Abstract
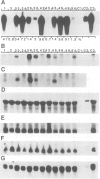
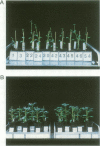
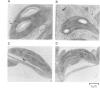
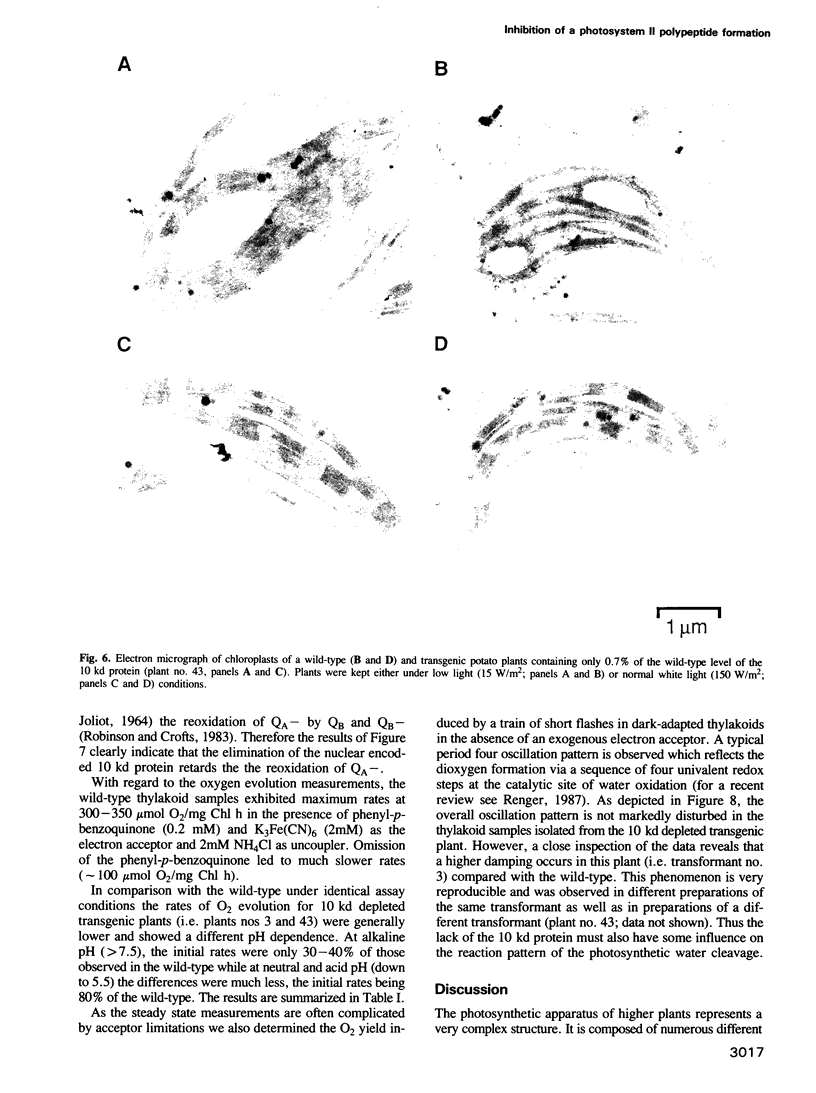
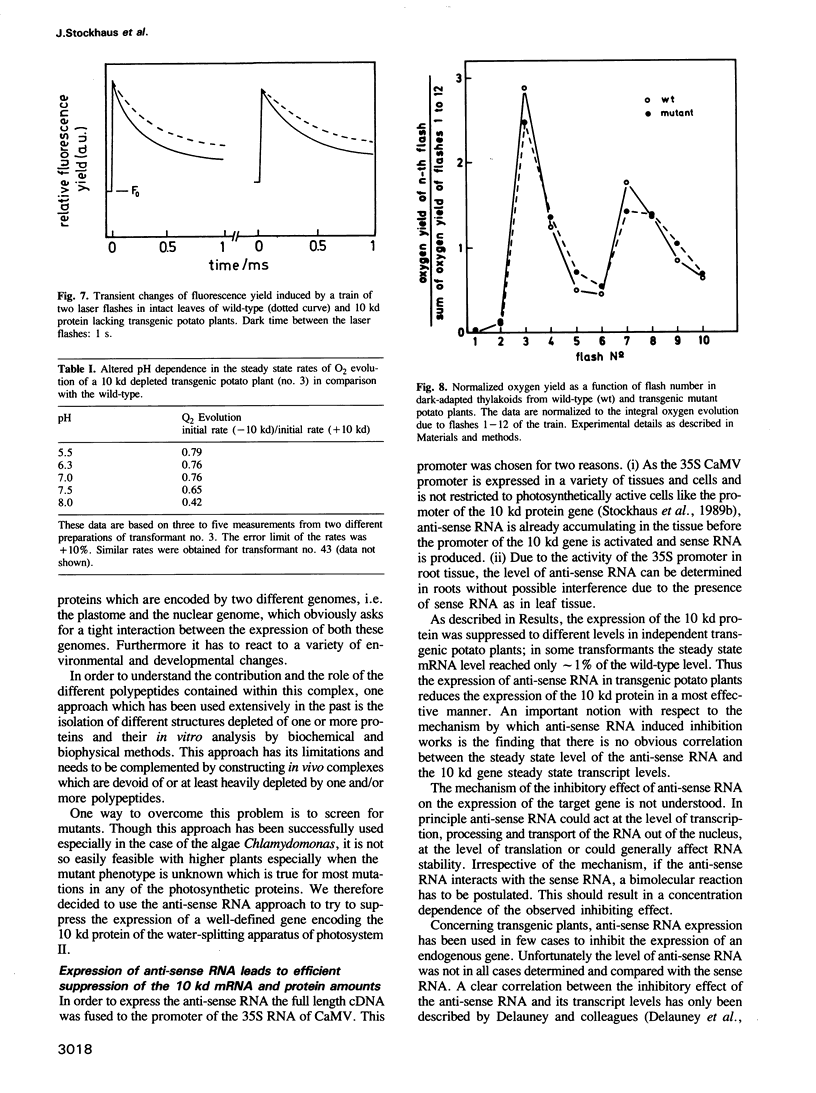
A chimeric gene encoding an anti-sense RNA of the 10 kd protein of the water-splitting apparatus of photosystem II of higher plants under the control of the CaMV 35S promoter was introduced into potato using Agrobacterium based vectors. The expression of the anti-sense RNA led to a significant reduction of the amounts of the 10 kd protein and RNA in a number of transgenic plants. In three out of 36 plants tested, the level of the 10 kd protein was only up to 1-3% compared with the wild-type control. The drastic reduction of the 10 kd protein did not influence the accumulation of other photosystem II associated polypeptides at both the RNA and protein level. Furthermore no phenotypic differences were observed between potato plants expressing wild-type and drastically reduced levels of the 10 kd protein with respect to growth rate, habitus or ultrastructure of the chloroplasts. Measurements of the relaxation of the flash-induced enhancement in the fluorescence quantum yield as determined in intact leaves and the rates and characteristic oscillation pattern of O2 evolution as determined in isolated thylakoid samples however, show that the elimination of the 10 kd protein on the one hand retards reoxidation of QA- and on the other hand introduces a general disorder into the PSII complex.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschauer A., Mösinger E., Kreuz K., Dörr I., Apel K. The implication of a plastid-derived factor in the transcriptional control of nuclear genes encoding the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1986 Feb 3;154(3):625–634. doi: 10.1111/j.1432-1033.1986.tb09444.x. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brudvig G. W., Beck W. F., de Paula J. C. Mechanism of photosynthetic water oxidation. Annu Rev Biophys Biophys Chem. 1989;18:25–46. doi: 10.1146/annurev.bb.18.060189.000325. [DOI] [PubMed] [Google Scholar]
