Abstract
HPV is the cause of almost all cervical cancer and is responsible for a substantial fraction of other anogenital cancers and oropharyngeal cancers. Understanding the HPV‐attributable cancer burden can boost programs of HPV vaccination and HPV‐based cervical screening. Attributable fractions (AFs) and the relative contributions of different HPV types were derived from published studies reporting on the prevalence of transforming HPV infection in cancer tissue. Maps of age‐standardized incidence rates of HPV‐attributable cancers by country from GLOBOCAN 2012 data are shown separately for the cervix, other anogenital tract and head and neck cancers. The relative contribution of HPV16/18 and HPV6/11/16/18/31/33/45/52/58 was also estimated. 4.5% of all cancers worldwide (630,000 new cancer cases per year) are attributable to HPV: 8.6% in women and 0.8% in men. AF in women ranges from <3% in Australia/New Zealand and the USA to >20% in India and sub‐Saharan Africa. Cervix accounts for 83% of HPV‐attributable cancer, two‐thirds of which occur in less developed countries. Other HPV‐attributable anogenital cancer includes 8,500 vulva; 12,000 vagina; 35,000 anus (half occurring in men) and 13,000 penis. In the head and neck, HPV‐attributable cancers represent 38,000 cases of which 21,000 are oropharyngeal cancers occurring in more developed countries. The relative contributions of HPV16/18 and HPV6/11/16/18/31/33/45/52/58 are 73% and 90%, respectively. Universal access to vaccination is the key to avoiding most cases of HPV‐attributable cancer. The preponderant burden of HPV16/18 and the possibility of cross‐protection emphasize the importance of the introduction of more affordable vaccines in less developed countries.
Keywords: human papillomavirus, cancer, attributable fraction, prevention, vaccine
Short abstract
What's new?
Most cervical cancers result from human papillomavirus (HPV) infection and therefore are preventable through screening and vaccination. Nonetheless, efforts toward HPV‐attributable cancer prevention frequently are undermined by limited access to necessary resources. The present study estimates that worldwide as many as 4.5% of new cancer cases, including cancers of the cervix, anogenital tract and head and neck, are associated with HPV infection. Cervical cancer alone accounts for 83% of those cases, most of which affect women in less‐developed countries. The findings emphasize the importance of HPV screening and vaccination and the need for less‐costly vaccines.
Abbreviations
- AF
attributable fraction
- ASR
age‐standardized incidence rates
- HDI
Human Development Index
- HPV
human papillomavirus
- 2‐V
two‐valent
Cervical cancer is one of the most preventable cancers. A comprehensive strategy based on vaccination against human papillomavirus (HPV) and HPV‐based screening has been demonstrated to be cost‐effective in nearly all countries.1 Yet progress toward prevention is often frustrating, with relatively low access to vaccine2 and limited use of cervical cancer screening,3 particularly in less developed countries.
HPV includes a family of DNA viruses that infect basal epithelial cells, causing benign and malignant lesions of the skin and mucosae of the anogenital and upper aero‐digestive tract.4, 5, 6 Epidemiological studies and mechanistic evidence led to the classification of HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59 as carcinogenic and HPV68 as probably carcinogenic. These types are referred to as high risk types. The three currently available vaccines are (i) Cervarix (GlaxoSmithKline), a two‐valent (2‐V) vaccine targeting HPV16 and 18, the most carcinogenic types (ii) Gardasil (Merck Inc.), a four valent (4‐V) vaccine targeting HPV16/18 and also low‐risk types HPV6 and 11 that cause genital warts and (iii) Gardasil 9 (Merck Inc.), a nine‐valent (9‐V) vaccine targeting HPV6/11/16/18 and the next five most carcinogenic types (HPV31/33/45/52/58). In addition to cervical cancer, a substantial proportion of cancers of the vulva, vagina, penis, anus and oropharynx are due to HPV, mainly HPV16. Although no effective screening exists for these cancers, they would also be prevented by HPV vaccination.7
The public health importance of infectious agents in causing cancer is often quantified using the population attributable fraction, hereafter referred to as AF. For HPV‐related cancers, the AF is the proportion of cancer cases that would not have occurred if HPV had been totally absent from the population. In this work, we estimate the AF for HPV globally and by anatomic site, age, sex and country. We also show the fraction of HPV‐attributable cancer that could be prevented in a few decades through global programs of vaccination of preadolescents using vaccines that target HPV16/18 or HPV6/11/16/18/31/33/45/52/58.
