel. Biomaterials 2016; 95: 60–73. [DOI] [PubMed] [Google Scholar]
- Haque S, Whittaker MR, McIntosh MP, Pouton CW, Kaminskas LM. Disposition and safety of inhaled biodegradable nanomedicines: Opportunities and challenges. Nanomedicine 2016; 12: 1703–24. [DOI] [PubMed] [Google Scholar]
- Matsumura Y. The drug discovery by nanomedicine and its clinical experience. Jpn J Clin Oncol 2014; 44: 515–25. [DOI] [PubMed] [Google Scholar]
- Suk JS, Xu QG, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliver Rev 2016; 99: 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wu QJ, Peng F, Liu L, Gong CY. Strategies of polymeric nanoparticles for enhanced internalization in cancer therapy. Colloid Surface B 2015; 135: 56–72. [DOI] [PubMed] [Google Scholar]
- Chow TH, Lin YY, Hwang JJ, Wang HE, Tseng YL, Wang SJ, et al. Improvement of biodistribution and therapeutic index via increase of polyethylene glycol on drug-carrying liposomes in an HT-29/luc xenografted mouse model. Anticancer Res 2009; 29: 2111–20. [PubMed] [Google Scholar]
- Peng Q, Mu H. The potential of protein-nanomaterial interaction for advanced drug delivery. J Control Release 2016; 225: 121–32. [DOI] [PubMed] [Google Scholar]
- Hood JL. Post isolation modification of exosomes for nanomedicine applications. Nanomedicine (Lond) 2016; 11: 1745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev 2016; 106: 148–56. [DOI] [PubMed] [Google Scholar]
- Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release 2015; 219: 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release 2015; 219: 278–94. [DOI] [PubMed] [Google Scholar]
- Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol-Cell Ph 2014; 306: C621–C33. [DOI] [PubMed] [Google Scholar]
- Desrochers LM, Antonyak MA, Cerione RA. Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev Cell 2016; 37: 301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol 2012; 44: 2060–4. [DOI] [PubMed] [Google Scholar]
- Malhotra H, Sheokand N, Kumar S, Chauhan AS, Kumar M, Jakhar P, et al. Exosomes: tunable nano vehicles for macromolecular delivery of transferrin and lactoferrin to specific intracellular compartment. J Biomed Nanotechnol 2016; 12: 1101–14. [DOI] [PubMed] [Google Scholar]
- Rupert DL, Claudio V, Lasser C, Bally M. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim Biophys Acta 2017; 1861: 3164–79. [DOI] [PubMed] [Google Scholar]
- Hood JL, Wickline SA. A systematic approach to exosome-based translational nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2012; 4: 458–67. [DOI] [PubMed] [Google Scholar]
- Morishita M, Takahashi Y, Matsumoto A, Nishikawa M, Takakura Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials 2016; 111: 55–65. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem 2016; 74: 103–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagoli GG, Zelante BB, Toniolo PA, Migliori IK, Barbuto JA. Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front Immunol 2015; 5: 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydrychowicz M, Kolecka-Bednarczyk A, Madejczyk M, Yasar S, Dworacki G. Exosomes — structure, biogenesis and biological role in non-small-cell lung cancer. Scand J Immunol 2015; 81: 2–10. [DOI] [PubMed] [Google Scholar]
- Mahaweni NM, Kaijen-Lambers ME, Dekkers J, Aerts JG, Hegmans JP. Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J Extracell Vesicles 2013; 2: 22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001; 7: 297–303. [DOI] [PubMed] [Google Scholar]
- Thomas Jefferson University. Pilot Immunotherapy trial for Recurrent Malignant Gliomas. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2016 Oct 20]. Available from: http://clinicaltrials.gov/show/ NCT01550523 NLM Identifier: NCT 01550523.
- Rana S, Yue SJ, Stadel D, Zoller M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell B 2012; 44: 1574–84. [DOI] [PubMed] [Google Scholar]
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015; 161: 1046–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DA, Patel SH, Gucek M, Hendrix A, Westbroek W, Taraska JW. Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS One 2015; 10: e0117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014; 26: 707–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey AB. Myeloid suppressor cells regulate the adaptive immune response to cancer. J Clin Invest 2006; 16: 2587–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironmen -ts. Semin Immunopathol 2011; 33: 441–54. [DOI] [PubMed] [Google Scholar]
- Tomihari M, Chung JS, Akiyoshi H. Cruz PD Jr, Ariizumi K. DC-HIL/glycoprotein Nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells. Cancer Res 2010; 70: 5778–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res 2005; 11: 1010–20. [PubMed] [Google Scholar]
- Ju SW, Mu JY, Dokland T, Zhuang XY, Wang QL, Jiang H, et al. Grape Exosome-like nanoparticles induce intestinal stem cells and protect mice from dss-induced colitis. Mol Ther 2013; 21: 1345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett 2016; 371: 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James Graham Brown Cancer Center; University of Louisville. Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2016 Oct 20]. Available from: http://clinicaltrials.gov/show/NCT01294072 NLM Identifier: NCT 01294072.
