Abstract
Titanium-45 (45Ti) with a three-hour half-life (t1/2=3.08 h), low maximum positron energy and high positron emission branching ratio, is a suitable positron emission tomography (PET) isotope whose potential has not yet been fully explored. Complicated radiochemistry and rapid hydrolysis continue to be major challenges to the development of 45Ti compounds based on a traditional chelator-based radiolabeling strategy. In this study we introduced an intrinsic (or chelator-free) radiolabeling technique for the successful labeling of 45Ti using mesoporous silica nanoparticle (MSN). We synthesized uniform MSN with an average particle size of ∼150 nm in diameter. The intrinsic 45Ti-labeling was accomplished through strong interactions between 45Ti (hard Lewis acid) and hard oxygen donors (hard Lewis bases), the deprotonated silanol groups (-Si-O-) from the outer surface and inner meso-channels of MSN. In vivo tumor-targeted PET imaging of as-developed PEGylated [45Ti]MSN was further demonstrated in the 4T1 murine breast tumor-bearing mice. This MSN-based intrinsic radiolabeling strategy could open up new possibilities and speed up the biomedical applications of 45Ti in the future.
Keywords: positron emission tomography, titanium-45, intrinsic radiolabeling, mesoporous silica nanoparticle, tumor targeting, 4T1 murine breast tumor
Introduction
Positron Emission Tomography (PET) is a highly quantitative, whole body functional imaging technique that plays a vital role in model clinical diagnosis and pre-clinical research1. During PET imaging, a radionuclide (or probe) produces positrons, which annihilate with surrounding electrons to release two 511 keV photons in opposing directions. The detectors of the PET scanner measure these photons and use this information to create an image of the probe distribution in the body. To date, positron emission radioisotopes, such as fluorine-18 (18F, t1/2=109.8 min), copper-64 (64Cu, t1/2=12.7 h), and zirconium-89 (89Zr, t1/2=78.4 h), have been actively used for clinical use and translational and pre-clinical research2.
Most current radiolabeling involves the use of exogenous chelators, which coordinate with radioisotopes to form a stable complex3. Nanoparticle-based intrinsic radiolabeling is an emerging chelator-free radiolabeling technique that takes advantages of the physical and chemical properties of both the nanoparticle and the selected radioisotopes4,5. Successful labeling of isotopes, such as 64Cu, arsenic-72 (72As, t1/2=26 h), germanium-69 (69Ge, t1/2=39.1 h) and 89Zr, using this technique has recently been achieved by us and other groups6,7,8,9,10,11,12,13.
Titanium-45 (45Ti, t1/2=3.08 h), with a 3-h half-life, low maximum positron energy (Emax=1040 keV, Eavg=439 keV) and high branching ratio (β+=84.8%), is a suitable PET isotope whose potential has not yet been fully explored due to challenges in achieving stable radiolabeling. Although it is relatively easy to generate on a cyclotron, its complicated radiochemistry and rapid oxidation of the metal in aqueous solution14 have resulted in few beingliterature reports during the last three decades on the radiolabeling and biomedical application of 45Ti15,16,17,18,19,20. For example, one of the very first 45Ti labeling studies was reported more than 30 years ago, when the 45Ti-titanium ascorbate complex (45Ti-AsA) was synthesized for tracking 45Ti in plants and live animals16. A doubly labeled complex was also prepared from 14C-ascorbic acid to form 45Ti-AsA-14C. Autoradiogram imaging in an osteogenic-disordered rat demonstrated that the 45Ti-AsA distribution did not coincide with that of 45Ti-AsA-14C, a clear indication of the low in vivo radiostability of the 45Ti-AsA complex16.
In a follow-up study by Ishiwata et al, four 45Ti-labeled compounds, 45Ti-labeled phytate (45TiO-phytate), 45Ti-labeled human serum albumin (45TiO-HAS), 45Ti-labeled diethylenetriaminepentaacetic acid (45TiO-DTPA), and 45Ti-labeled citric acid (45TiO-CA), were developed and tested in male Donryu rats17. Given large colloid formation, 45TiOCl2 and 45TiO-phytate shared a similar biodistribution pattern with high uptake in liver, spleen and lungs. In contrast, the remaining three 45Ti compounds (ie, 45TiO-DTPA, 45TiO-HAS and 45TiO-CA) showed similar tissue distributions, with high blood activity and low uptake in the reticuloendothelial system (RES). Further plasma protein binding and in vivo tumor targeting studies provided evidence of DTPA dissociation from the 45TiO-DTPA compound and the strong binding of free 45Ti with transferrin in the plasma. It was concluded that 45TiO-phytate could be used for imaging the RES, whereas 45TiO-DTPA has the potential for measuring blood volume or as a blood-brain-barrier indicator.
Perhaps inspired by Ishiwata's work, very recently, Vavere and co-workers reported a more systematic investigation on the in vivo biodistribution of 45Ti-transferrin, aiming to provide deeper insight into the mechanism of action of titanocene dichloride (a chemotherapeutic agent currently in clinical trials)19. 45Ti-Transferrin was prepared by re-dissolving the processed 45Ti in apotransferrin or by in vivo incorporation through the introduction of 45Ti-citrate. Similar biodistribution patterns and in vivo tumor (EMT-6, expresses high levels of transferrin receptors) uptakes were observed when directly injecting 45Ti-transferrin and 45Ti-citrate, which could transchelate to transferrin before the time of imaging. Despite these previous efforts, no attempts have been made to date using nanoparticles for the radiolabeling of 45Ti.