This report is part of an ongoing project to estimate the global burden of cancer attributable to all infections, for which the latest estimates are for the year 2012.8 Here, we present a more granular approach (e.g., by country and cancer subsites) to HPV than in previous reports, reflecting the growth in the literature on cancer sites associated with HPV and using the latest available incidence estimates.
Methods
Methods have previously been described.8 Briefly, estimates of the number of new cancer cases that occurred worldwide in 2012 were directly obtained from GLOBOCAN 2012 version 1.09 for cervical, penile and laryngeal cancer; for other sites (anus, vulva and vagina) and subsites (oral cavity, oropharynx and other pharynx) they were derived from data from high‐quality cancer registries in the Cancer in Five Continents database.10 We calculated the number of cases attributable to HPV by country and then aggregated them into eight geographical regions based on the United Nations classification used in GLOBOCAN. We also created a dichotomous classification of countries by development status using the 2012 Human Development Index (HDI): “less developed” countries, comprising low‐ and medium‐HDI countries and “more developed” countries, comprising high‐ and very high‐HDI countries.11
Methods for AF calculation are also described in detail in our previous work.8 Briefly, we used the simplified formula AF=p c as the prevalence (p) in cancer tissue (c) of transforming HPV infection. We considered that for the cervix, 100% of cancers are attributable to HPV; for other cancer sites, we reviewed and selected published case series that have used the following detection methods in cancer tissue: PCR‐based HPV DNA for penile cancer; HPV DNA and p16INK4a overexpression for vulva, vagina and anal cancer; HPV DNA and viral oncoproteins E6 and E7 mRNA for all head and neck cancers. The evidence base for estimating the prevalence of HPV in penile cancer (Supporting Information Table S1) and cancers of the oral cavity and larynx (Supporting Information Table S2) was updated by extending the literature to include recently published articles.
On account of substantial differences in incidence, sex‐ or country‐specific distribution and methods for causal attribution, results on HPV‐attributable cancer cases are presented separately for: (i) the cervix, (ii) other anogenital tract (vulvar, vaginal, anal and penile) and (iii) head and neck (oropharynx, oral cavity and larynx). Maps of age‐standardized incidence rates (ASR) of HPV‐attributable cancer by country were produced for these three cancer groups.
The relative contribution of HPV16/18 and HPV6/11/16/18/31/33/45/52/58 to the HPV‐attributable cancer burden was derived from published meta‐analyses, based on type distribution in HPV‐positive cancer cases.12, 13, 14 HPV6 and 11 are included in the relative contribution of the 9‐V vaccine types, even though they are not classified as carcinogenic4, 5, 6 because of their possible involvement in some noncervical carcinomas, notably penile cancer,12 and because only the combined contribution of the 9‐V vaccine types was reported in the meta‐analyses.12, 13, 14
Results
Globally, 570,000 cases per year in women and 60,000 cases in men are attributable to HPV (Table 1), respectively, 8.6% and 0.8% of all cancers occurring worldwide (Table 2). HPV AF in women ranges from <3% in Australia/New Zealand and the US to 26% in sub‐Saharan Africa. Globally, the relative contribution of HPV16/18 and of the 9‐V types are 460,000 and 570,000 cases, respectively, corresponding to 72% and 90% of all HPV‐attributable cases (Table 3).
Table 1.