- Deblaere R., Bytebier B., De Greve H., Deboeck F., Schell J., Van Montagu M., Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 1985 Jul 11;13(13):4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. Nobel lecture. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1989 Aug;8(8):2149–2170. doi: 10.1002/j.1460-2075.1989.tb08338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delauney A. J., Tabaeizadeh Z., Verma D. P. A stable bifunctional antisense transcript inhibiting gene expression in transgenic plants. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4300–4304. doi: 10.1073/pnas.85.12.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann P. H. Explorations in the "inner sanctum of the photosynthetic process," the water oxidizing system. Plant Physiol. 1988 Sep;88(1):1–5. doi: 10.1104/pp.88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfgen R., Willmitzer L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 1988 Oct 25;16(20):9877–9877. doi: 10.1093/nar/16.20.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOLIOT A., JOLIOT P. ETUDE CIN'ETIQUE DE LA R'EACTION PHOTOCHIMIQUE LIB'ERANT L'OXYG'ENE AU COURS DE LA PHOTOSYNTH'ESE. C R Hebd Seances Acad Sci. 1964 May 4;258:4622–4625. [PubMed] [Google Scholar]
- Jansson C., Andersson B., Akerlund H. E. Trypsination of inside-out chloroplast thylakoid vesicles for localization of the water-splitting site. FEBS Lett. 1979 Sep 1;105(1):177–180. doi: 10.1016/0014-5793(79)80912-6. [DOI] [PubMed] [Google Scholar]
- Kuchka M. R., Goldschmidt-Clermont M., van Dillewijn J., Rochaix J. D. Mutation at the Chlamydomonas nuclear NAC2 locus specifically affects stability of the chloroplast psbD transcript encoding polypeptide D2 of PS II. Cell. 1989 Sep 8;58(5):869–876. doi: 10.1016/0092-8674(89)90939-2. [DOI] [PubMed] [Google Scholar]
- Ljungberg U., Akerlund H. E., Andersson B. Isolation and characterization of the 10-kDa and 22-kDa polypeptides of higher plant photosystem 2. Eur J Biochem. 1986 Aug 1;158(3):477–482. doi: 10.1111/j.1432-1033.1986.tb09779.x. [DOI] [PubMed] [Google Scholar]
- Mayfield S. P., Bennoun P., Rochaix J. D. Expression of the nuclear encoded OEE1 protein is required for oxygen evolution and stability of photosystem II particles in Chlamydomonas reinhardtii. EMBO J. 1987 Feb;6(2):313–318. doi: 10.1002/j.1460-2075.1987.tb04756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield S. P., Rahire M., Frank G., Zuber H., Rochaix J. D. Expression of the nuclear gene encoding oxygen-evolving enhancer protein 2 is required for high levels of photosynthetic oxygen evolution in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1987 Feb;84(3):749–753. doi: 10.1073/pnas.84.