Here, we introduce a mesoporous silica nanoparticle (MSN, a highly attractive, widely studied drug delivery nanoplatform21,22)-based intrinsic 45Ti radiolabeling technique and investigate the potential of PEGylated [45Ti]MSN for in vivo tumor passively targeted PET imaging.
Materials and methods
Synthesis of mesoporous silica nanoparticles (MSN)
In a typical synthesis of ∼150 nm sized MSN with 4- to 5-nm pore size, 24 mL of hexadecyl trimethyl ammonium chloride (CTAC, 25 wt%) solution and 0.18 g of triethylamine (TEA) were added to 36 mL of water and stirred gently at 60 °C for 3 h in a 100-mL round bottom flask. Twenty mL of (20% v/v) tetraethyl orthosilicate (TEOS) in cyclohexane was carefully added to the water-CTAC-TEA solution and incubated at 60 °C in a water bath for 12 h (stirring rate was set to 125 r/min). Afterwards, milky white samples were collected by centrifugation (at 12 500×g for 10 min). The samples were subjected to CTAC removal process by stirring in 1 wt% NaCl/methanol solution 3 times (24 h/time).
To functionalize the MSN surface with -NH2 groups, as-synthesized MSN was first dispersed in 20 mL of absolute ethanol followed by the addition of 1 mL of (3-aminopropyl)triethoxysilane (APS). The system was sealed and maintained at 86 to 90 °C in a water bath for 24 h. Afterward, the mixture was centrifuged and washed with ethanol several times to remove the residual APS. The MSN-NH2 was well dispersed in water, and the concentration of -NH2 groups (nmol/mL) was measured using a Kaiser test kit.
Synthesis of dense silica nanoparticles (dSiO2)
In a typical synthesis of uniform ∼90 nm sized dSiO2, 35.7 mL of absolute ethanol was mixed with 5 mL of water and 0.8 mL of ammonia and stirred for 5 to 10 min at room temperature. One mL of TEOS was then added, and the mixture was allowed to react at room temperature for 1 h. Afterward, dSiO2 nanoparticles were collected by centrifugation (at 12 500×g for 10 min), washed with water/ethanol 3 times, and re-suspended in 20 mL of water before use.
Synthesis of sodium citrate-capped CuS nanoparticles (CuS-Cit)
Previously reported procedures were used for the synthesis of CuS-Cit with slight modifications23,24. For a typical synthesis, 10 mL of CuCl2 water solution (0.85 mg/mL) and 10 mL of sodium citrate (1.0 mg/mL) were added to 30 mL water. The mixture was stirred for 30 min at room temperature. After that, 50 μL Na2S (60.54 mg/250 μL) was added to the mixture and stirred for an additional 5 min before transferring to a 90 °C water bath. The reaction was incubated for 15 min before cooling down with ice, forming green-colored CuS-Cit nanoparticles.
Synthesis of [45Ti]MSN-PEG5k
MSN was first labeled with 45Ti using the procedures previously described by incubating 45Ti with MSN-NH2 for one hour at pH 7–8 and 75 °C. The sample was then collected by centrifugation (at 12 500×g for 10 min) and washed with water 3 times. Then, a well-established PEGylation process was introduced by reacting [45Ti]MSN-NH2 with 5 mg (1000 nmol) of SCM-PEG5k-Mal in pH 7 for 60 min. The PEGylated sample was isolated by centrifugation at 12 500×g for 10 min. Free PEG was removed by washing with PBS at least 2 times. As synthesized [45Ti]MSN-PEG5k was then used for in vivo passively targeted tumor imaging.
4T1 tumor model
To generate the 4T1 tumor model, 4- to 5-week-old female BALB/c mice were purchased from Harlan (Indianapolis, IN, USA), and tumors were established by subcutaneously injecting 2×106 cells suspended in 100 μL of 1:1 mixture of RPMI-1640 and Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) into the front flank of mice. The tumor sizes were monitored every other day, and the animals were subjected to in vivo experiments when the tumor diameter reached 5 to 8 mm.
In vivo PET imaging
All animal studies were conducted under a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee. PET scans at various time points post-injection (pi) using a microPET/microCT Inveon rodent model scanner (Siemens Medical Solutions, USA, Inc), image reconstruction, and region-of-interest (ROI) analysis of the PET data were performed as previously described25. Quantitative PET data were presented as percentage injected dose per gram of tissue (%ID/g). 4T1 murine breast tumor-bearing mice, a fast-growing tumor model, were each intravenously injected with ∼300 μCi (or 11.1 MBq) [45Ti]MSN-PEG5k or free 45Ti (diluted with PBS) before serial PET scans.
Ex vivo biodistribution study
Biodistribution studies were performed to confirm that the %ID/g values based on PET imaging truly represented the radioactivity distribution in tumor-bearing mice. Mice were euthanized, and blood, 4T1 tumor, and major organs/tissues were collected and wet-weighed. The radioactivity in the tissue was measured using a gamma-counter (Perkin-Elmer) and presented as%ID/g (%ID/g=percentage injected dose per gram, mean±SD).
Results and discussion
Generation and characterizations of titanium-45
45Ti was produced by proton irradiation of a 0.25-mm-thick scandium foil (99.9%, 96–140 mg) with a current of 20 μA on a GE PETtrace. A 254-μm niobium degrader was used to reduce the proton energy from the nominal 16.0 MeV to 11.8 MeV to avoid the production of 44Ti from 45Sc(p,2n). During irradiation, heat from the scandium foil was removed by contact with a water-cooled silver disk. The activated foil was dissolved in 3 to 4 mL of 6 mol/L HCl. The solution was then diluted with 18 MΩ·cm water (2 to 4 mL) to a concentration of ∼2 mol/L HCl.