Number of cancer cases attributable to HPV and corresponding attributable fraction (AF) by cancer site, sex and age; World, 2012
| HPV‐related cancer site (ICD‐10 code) | Number of incident casesa, b | Number attributable to HPV | AF (%) | Number attributable to HPV by gender | Number attributable to HPV by age group | |||
|---|---|---|---|---|---|---|---|---|
| Males | Females | <50 years | 50–69 years | 70+ years | ||||
| Cervix uteri (C53) | 530,000 | 530,000 | 100.0 | 0 | 530,000 | 250,000 | 220,000 | 58,000 |
| Anusc (C21) | 40,000 | 35,000 | 88.0 | 17,000 | 18,000 | 6,600 | 17,000 | 12,000 |
| Vulvac (C51) | 34,000 | 8,500 | 24.9 | 0 | 8,500 | 2,600 | 3,400 | 2,500 |
| Vaginac (C52) | 15,000 | 12,000 | 78.0 | 0 | 12,000 | 2,500 | 5,200 | 3,900 |
| Penisc (C60) | 26,000 | 13,000 | 50.0 | 13,000 | 0 | 2,700 | 5,800 | 4,400 |
| Oropharynxc (C01, C09–10) | 96,000 | 29,000 | 30.8 | 24,000 | 5,500 | 5,400 | 18,000 | 6,000 |
| Oral cavityc (C02–06) | 200,000 | 4,400 | 2.2 | 2,900 | 1,500 | 890 | 2,300 | 1,200 |
| Larynx (C32) | 160,000 | 3,800 | 2.4 | 3,300 | 460 | 420 | 2,200 | 1,200 |
| Other pharynxc (C12–C14) | 78,000 | 0 | 0 | – | – | – | – | – |
| Total HPV‐related sites | 1,200,000 | 630,000 | 54.0 | 60,000 | 570,000 | 270,000 | 270,000 | 88,000 |
Source: Globocan 2012.
Numbers are rounded to two significant digits.
These cancer sites were not directly available in GLOBOCAN 2012; therefore, data from the Cancer Incidence in Five Continents (CI5‐X) database were used to estimate the corresponding number of cases.
Table 2.
Number of all cancer cases attributable to HPV and corresponding attributable fraction (AF) for all cancers, by region, cancer site(s) and sex; World, 2012
| Region | Cervix uteri | Anus | Penis | Vulva/vagina | Head and neck | All cancer Attributable to HPV | AF (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | Both sexes | M | F | Both | |
| Africa | |||||||||||
| Sub‐Saharan Africa | 93,000 | 1,000 | 1,200 | 1,000 | 2,100 | 360 | 150 | 99,000 | 0.9 | 26.1 | 15.8 |
| Northern Africa/Western Asia | 10,000 | 430 | 350 | 70 | 650 | 240 | 80 | 12,000 | 0.3 | 4.3 | 2.2 |
| Asia | |||||||||||
| India | 120,000 | 2,600 | 1,900 | 3,200 | 2,800 | 5,600 | 1,000 | 140,000 | 2.4 | 23.9 | 13.8 |
| Other Central Asia | 29,000 | 490 | 410 | 30 | 460 | 760 | 300 | 31,000 | 0.5 | 11.4 | 6.3 |
| China | 62,000 | 5,900 | 3,600 | 1,300 | 1,600 | 950 | 270 | 75,000 | 0.5 | 5.4 | 2.5 |
| Japan/Republic of Korea | 13,000 | 600 | 560 | 250 | 460 | 1,500 | 350 | 16,000 | 0.5 | 3.5 | 1.8 |
| Other Eastern Asia | 54,000 | 550 | 530 | 1,100 | 1,000 | 1,000 | 280 | 59,000 | 0.6 | 11.7 | 6.2 |
| America | |||||||||||
| Latin America | 69,000 | 1,000 | 1,900 | 2,000 | 2,500 | 980 | 280 | 78,000 | 0.8 | 13.0 | 7.1 |
| Northern America | 14,000 | 1,800 | 2,700 | 1,100 | 3,300 | 7,000 | 1,900 | 32,000 | 1.1 | 2.6 | 1.8 |
| Europe | |||||||||||
| Europe | 58,000 | 2,700 | 4,200 | 2,700 | 5,100 | 11,000 | 2,800 | 87,000 | 0.9 | 4.4 | 2.5 |
| Oceania | |||||||||||
| Australia/New Zealand | 940 | 150 | 190 | 50 | 150 | 290 | 80 | 1,900 | 0.6 | 2.2 | 1.3 |
| Other Oceania | 1,300 | 10 | 10 | 10 | 30 | 30 | 10 | 1,300 | 0.8 | 18.5 | 11.1 |
| Less developed countries | 370,000 | 10,000 | 7,600 | 6,800 | 8,300 | 8,600 | 2,100 | 410,000 | 0.8 | 13.2 | 6.7 |
| More developed countries | 160,000 | 6,800 | 10,000 | 6,100 | 12,000 | 22,000 | 5,500 | 220,000 | 0.8 | 5.0 | 2.8 |
| World | 530,000 | 17,000 | 18,000 | 13,000 | 20,000 | 30,000 | 7,500 | 630,000 | 0.8 | 8.6 | 4.5 |
1Numbers over 100 are rounded to two significant digits; numbers <100 are rounded to the closest ten.