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield S. P., Taylor W. C. Carotenoid-deficient maize seedlings fail to accumulate light-harvesting chlorophyll a/b binding protein (LHCP) mRNA. Eur J Biochem. 1984 Oct 1;144(1):79–84. doi: 10.1111/j.1432-1033.1984.tb08433.x. [DOI] [PubMed] [Google Scholar]
- Pakrasi H. B., Diner B. A., Williams JGK., Arntzen C. J. Deletion Mutagenesis of the Cytochrome b559 Protein Inactivates the Reaction Center of Photosystem II. Plant Cell. 1989 Jun;1(6):591–597. doi: 10.1105/tpc.1.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger G., Hagemann R., Dohnt G. Properties of the proteinaceous component acting as apoenzyme for the functional plastoquinone redox groups on the acceptor side of system II. Biochim Biophys Acta. 1981 Jun 12;636(1):17–26. doi: 10.1016/0005-2728(81)90070-0. [DOI] [PubMed] [Google Scholar]
- Rocha-Sosa M., Sonnewald U., Frommer W., Stratmann M., Schell J., Willmitzer L. Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J. 1989 Jan;8(1):23–29. doi: 10.1002/j.1460-2075.1989.tb03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J. D., Erickson J. Function and assembly of photosystem II: genetic and molecular analysis. Trends Biochem Sci. 1988 Feb;13(2):56–59. doi: 10.1016/0968-0004(88)90029-1. [DOI] [PubMed] [Google Scholar]
- Rutherford A. W. Photosystem II, the water-splitting enzyme. Trends Biochem Sci. 1989 Jun;14(6):227–232. doi: 10.1016/0968-0004(89)90032-7. [DOI] [PubMed] [Google Scholar]
- Sanchez-Serrano J. J., Keil M., O'Connor A., Schell J., Willmitzer L. Wound expression of a potato proteinase inhibitor II gene in transgenic tobacco plants. EMBO J. 1987 Feb;6(2):303–306. doi: 10.1002/j.1460-2075.1987.tb04754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stockhaus J., Schell J., Willmitzer L. Correlation of the expression of the nuclear photosynthetic gene ST-LS1 with the presence of chloroplasts. EMBO J. 1989 Sep;8(9):2445–2451. doi: 10.1002/j.1460-2075.1989.tb08379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhaus J., Schell J., Willmitzer L. Identification of enhancer elements in the upstream region of the nuclear photosynthetic gene ST-LS1. Plant Cell. 1989 Aug;1(8):805–813. doi: 10.1105/tpc.1.8.805. [DOI] [PMC free article] [PubMed] [Google Scholar]