The separation of 45Ti was performed on a hydroxamic acid resin26. Briefly, the hydroxamic acid resin was prepared and equilibrated with 2 mol/L HCl27. The target dissolution in 2 mol/L HCl (5 to 8 mL) was passed at a flow rate of 1.1 mL/min over 100 mg of resin loaded in a 5-mm-diameter column using the peristaltic pump-driven, automated module28. Upon passing the target solution through the column, a wash of 5 mL of 2 mol/L HCl was passed before elution with 1 mol/L oxalic acid in fractions of 200 μL. The entire separation process required approximately one hour. The radionuclidic purity assay employed a high-purity germanium (HPGe) detector (Canberra C1519) and an ion chamber (Capintec) connected to an electrometer (Keithley 6517A) for decay logging. MicroPET images of a Derenzo phantom were also obtained for resolution evaluation.
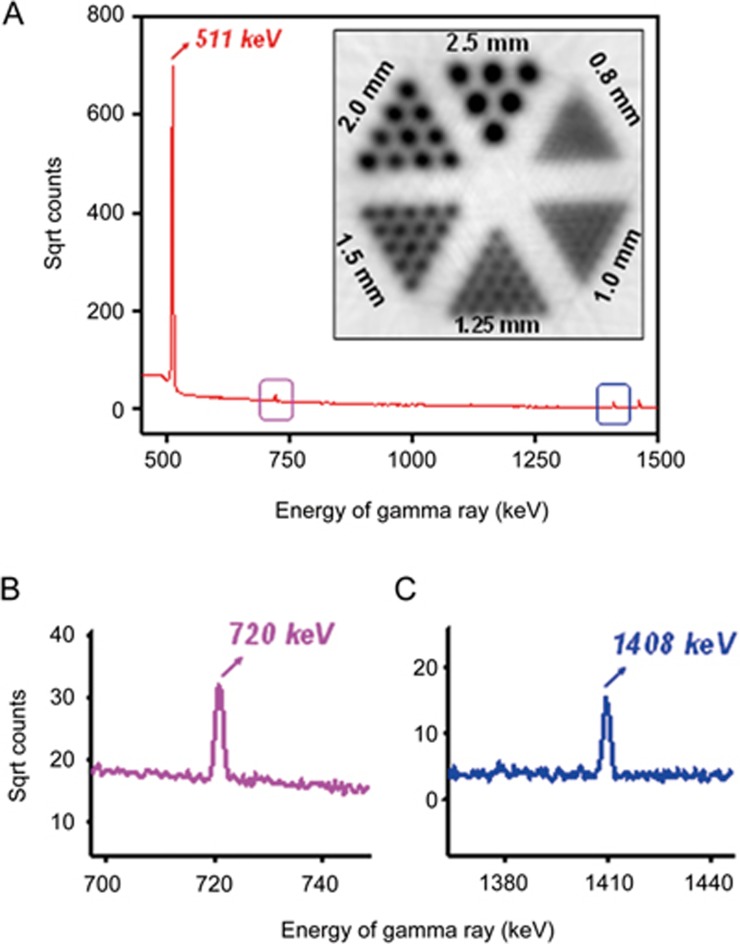
Results showed that one-hour irradiations generate 4.6±0.4 GBq of 45Ti (n=6). In addition, 42%±6% of this activity was separated in 600 μL of 1 mol/L oxalic acid. The gamma spectrum (Figure 1A) obtained with the HPGe detector exhibited expected 511 keV photons and the two characteristic gammas from 45Ti at 720 and 1408 keV after a 2.3-h assay, as shown in Figure 1B and 1C. A single exponential fit to the data from the decay logging indicated that the half-life of the separated radionuclide is 3.067±0.004 h, which is only 0.4% less than the value reported in the National Nuclear Data Center29. MicroPET images of a miniature Derenzo phantom exhibited excellent resolution down to a rod diameter of 2 mm (Figure 1A, inset).
Figure 1.
Generation and characterization of Titanium-45. (A) A gamma spectrum of 45Ti obtained with the HPGe detector. (B) and (C) show the enlarged spectra of two characteristic gammas from 45Ti at 720 and 1408 keV (pointed by link and blue arrows). Inset in (A) shows the MicroPET image of a Derenzo phantom of 45Ti. The distance values shown in the phantom image are the diameters of the cylinders in each region. The distance between axes of the cylinders is twice their diameter.