Table 3.
Relative contribution of HPV 16/18 or HPV6/11/16/18/31/33/45/52/58 to HPV‐associated cancers by site and by sex; World, 2012
| HPV‐related cancer site (ICD‐10 code) | Number attributable to HPVa | Relative contribution of HPV16/18b | Relative contribution of HPV6/11/16/18/31/33/45/52/58b | ||
|---|---|---|---|---|---|
| Percent | Number | Percent | Number | ||
| Cervix uteri (C53) | 530,000 | 70.8 | 370,000 | 89.5 | 470,000 |
| Anus (C21) | 35,000 | 87.0 | 30,000 | 95.9 | 33,000 |
| Vulva (C51) | 8,500 | 72.6 | 6,200 | 87.1 | 7,400 |
| Vagina (C52) | 12,000 | 63.7 | 7,400 | 85.3 | 9,900 |
| Penis (C60) | 13,000 | 70.2 | 9,100 | 84.6 | 11,000 |
| Head and neck (C01‐06, C09‐10, C32) | 38,000 | 84.9 | 32,000 | 89.7 | 34,000 |
| Total HPV‐related sites in women | 570,000 | 71.4 | 410,000 | 89.6 | 510,000 |
| Total HPV‐related sites in men | 60,000 | 82.3 | 50,000 | 90.4 | 55,000 |
| Total HPV‐related sites | 630,000 | 72.4 | 460,000 | 89.7 | 570,000 |
Derived from Plummer, de Martel et al.8; numbers are rounded to two significant digits.
Derived from Serrano et al.,14 Alemany et al.12 and Castellsague et al.13
Country‐specific ASR estimates for HPV‐attributable cancer are shown separately for cervical cancer (Fig. 1), other anogenital cancers (Fig. 2 a) and head and neck cancers (Fig. 2 b). Due to the much higher ASR of cervical cancer, different cut‐points are used for Figure 1 versus Figure 2 panels (a) and (b). This difference is also reflected in the different color palettes. Country‐specific ASRs are given in Supporting Information Table S3 together with quality scores for cancer incidence data as in GLOBOCAN 2012 version 1.0.9
Figure 1.
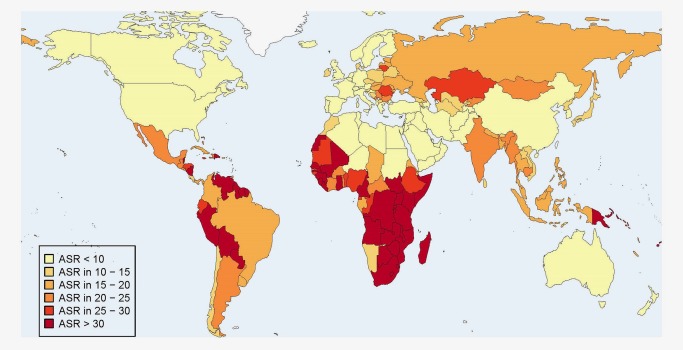
Age standardized (world) incidence rates (per 100,000) of cervical cancer cases attributable to HPV in 2012.
Figure 2.