- Wang QL, Ren Y, Mu JY, Egilmez NK, Zhuang XY, Deng ZB, et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res 2015; 75: 2520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med 2005; 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Terme M, Flament C, Taieb J, Andre F, Novault S, et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15R alpha. PLoS One 2009; 4: e4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, et al. Dendritic cells release hla-b-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One 2008; 3: e3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoda BB, Ajit SK. Modulation of immune responses by exosomes derived from antigen-presenting cells. Clin Med Insights Pathol 2016; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney MJ, Klyachko NL, Zhaoa YL, Gupta R, Plotnikova EG, He ZJ, et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release 2015; 207: 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustave Roussy, Cancer Campus, Grand Paris. Trial of a Vaccination With Tumor Antigen-loaded Dendritic Cell-derived Exosomes (CSET 1437). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2016 Oct 20]. Available from: http://clinicaltrials.gov/show/ NCT01159288 NLM Identifier: NCT 01159288.
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4(+) T cells by dendritic cell-derived exosomes. Nat Immunol 2002; 3: 1156–62. [DOI] [PubMed] [Google Scholar]
- Sun D, Zhuang XY, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 2010; 18: 1606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci L, Cocce V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J Control Release 2014; 192: 262–70. [DOI] [PubMed] [Google Scholar]
- Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed-Nanotechnol 2016; 12: 655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 2015; 205: 35–44. [DOI] [PubMed] [Google Scholar]
- Sato YT, Umezaki K, Sawada S, Mukai S, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep 2016; 6: 21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren J, Karlson TD, Brisslert M, Sani FV, Telemo E, Sunnerhagen P, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res 2012; 40: e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen KB, Gudbergsson JM, Skov MN, Christiansen G, Gurevich L, Moos T, et al. Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology 2016; 68: 2125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev 2010; 9: 425–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Altinoglu S, Takeda YS, Xu QB. Integrating protein engineering and bioorthogonal click conjugation for extracellular vesicle modulation and intracellular delivery. PLoS One 2015; 10: e0141860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth T, Petrova K, Payton NM, Persaud I, Redzic JS, Gruner MW, et al. Surface functionalization of exosomes using click chemistry. Bioconjugate Chem 2014; 25: 1777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham JN, Zhang Q, Jeppesen DK, Scott AM, Manning HC, Ochieng J, et al. Identification and characterization of EGF receptor in individual exosomes by fluorescence-activated vesicle sorting. J Extracell Vesicles 2016; 5: 29254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett 2016; 371: 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome mdr in cancer cells. Nanomedicine 2016; 12: 655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J, Hirabayashi K, Nakagawa S, Yamaguchi T, Nogawa M, Kashimori I, et al. Antitumor activity of small interfering RNA/cationic liposome complex in mouse models of cancer. Clin Cancer Res 2004; 10: 7721–6. [DOI] [PubMed] [Google Scholar]
- Sioud M, Sorensen DR. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem Biophys Res Commun 2003; 312: 1220–5. [DOI] [PubMed] [Google Scholar]
- Patil ML, Zhang M, Taratula O, Garbuzenko OB, He H, Minko T. Internally cationic polyamidoamine PAMAM-OH dendrimers for siRNA delivery: effect of the degree of quaternization and cancer targeting. Biomacromolecules 2009; 10: 258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci U S A 2005; 102: 5679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesharwani P, Gajbhiye V, Jain NK. A review of nanocarriers for the delivery of small interfering RNA. Biomaterials 2012; 33: 713–50. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011; 29: 341–5. [DOI] [PubMed] [Google Scholar]
- Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 2013; 21: 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014; 8: 483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuyzen W, Sime NE, Ivy JR, Turtle EJ, Street JM, Pound J, et al. Quantification of human urinary exosomes by nanoparticle tracking analysis. J Physiol 2013; 591: 5833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Guo R, Yang Y, Jacobs B, Chen S, Iwuchukwu I, et al. The novel methods for analysis of exosomes released from endothelial cells and endothelial progenitor cells. Stem Cells Int 2016; 2016: 2639728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CF, Makila EM, Kaasalainen MH, Liu D, Sarparanta MP, Airaksinen AJ, et al. Copper-free azide-alkyne cycloaddition of targeting peptides to porous silicon nanoparticles for intracellular drug uptake. Biomaterials 2014; 35: 1257–66. [DOI] [PubMed] [Google Scholar]
- Amstrong JP, Holme MN, Stevens MM. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano 2017; doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed]
- Nakase I, Futaki S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci Rep 2015; 5: 10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano 2016; 10: 3323–33. [DOI] [PubMed] [Google Scholar]
- Shao Y, Shen Y, Chen T, Xu F, Chen X, Zheng S. The functions and clinical application of tumor-derived exosomes. Oncotarget 2016; 7: 60736–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem 2016; 74: 103–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest 2016; 126: 1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meel R, Fens MH, Vader P, van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release 2014; 195: 72–85. [DOI] [PubMed] [Google Scholar]
- Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods 2002; 270: 211–26. [DOI] [PubMed] [Google Scholar]