MSN-based intrinsic radiolabeling of titanium-45
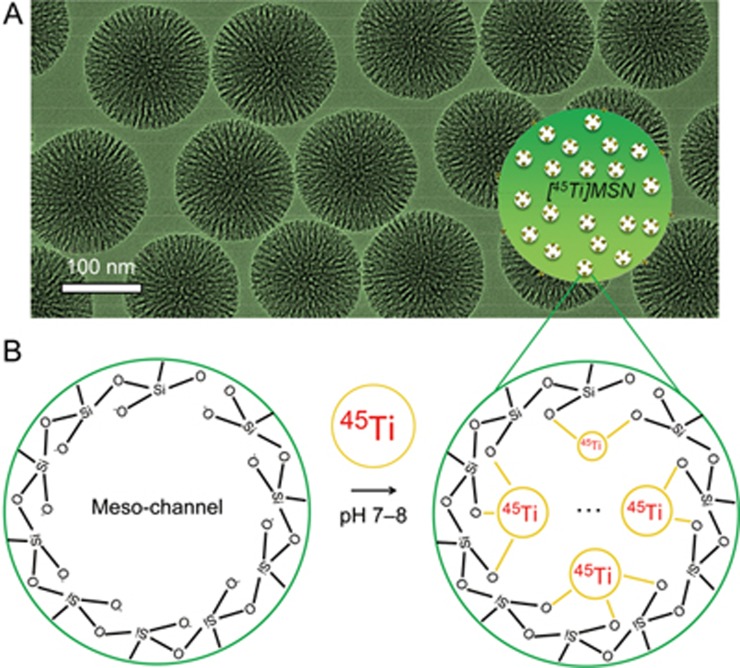
Uniform MSN with an average particle size of ∼150 nm in diameter was synthesized using previously reported procedures (Figure 2A)9,30. The Brunauer-Emmett-Teller (BET) surface area of as-synthesized MSN was 581.5 m2/g, with a high pore volume of 1.36 cm3/g and an average pore size of 4 to 5 nm. Approximately 2.6×106 -Si-OH groups in each MSN particle was estimated based on our previous calculation9.
Figure 2.
Synthesis and characterization of MSN. (A) A transmission electron microscopy (TEM) image of ∼150 nm sized MSN particles. Surface area: 581.5 m2/g. Pore volume: 1.36 cm3/g. Average pore size: 4–5 nm. (B) A schematic illustration showing the labeling of 45Ti to the deprotonated silanol groups (-Si-O-) from the outer surface and inner meso-channels of MSN. Please note the real coordination chemistry of 45Ti4+ with the silanol groups could be much more complex than the scheme shown in (B).
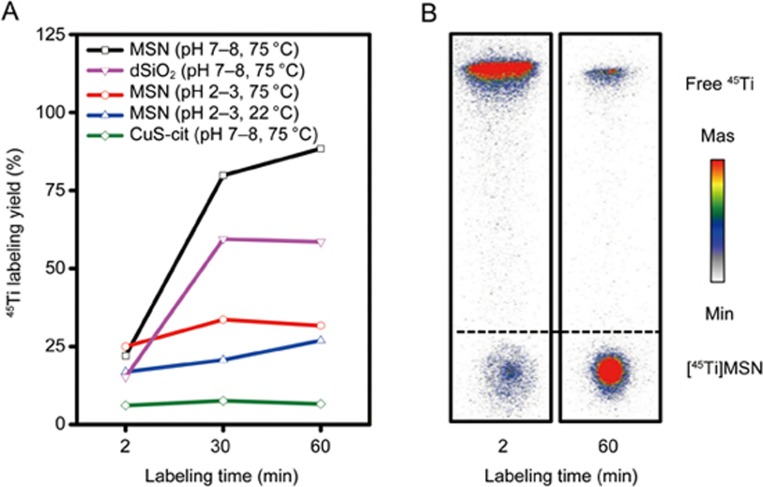
Previously, we reported the successful chelator-free radiolabeling of 89Zr to the abundant deprotonated silanol groups (-Si-O−) from MSN9. Like 89Zr4+, 45Ti4+ is also a hard Lewis acid and thus prefers hard Lewis bases, such as -Si-O−, as the donor groups (Figure 2B). To demonstrate this feature, we mixed 250 μL of MSN (2 mg/mL) with 0.5 mCi (or 18.5 MBq) 45Ti-oxalate, and the solution was shaken under varied labeling conditions. Approximately 80% labeling yield was achieved within 30 min of incubating at 75 °C, pH 7–8 (Figure 3A, black line). The labeling yield further increased to ∼90% after an additional 30-min incubation, which was confirmed by the radio-iTLC study (Figure 3B). As expected, a significantly reduced labeling yield (∼25%) was observed when labeling was performed at pH 2–3 (75 °C), indicating the inhibition of 45Ti labeling if -Si-O− groups become protonated (Figure 3, left, red line). Such protonation was also confirmed by the surface charge change of MSN from −48.4±0.3 mV (pH 7–8) to 3.6±0.3 mV (pH 2–3).
Figure 3.
MSN-based intrinsic radiolabeling of titanium-45. (A) 45Ti labeling yields of different samples under varied labeling conditions. (B) An autoradiograph of [45Ti]MSN iTLC plates after 2 min and 60 min incubation (pH 7–8, 75 °C).
To further demonstrate the specific labeling of 45Ti to the deprotonated silanol groups, dense silica nanoparticles (dSiO2) and copper sulfide (CuS) nanoparticles with less (∼1.5×105 -Si-O− per dSiO2)9 or almost no oxygen donors were introduced as control groups. Our results revealed ∼60% and <10% labeling yield under the optimized labeling conditions (pH 7–8, 75 °C, 60 min, Figure 3, left, pink and green lines) for dSiO2 and CuS, respectively, clearly demonstrating the -Si-O−-dependent 45Ti labeling process. More detailed labeling yields and autoradiography images are presented in Figure S1 (Supporting Information). Given that the intrinsic 45Ti-labeling mechanism is based on the strong interaction between 45Ti (hard Lewis acid) and hard oxygen donors (hard Lewis bases), successful chelator-free 45Ti radiolabeling might also be achievable using other oxygen-containing nanoparticles. This finding was confirmed by successful chelator-free 45Ti labeling to water-soluble SPION@PAA [SPION: superparamagnetic iron oxide nanoparticle, PAA: poly(acrylic acid)], as shown in Figure S2.