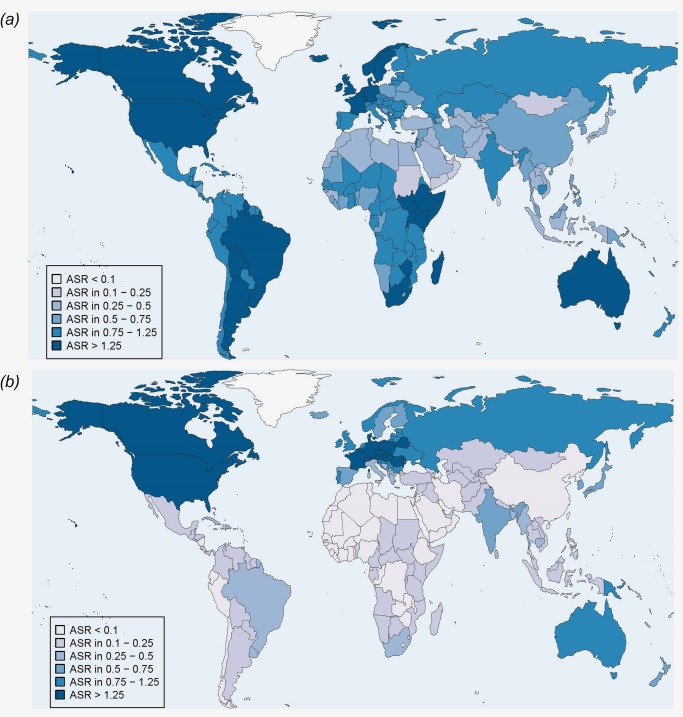
Age standardized (world) incidence rates (per 100,000) of cancer cases attributable to HPV in 2012, both sexes. Panel (a) Anogenital cancer cases (vulvar, vaginal, anal and penile). Panel (b) Head and neck cancer cases (oropharynx, oral cavity and larynx).
Cervical cancer
Cervical cancers represent 530,000 new cases per year and account for the vast majority of all HPV‐attributable cancer cases worldwide. Nearly half of the cases are diagnosed in women <50 years old (Table 1), and more than two‐thirds are diagnosed in less developed countries (Table 2). The majority of cervical cancer occurs in South‐Eastern Asia (with an especially large burden in India), Latin America and sub‐Saharan Africa (Table 2). Countries in which the ASR is over 30 per 100,000 are mainly located in sub‐Saharan Africa but a few are also found in Latin America and Oceania (Fig. 1). HPV 16 and 18 together are responsible globally for 71% of cervical cancer. This percentage rises to 90% for HPV6/11/16/18/31/33/45/52/58 (Table 3).
Other anogenital cancers: anus, vulva, vagina and penis
Globally, approximately 8,500 cases of vulvar carcinoma, 12,000 of vaginal cancer, 35,000 of anal cancer and 13,000 of penile cancer are attributable to HPV (Table 1). As for cervical cancer, the burden of HPV‐attributable anogenital cancers varies by region (Table 2). Countries in which the ASR of HPV‐attributable anogenital cancers is relatively high (over 1.25 per 100,000) are mainly located in Latin and Northern America and Australia but a few are also found in Europe and sub‐Saharan Africa (Fig. 2 a).
Nearly 90% of anal cancers are attributable to HPV and globally the malignancy is equally distributed in the two sexes (Table 1). However, anal cancer occurs slightly more frequently in males in less developed countries and in females in more developed countries (Table 2). Compared to cervical carcinoma, a stronger predominance of HPV16 is constantly reported. HPV 16 and 18 are together responsible for 87% of anal cancer while the relative contribution of HPV6/11/16/18/31/33/45/52/58 is 96% (Table 3). Vulvar cancers and penile cancers are relatively rare, and the HPV AFs of 25% for vulva and 50% for penis are lower than for other anogenital sites (Table 1). However, in several regions such as Europe, Latin America and India, HPV‐attributable cases of penile cancer are at least as frequent as anal cancer in males (Table 2). Vaginal cancer is rarer than cancer of the vulva but its AF is higher (AF 78%) (Table 1). The relative contributions of both HPV 16/18 (approximately 70%) and HPV6/11/16/18/31/33/45/52/58 (approximately 85%) are similar in vulvar, vaginal and penile cancer (Table 3).
Head and neck cancers
Three cancer sites in the head and neck have been associated with HPV: oropharynx and, to a much weaker extent, oral cavity and larynx. Globally, approximately 38,000 cases of head and neck cancer are attributable to HPV (Table 1). Their geographical distribution is diametrically different from that of cervical cancer as it shows much higher burden in more developed than less developed countries (Table 2). Countries in which the ASR of HPV‐attributable head and neck cancer is relatively high (over 1.25 per 100,000) are located in Northern America and Europe (Fig. 2 b).
Around 30% of oropharyngeal cancers (which mainly comprises the tonsils and base of tongue sites) are caused by HPV (29,000 cases per year) (Table 1). AF varies greatly worldwide, being highest in more developed countries (over 40% in Europe, Northern America, Australia, New Zealand, Japan and Republic of Korea), but much lower (<20%) and still uncertain in many countries (Supporting Information Table S2). For cancers of the oral cavity (4,400 cases per year attributed to HPV) and larynx (3,800 cases), the prevalence of HPV in cancer cases is derived from a small number of case series. Most of the studies were conducted in Europe and North America and yielded an average prevalence of approximately 4% at both sites. In the rest of the world, HPV AF in cancers of the oral cavity and larynx is even lower (1–2%) (Supporting Information Table S2).
On account of a greater predominance of HPV16 compared to cervical cancer, HPV 16 and 18 are globally responsible for 85% of cancer of the head and neck while the relative contribution of HPV6/11/16/18/31/33/45/52/58 is 90% (Table 3).
Discussion
This report provides updated estimates of the burden of cancer attributable to HPV at country and regional level for three groups of HPV‐related malignancies: cervical cancer, other anogenital cancers and head and neck cancers, which together are responsible for 630,000 new cases of cancer per year worldwide. The fraction of cancer attributable to HPV is dominated by cervical cancer, which represents 83% of the total burden of cancer attributable to HPV. The geographical variation highlights the contrast between cervical cancer (occurring predominantly in less developed countries) and HPV‐attributable head and neck cancer (occurring mostly in North America and Northern Europe). Countries at relatively high risk for other anogenital cancers can be found in all regions. Variation in cervical cancer incidence rates are mainly due to differences in the population prevalence of cervical HPV infection (>10‐fold differences are observed between countries)15 and the presence of adequate cervical cancer screening. HPV‐attributable head and neck cancer is mainly represented by oropharyngeal cancer for which consistent evidence suggests that two main types exist: one is driven by HPV and the other by tobacco and alcohol use.16, 17 The fraction of oropharyngeal cancer related to HPV has been increasing over the past two decades in some high‐income countries concomitant to the decline of tobacco smoking and increase of HPV infection.18 The pattern of anogenital cancers is probably due to a combination of variation in the burden of HPV infection in women and men and in the completeness of disease detection.
At variance with our previous report on cancer attributable to all infections,8 we used ASR instead of AF to explore the geographic distribution of different cancer sites attributable to HPV. AF is strongly affected by variations in the denominator, i.e., total cancer incidence. When AF is used, large differences in the incidence of noninfection‐related cancers between less developed and more developed countries can distort international comparisons of the burden. ASR can also better allow for variations in age structure between populations than AF.