In vivo tumor-targeted PET Imaging
An in vitro serum stability study was performed prior to the in vivo 45Ti-based PET imaging. The in vitro radiostability of [45Ti]MSN was less stable than that of [89Zr]MSN9. As shown in Figure S3, approximately 15% of 45Ti was detached from the [45Ti]MSN after incubated in mouse serum at 37 °C for 3 h. Approximately 20% of detached free 45Ti was observed within 14 h (more than 4 half-lives of 45Ti).
To demonstrate the feasibility of using 45Ti-labeled MSN for in vivo tumor-targeted PET imaging, the nanoparticle was first modified with -NH2 groups and then labeled with 45Ti (pH 7–8, 75 °C) followed by a well-established PEGylation step9. Surface PEGylation provides significantly improved nanoparticle stability in biological buffers and in vivo and does not affect the labeling of 45Ti due to the abundant silanol groups in each MSN particles (especially those inside the meso-channels). The blood circulation half-life (t1/2) of PEGylated MSN was estimated to be approximately 4 h, which was very close to the decay half-life of 45Ti (t1/2=3.08 h), but significantly longer than that of the same sample with no PEG protective layer (t1/2<10 min). Synthesized [45Ti]MSN-PEG5k was then intravenously injected to 4T1 murine breast tumor-bearing mice. To compare the biodistribution patterns between the free 45Ti and [45Ti]MSN-PEG5k (HD: ∼200 nm), we further injected free 45Ti-oxalate (diluted with PBS, pH 7.4) into another group of 4T1 murine breast tumor-bearing mice and compared organ uptake over time.
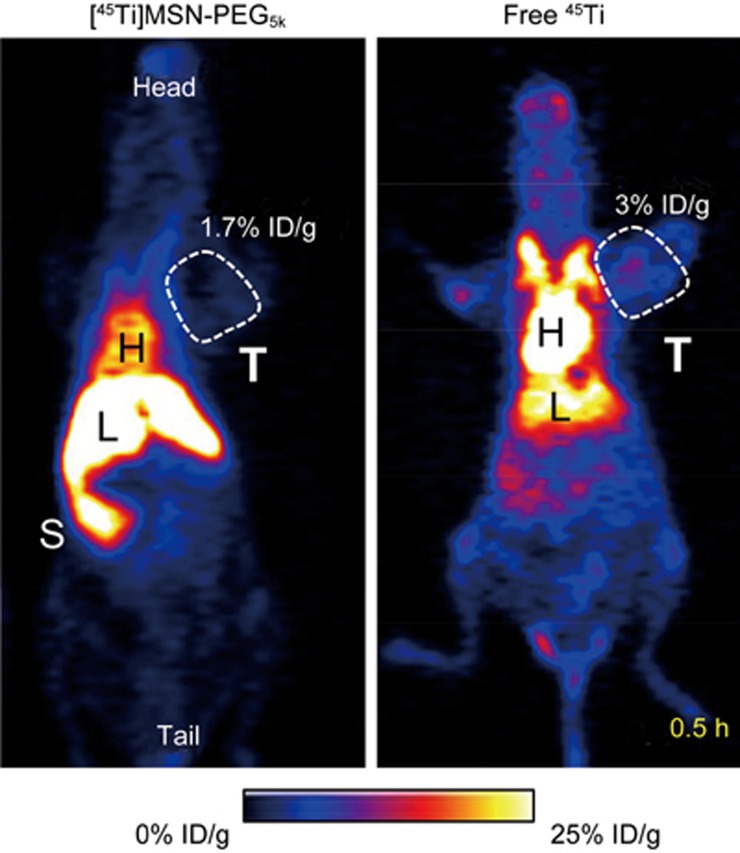
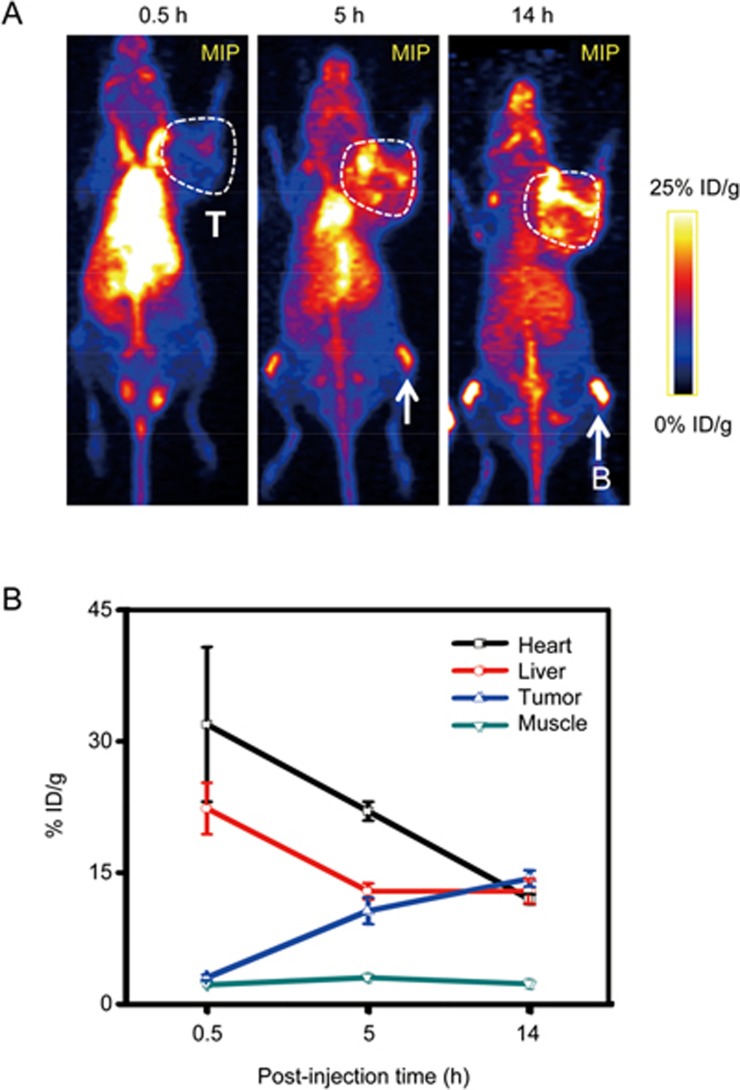
The PET imaging in Figure 4 clearly revealed differences in biodistribution at 30 min post-injection. As expected, mice injected with [45Ti]MSN-PEG5k shared a similar PK (dominant uptake in liver [43.2%±6.6% ID/g] and heart [16.7%±2.6% ID/g]) compared with that injected with our previously reported 89Zr chelator-free labeled MSN-PEG5k31. Mice injected with free 45Ti exhibited a significantly different PK, with an approximately two-fold increased uptake in the blood (31.9%±8.8% ID/g) and a two-fold reduced uptake in the liver (22.3%±3.0% ID/g). Interestingly, cold Ti and radioactive 45Ti have a strong interaction with unsaturated transferrin in mouse (or human) blood, where transferrin could act as a mediator for titanium delivery to tumor cells19,32,33. Like 45Ti-citrate19, 45Ti-oxalate was expected to transchelate to transferrin to form 45Ti-transferrin in vivo. These features explain the high initial blood retention of free 45Ti. The tumor uptake of [45Ti]MSN-PEG5k at 30 min pi was approximately 1.7% ID/g, which is approximately half of that in mice injected with free 45Ti (Figure 4). Possibly due to the longer blood circulation half-life of 45Ti-transferrin, mice injected with free 45Ti exhibited significantly increased tumor accumulation over time (from 3.0%±0.3% ID/g at 0.5 h pi to 14.3%±0.9% ID/g at 14 h pi), as shown in Figure 5A, 5B and Table S1.
Figure 4.
Comparison of the in vivo biodistribution between [45Ti]MSN-PEG5k (Left) and free 45Ti (Right) at early time point (0.5 h post-injection) by using PET imaging. H: heart; L: liver; S: spleen; T: tumor.
Figure 5.
(A) In vivo serial coronal maximum intensity projection (MIP) PET images of 4T1 tumor-bearing mice (n=3) injected with free 45Ti. Tumor (T) was marked with a white box. Bone (B) uptake was indicated with white arrows. (B) Time-activity curves of heart, liver, tumor and muscle.
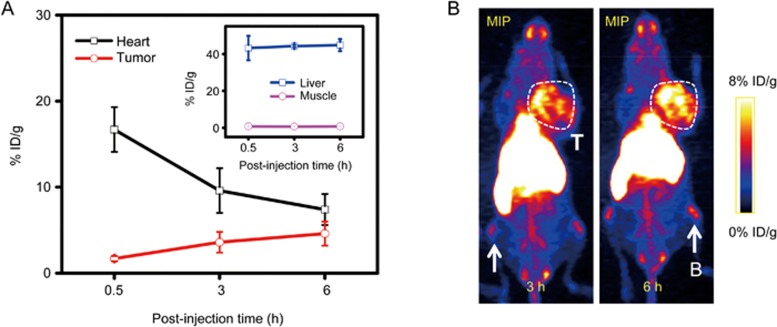
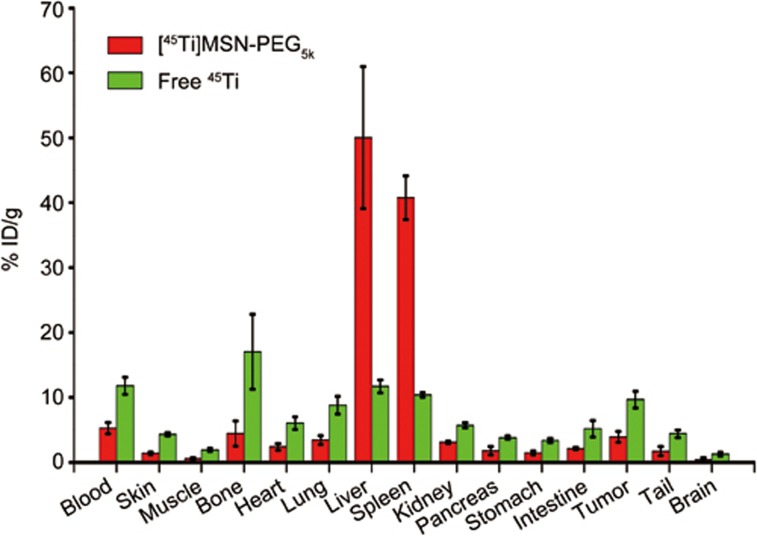