The estimation of AF and of the number of attributable cases is relatively accurate for HPV in comparison with other infectious agents and most other cancer risk factors. This is due to the accuracy of HPV detection methods and the predominant weight of cervical cancer, which is relatively easy to diagnose and for which HPV is responsible for the vast majority of cases. We note that a comprehensive genomic survey of 228 primary cervical cancers has confirmed the existence of a small fraction of tumors with no HPV DNA and a gene expression profile similar to endometrial cancer.19 Nevertheless, for public health purposes, the AF of 100% is a useful approximation. In contrast to cervical cancer, rarer anogenital cancers are probably underreported in less developed countries particularly anal cancer (difficult to separate from rectal cancer) and oropharyngeal cancer (hard to distinguish from other head and neck cancers when the disease is diagnosed in advanced stages). Notwithstanding these limitations, our study highlights some little‐appreciated differences between HPV‐attributable cancers. Compared to the cervix, noncervical HPV‐attributable cancers tend to be more frequent in men and in older age groups. The male‐to‐female ratio for anal cancer, which has been intensively studied in more developed countries on account of its extremely high incidence in HIV‐positive men who have sex with men,20 is 0.7 in more developed countries but 1.3 in less developed countries. The combined incidence of vulvar and vaginal cancer is two‐times higher than that of penile cancer in more developed countries but approximately equal to that of penile cancer in less developed countries.
For decades, screening has been the cornerstone of cervical cancer prevention whereas other HPV‐attributable cancers are too rare for population‐based screening and/or high risk populations have not been identified (with the possible exception of anal cancer in men who have sex with men).20 Screening can reduce cases of and deaths from cervical cancer faster than HPV vaccination but it is much more logistically demanding. Combinations of HPV‐based screening and HPV vaccination have also been proposed to exploit the advantages of both interventions.21
Between 70% and 90% of all HPV‐attributable cancer cases may be prevented by universal high‐coverage HPV vaccination. Compared to vaccines targeting HPV16/18 only, the additional protection from the 9‐V vaccine is more substantial for cervical cancer than for other HPV‐attributable cancers and hence in women more than men. However, estimates of the relative contribution of HPV types suffer from uncertainty in multiple‐type infections.22 The combined contribution of many HPV types may be over‐estimated by highly sensitive HPV assays, which may detect HPV types that are present but not clinically relevant. In addition, findings from randomized controlled trials23, 24 and population‐based surveys conducted after the implementation of HPV vaccination programs25 showed that 2‐V and, to a lesser extent, 4‐V vaccines, also offer some cross‐protection against other high risk HPV types that are phylogenetically related to HPV16 or 18, such as HPV31, 33 and 45. Among high risk HPV types that are not targeted by the 9‐V vaccine, HPV35 deserves a special mention for being the next most frequent type in invasive cervical cancer worldwide and especially important in sub‐Saharan Africa in both HIV‐negative and HIV‐positive women with cervical cancer.26
Conclusion
Universal vaccination of girls is the most effective strategy to avoid cases of and deaths from cancer caused by HPV. It is estimated that 118 million women aged 10–20 have been targeted by HPV vaccination programs, but only 1% of these are in less developed countries.2 However, vaccination programs reach a higher proportion of the target population in less developed countries (74%) than in more developed countries (48%).2 The preponderant role of HPV16/18 in HPV‐attributable cancer emphasizes the importance of new, more affordable HPV vaccines, such as the 2‐V vaccine announced by the Developing Countries Vaccine Manufacturers' Network.27
Supporting information
Supporting Information
We declare that we have no conflicts of interest.
References
- 1. Jit M, Brisson M, Portnoy A, et al. Cost‐effectiveness of female human papillomavirus vaccination in 179 countries: a PRIME modelling study. Lancet Glob Health 2014;2:e406–14. [DOI] [PubMed] [Google Scholar]
- 2. Bruni L, Diaz M, Barrionuevo‐Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 2016;4:e453–63. [DOI] [PubMed] [Google Scholar]
- 3. Santesso N, Mustafa RA, Schunemann HJ, et al. World Health Organization Guidelines for treatment of cervical intraepithelial neoplasia 2‐3 and screen‐and‐treat strategies to prevent cervical cancer. Int J Gynaecol Obstet 2016;132:252–8. [DOI] [PubMed] [Google Scholar]
- 4. IARC . Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum 1995;64:1–378 (Accessed May 12, 2015 at http://monographs.iarc.fr/ENG/Monographs/vol64/index.php.) [PMC free article] [PubMed] [Google Scholar]
- 5. IARC . Human Papillomaviruses. IARC Monogr Eval Carcinog Risks Hum 2007;90:1–670 (Accessed May 12, 2015 at http://monographs.iarc.fr/ENG/Monographs/vol90/index.php.) [PMC free article] [PubMed] [Google Scholar]
- 6. IARC . Biological Agents. IARC Monogr Eval Carcinog Risks Hum 2012;100B:1–475 (Accessed May 12, 2015 at http://monographs.iarc.fr/ENG/Monographs/vol100B/index.php.) [PMC free article] [PubMed] [Google Scholar]
- 7. Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral hpv infection. J Natl Cancer Inst 2016;108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 2016;4:e609–16. [DOI] [PubMed] [Google Scholar]
- 9. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. International Agency for Research on Cancer, 2013. (Accessed May 20, 2015, at http://GLOBOCAN.iarc.fr.)
- 10. Forman D, Bray F, Brewster DH, et al. Cancer Incidence in Five Continents, Vol. X. International Agency for Research on Cancer, 2013. (Accessed 20 May, 2015, at http://ci5.iarc.fr.)
- 11. United Nations Development Program . Human Development Report 2013: The Rise of the South: Human Progress in a Diverse World. New York: UN Development Program, 2013. (Accessed May 20, 2015, at http://hdr.undp.org/en/2013-report.)
- 12. Alemany L, Cubilla A, Halec G, et al. Role of Human Papillomavirus in Penile Carcinomas Worldwide. Eur Urol 2016;69:953–61. [DOI] [PubMed] [Google Scholar]
- 13. Castellsague X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst 2016;108:djv403. [DOI] [PubMed] [Google Scholar]
- 14. Serrano B, de Sanjose S, Tous S, et al. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur J Cancer 2015;51:1732–41. [DOI] [PubMed] [Google Scholar]
- 15. Crosbie EJ, Einstein MH, Franceschi S, et al. Human papillomavirus and cervical cancer. Lancet 2013;382:889–99. [DOI] [PubMed] [Google Scholar]
- 16. Marur S, D'Souza G, Westra WH, et al. HPV‐associated head and neck cancer: a virus‐related cancer epidemic. Lancet Oncol 2010;11:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rietbergen MM, Braakhuis BJ, Moukhtari N, et al. No evidence for active human papillomavirus (HPV) in fields surrounding HPV‐positive oropharyngeal tumors. J Oral Pathol Med 2014;43:137–42. [DOI] [PubMed] [Google Scholar]
- 18. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cancer Genome Atlas Research Network . Integrated genomic and molecular characterization of cervical cancer. Nature 2017; doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Martel C, Shiels MS, Franceschi S, et al. Cancers attributable to infections among adults with HIV in the United States. AIDS 2015;29:2173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bosch FX, Robles C, Diaz M, et al. HPV‐FASTER: broadening the scope for prevention of HPV‐related cancer. Nat Rev Clin Oncol 2016;13:119–32. [DOI] [PubMed] [Google Scholar]
- 22. Guan P, Howell‐Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV‐positive women: a meta‐analysis from cervical infection to cancer. Int J Cancer 2012;131:2349–59. [DOI] [PubMed] [Google Scholar]
- 23. Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nat Rev Clin Oncol 2013;10:400–10. [DOI] [PubMed] [Google Scholar]
- 24. Kreimer AR, Struyf F, Del Rosario‐Raymundo MR, et al. Efficacy of fewer than three doses of an HPV‐16/18 AS04‐adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol 2015;16:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cameron RL, Kavanagh K, Pan J, et al. Human Papillomavirus Prevalence and Herd Immunity after Introduction of Vaccination Program, Scotland, 2009‐2013. Emerg Infect Dis 2016;22:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clifford GM, de Vuyst H, Tenet V, et al. Effect of HIV infection on human papillomavirus types causing invasive cervical cancer in Africa. J Acquir Immune Defic Syndr 2016;73:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pagliusi S, Ting CC, Khomvilai S. Quality vaccines for all people: Report on the 16th annual general meeting of the Developing Countries Vaccine Manufacturers' Network, 05‐07th October 2015, Bangkok, Thailand. Vaccine 2016;34:3562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