For mice injected with [45Ti]MSN-PEG5k, our region-of-interest (ROI) quantification revealed reduced heart uptake (from 16.7%±2.6% ID/g to 7.4%±1.8% ID/g) and increased tumor uptake (from 1.7%±0.3% ID/g to 4.6%±1.4% ID/g, Figure 6, Table S2). The accumulation of [45Ti]MSN-PEG5k is primarily based on the well-known enhanced permeability and retention effect34. The liver uptake was maintained at constant levels at approximately 44% ID/g, which also indicates the high radio-stability of [45Ti]MSN-PEG5k in vivo. Similar tumor (∼4% ID/g) and high liver uptake (50%–60% ID/g) were observed for 89Zr chelator-free labeled MSN-PEG5k31. A medium level (4.4%±1.9% ID/g) of bone uptake was also observed based on the maximum intensity projection (MIP) PET images (Figure 6B, pointed by white arrows) and ex vivo biodistribution study (Figure 7, Table S3). However, significantly increased bone and joint uptakes were observed in mice injected with free 45Ti (Figures 5A and 7, Table S4), which could indicate a relatively lower radiostability of 45Ti-transferrin compared with [45Ti]MSN-PEG5k. Although the current silica-based 45Ti radiolabeling is not designed for labeling 45Ti to free antibody or peptides, it might still be quite useful given that silica surface modification has been widely used in developing multifunctional nanostructures35,36. In addition, considering that ultrasmall (6–7 nm sized) renal clearable Cornell dots (which have been approved by the US Food and Drug Administration as Investigational New Drug for the first-in-human clinical trial studies) are also based on silica37,38, the developed 45Ti (or 89Zr) chelator-free radiolabeling technique might become a highly useful tool given that no extra chelator or surface modification is needed and size-controlling is vital when labeling these ultrasmall particles. Taken all together, we demonstrated the successful chelator-free 45Ti radiolabeling to mesoporous silica nanoparticles and the potential use of [45Ti]MSN-PEG5k for future biomedical applications.
Figure 6.
(A) Time-activity curves. (B) In vivo serial coronal maximum intensity projection PET images of 4T1 tumor-bearing mice injected with [45Ti]MSN-PEG5k. Tumor (T) was marked with a white box. Bone (B) uptake was indicated with white arrows.
Figure 7.
Ex vivo biodistribution of [45Ti]MSN-PEG5k and free 45Ti in 4T1 tumor-bearing mice at 20 and 18 h post-injection, respectively (n=3 for both groups).
In conclusion, an MSN-based intrinsic 45Ti radiolabeling technique was developed. The mechanism was based on the strong interaction between 45Ti (hard Lewis acid) and the hard oxygen donors (hard Lewis bases) of the deprotonated silanol groups from the outer surface and inner meso-channels of MSN. Successful chelator-free 45Ti radiolabeling might also be achievable using other oxygen-containing nanoparticles. We hope these findings inspire researchers in the field of nano-oncology to create other 45Ti-based multifunctional nanomedicine.
Author contribution
The manuscript was written by Feng CHEN, Hector F VALDOVINOS, and Wei-bo CAI. All authors have given approval to the final version of the manuscript. Feng CHEN, and Hector F VALDOVINOS contributed equally to this work.
Acknowledgments
This work is supported in part by the University of Wisconsin-Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365, P30CA014520, and 5T32GM08349), the National Science Foundation (No DGE-1256259), and the American Cancer Society (No 125246-RSG-13-099-01-CCE).
Footnotes
Supplementary Figures and Tables are available at the website of Acta Pharmacologica Sinica.
Supplementary Information
Intrinsic Radiolabeling of Titanium-45 using Mesoporous Silica Nanoparticles
References
- Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer 2002; 2: 683–93. [DOI] [PubMed] [Google Scholar]
- Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med 2008; 49 Suppl 2: 113s–28s. [DOI] [PubMed] [Google Scholar]
- Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem Rev 2010; 110: 2858–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Chen F, Ehlerding EB, Cai W. Intrinsically radiolabeled nanoparticles: an emerging paradigm. Small 2014; 10: 3825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Cai W, Chen X. Positron emission tomography imaging using radiolabeled inorganic nanomaterials. Acc Chem Res 2015; 48: 286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Ellison PA, Lewis CM, Hong H, Zhang Y, Shi S, et al. Chelator-free synthesis of a dual-modality PET/MRI agent. Angew Chem Int Ed Engl 2013; 52: 13319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty R, Valdovinos HF, Chen F, Lewis CM, Ellison PA, Luo H, et al. Intrinsically germanium-69-labeled iron oxide nanoparticles: synthesis and in-vivo dual-modality PET/MR imaging. Adv Mater 2014; 26: 5119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Huang X, Guo J, Zhu W, Ding Y, Niu G, et al. Self-illuminating 64Cu-doped CdSe/ZnS nanocrystals for in vivo tumor imaging. J Am Chem Soc 2014; 136: 1706–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Goel S, Valdovinos HF, Luo H, Hernandez R, Barnhart TE, et al. In vivo integrity and biological fate of chelator-free zirconium-89-labeled mesoporous silica nanoparticles. ACS Nano 2015; 9: 7950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Sun X, Jacobson O, Yan X, Min K, Srivatsan A, et al. Intrinsically radioactive [64Cu]CuInS/ZnS quantum dots for PET and optical imaging: improved radiochemical stability and controllable Cerenkov luminescence. ACS Nano 2015; 9: 488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieffel J, Chen F, Kim J, Chen G, Shao W, Shao S, et al. Hexamodal imaging with porphyrin-phospholipid-coated upconversion nanoparticles. Adv Mater 2015; 27: 1785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Fliss BC, Gu Z, Zhu Y, Hong H, Valdovinos HF, et al. Chelator-free labeling of layered double hydroxide nanoparticles for in vivo PET imaging. Sci Rep 2015; 5: 16930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Li J, Liang S, Sood AK, Liang D, Li C. CuS Nanodots with ultrahigh efficient renal clearance for positron emission tomography imaging and image-guided photothermal therapy. ACS Nano 2015; 9: 7085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner KM, Valentine AM. Bioinorganic chemistry of titanium. Chem Rev 2012; 112: 1863–81. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Ido T, Monma M, Murakami M, Kameyama M, Fukuda H, et al. Preparation of 45Ti-labeled compounds and their medical application. J Labelled Comp Radiopharm 1982; 19: 1539–41. [Google Scholar]
- Kawamura M, Inoue K, Kimura S, Ido T, Ishiwata K, Kawashima K, et al. Metabolism of 45Ti-labeled compounds: effect of ascorbic acid. J Labelled Comp Radiopharm 1986; 23: 1360–62. [Google Scholar]
- Ishiwata K, Ido T, Monma M, Murakami M, Fukuda H, Kameyama M, et al. Potential radiopharmaceuticals labeled with titanium-45. Int J Rad Appl Instrum A 1991; 42: 707–12. [DOI] [PubMed] [Google Scholar]
- Vavere AL, Laforest R, Welch MJ. Production, processing and small animal PET imaging of titanium-45. Nucl Med Biol 2005; 32: 117–22. [DOI] [PubMed] [Google Scholar]
- Vavere AL, Welch MJ. Preparation, biodistribution, and small animal PET of 45Ti-transferrin. J Nucl Med 2005; 46: 683–90. [PubMed] [Google Scholar]
- Severin GW, Nielsen CH, Jensen AI, Fonslet J, Kjaer A, Zhuravlev F. Bringing radiotracing to titanium-based antineoplastics: solid phase radiosynthesis, PET and ex vivo evaluation of antitumor agent [45Ti](salan)Ti(dipic). J Med Chem 2015; 58: 7591–5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen H, Shi J. In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv Mater 2013; 25: 3144–76. [DOI] [PubMed] [Google Scholar]
- Yang P, Gai S, Lin J. Functionalized mesoporous silica materials for controlled drug delivery. Chem Soc Rev 2012; 41: 3679–98. [DOI] [PubMed] [Google Scholar]
- Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, et al. A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J Am Chem Soc 2010; 132: 15351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Hong H, Goel S, Graves SA, Orbay H, Ehlerding EB, et al. In vivo tumor vasculature targeting of CuS@MSN based theranostic nanomedicine. ACS Nano 2015; 9: 3926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Zhang Y, Severin GW, Yang Y, Engle JW, Niu G, et al. Multimodality imaging of breast cancer experimental lung metastasis with bioluminescence and a monoclonal antibody dual-labeled with 89Zr and IRDye 800CW. Mol Pharm 2012; 9: 2339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon K, Severin GW, Barnhart TE, Engle JW, Valdovinos HF, Nickles RJ. 45Ti extraction using hydroxamate resin. AIP Conference Proceedings 2012; 1509: 211–4. [Google Scholar]
- Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol 2009; 36: 729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdovinos HF, Hernandez R, Barnhart TE, Graves S, Cai W, Nickles RJ. Separation of cyclotron-produced 44Sc from a natural calcium target using a dipentyl pentylphosphonate functionalized extraction resin. Appl Radiat Isot 2015; 95: 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows TW. Nuclear data sheets for A=45. Nuclear Data Sheets 2008; 109: 171–296. [Google Scholar]
- Shen D, Yang J, Li X, Zhou L, Zhang R, Li W, et al. Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres. Nano Lett 2014; 14: 923–32. [DOI] [PubMed] [Google Scholar]
- Goel S, Chen F, Luan S, Valdovinos HF, Shi S, Graves SA, et al. Engineering intrinsically zirconium-89 radiolabeled self-destructing mesoporous silica nanostructures for in vivo biodistribution and tumor targeting studies. Adv Sci (Weinh) 2016; 3: 1600122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1Guo M, Sun H, McArdle HJ, Gambling L, Sadler PJ. Ti(IV) uptake and release by human serum transferrin and recognition of Ti(IV)-transferrin by cancer cells: understanding the mechanism of action of the anticancer drug titanocene dichloride. Biochemistry 2000; 39: 10023–33. [DOI] [PubMed] [Google Scholar]
- Sun H, Li H, Weir RA, Sadler PJ. The first specific tiiv-protein complex: potential relevance to anticancer activity of titanocenes. Angew Chem Int Ed Engl 1998; 37: 1577–9. [DOI] [PubMed] [Google Scholar]
- Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 2011; 63: 136–51. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hsu BY, Ren C, Li X, Wang J. Silica-based nanocapsules: synthesis, structure control and biomedical applications. Chem Soc Rev 2015; 44: 315–35. [DOI] [PubMed] [Google Scholar]
- Bitar A, Ahmad NM, Fessi H, Elaissari A. Silica-based nanoparticles for biomedical applications. Drug Discov Today 2012; 17: 1147–54. [DOI] [PubMed] [Google Scholar]
- Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J Clin Invest 2011; 121: 2768–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci Transl Med 2014; 6: 260ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intrinsic Radiolabeling of Titanium-45 using Mesoporous Silica Nanoparticles