Abstract
Background
The correlation between proteinuria and contrast-induced acute kidney injury (CI-AKI) in patients with cerebrovascular disease is still unknown.
Objective
To determine whether proteinuria is a risk factor for CI-AKI and death in patients with stroke undergoing cerebral angiography.
Methods
Data from 2015 patients with stroke undergoing cerebral angiography between January 2009 and December 2013 were retrospectively collected. Clinical parameters were obtained from the hospital's computerized database. All variables were analyzed by univariate analysis and multivariate logistic regression analysis.
Results
CI-AKI was seen in 85 patients (4.2%). After adjustment for potential confounding risk factors, patients with proteinuria had a fivefold higher risk of CI-AKI than patients without proteinuria (OR=5.74; 95% CI 2.23 to 14.83; p<0.001). Other independent risk factors for CI-AKI were estimated glomerular filtration rate <60 mL/min/1.73 m2, anemia, and a high National Institute of Health Stroke Scale score. Proteinuria did not increase in-hospital mortality (OR=1.25; 95% CI 0.49 to 3.17; p=0.639) but did increase 1-year mortality (HR=2.30, 95% CI 1.55 to 3.41, p<0.001).
Conclusions
Proteinuria is an independent risk factor for CI-AKI and 1-year mortality in patients with stroke undergoing cerebral angiography. More attention should be paid to the development of CI-AKI in patients with stroke with proteinuria.
Keywords: Angiography, Stroke, Intervention, Complication
Introduction
Acute kidney injury (AKI) is a common clinical event and is associated with high morbidity and mortality.1 The mortality rates of patients with AKI in the intensive care unit are extremely high, reaching 80%.2 Contrast-induced AKI (CI-AKI) is the third leading cause of hospital-acquired AKI, accounting for 11% of cases.3 CI-AKI is often related to high in-hospital mortality and long-term mortality.4–6 Because there is no effective treatment for CI-AKI, the detection and management of factors related to CI-AKI are important concerns for clinicians.
The importance of cerebral angiography and endovascular therapy in the diagnosis and treatment of cerebrovascular diseases is increasing. Previous studies revealed some CI-AKI indicators (eg, renal failure, anemia, advanced age, and diabetes), but most of these studies focused on patients with cardiovascular disease.7–9 Few studies investigated the risk factors for CI-AKI in patients with cerebrovascular disease.
The Kidney Disease Improving Global Outcomes (KDIGO) guidelines define and stage chronic kidney diseases (CKDs) according to glomerular filtration rate (GFR) and proteinuria.10 Proteinuria aggravates renal outcomes11 12 and also increases the rate of cardiovascular events and long-term mortality.13 14 In addition, multiple studies have shown that proteinuria is independently associated with AKI development and mortality.15–17 However, the correlation between proteinuria and CI-AKI in patients with cerebrovascular disease is still unknown. Therefore, we conducted this retrospective observational study to evaluate the correlation between proteinuria and CI-AKI in patients with stroke undergoing cerebral angiography.
Materials and methods
Study population
We conducted this retrospective observational study at Guangdong General Hospital. The study population consisted of consecutive patients with cerebral ischemic stroke or hemorrhagic stroke undergoing cerebral angiography with/without endovascular therapy between January 2009 and December 2013. The exclusion criteria were as follows: (1) age <18 years; (2) lack of serum creatinine measurements before and within 48 h after angiography or intervention; (3) lack of either a routine urine analysis or a urine albumin–creatinine ratio (ACR); (4) loss to 1-year follow-up; (5) history of end-stage renal disease and dialysis; (6) history of kidney transplantation; (7) AKI before the procedure; (8) administration of nephrotoxic drugs (non-steroidal anti-inflammatory drugs, glycopeptide or aminoglycoside antibiotics, mannitol) 72 h before and after the procedure; (9) iodic contrast administration in the 72 h preceding the procedure; (10) pregnancy; and (11) severe hepatic insufficiency, heart failure, acute myocardial infarction, shock, urinary tract obstruction, sepsis, hypotension, or hypovolemia at the time of hospitalization.
The ethics research committee at Guangdong General Hospital approved the study and agreed that informed consent was not necessary because of the purely observational nature of this study. All patient information was anonymized and de-identified before analysis.
Definition of CI-AKI
The diagnosis of CI-AKI was guided by the diagnostic criteria from the 2011 European Society of Urogenital Radiology Contrast Media Safety Committee guidelines.8 CI-AKI was defined as an increase in serum creatinine by 44 μmol/L or ≥25% of the baseline value within 48 h after exposure to contrast medium in the absence of an alternative etiology. The last serum creatinine measurement before angiography was considered the baseline measurement in this study.
Definition of proteinuria
Proteinuria was defined as urine dipstick trace to 3+ protein based on routine urine analysis, or an ACR of >30 mg/g.
Data collection
Clinical parameters, including patient demographics, medical history (smoking, diabetes, hypertension, and previous cerebrovascular diseases), physical examination, National Institute of Health Stroke Scale (NIHSS) score of neurological deficit, etiology of stroke, medication use, laboratory findings, length of stay in the hospital, requirement for renal replacement therapy (RRT), and date of in-hospital death, were obtained from the hospital's computerized database. An estimated GFR (eGFR) was obtained for each patient using the CKD Epidemiology Collaboration Creatinine Equation (2009).18 Hydration therapy is defined as IV administration of isotonic sodium chloride or sodium bicarbonate solutions at 1–1.5 mL/kg/h at least 6 h after cerebral angiography. RRT is defined as intermittent hemodialysis or continuous RRT. Electronic medical records were retrospectively reviewed to gather data on contrast type, contrast amount, procedure time, complications, and whether patients had received intervention therapy. We acquired the data on 1-year mortality from the hospital's computerized database or by telephone follow-up.
Statistical analysis
All the analyses and calculations were performed using SPSS software (IBM, V.21.0). Continuous variables are expressed as the mean±SD or median (with 25th and 75th centiles) and were analyzed by the t test or Mann–Whitney U test, as appropriate. Categorical variables are described as numbers and proportions and were compared using the χ2 test. Univariate and multivariate logistic regression analyses were used to identify the risk factors for CI-AKI and in-hospital death. One-year mortality curves were generated using the Kaplan–Meier method, and curves were compared with the log-rank test. Univariate and multivariate Cox proportional regression analyses were performed to assess the relationship between proteinuria and 1-year mortality. All probability values are two-tailed, and statistical significance was defined as p<0.05.
Results
Characteristics of the study population
A total of 2015 patients with stroke undergoing cerebral angiography with or without endovascular therapy were analyzed. Their mean age was (51.9±16.8) years and 995 (49.4%) patients were women. Ischemic stroke was the leading cause, with 1820 patients accounting for 90.3% of the cases. Only 195 patients had hemorrhagic stroke, and 98.5% of them were diagnosed with subarachnoid hemorrhage. The mean eGFR was (64.5±33.7) mL/min/1.73 m2 at baseline. The median NIHSS score was 1 (1, 3). A total of 493 (24.5%) patients received endovascular therapy. The contrast medium used in 96.2% of the patients was iopromide (Ultravist 370), while the others received iodixanol (Visipaque). Hydration therapy was performed in 601 patients (29.8%).
Overall, CI-AKI was observed in 85 patients (4.2%). The differences between patients according to the development of CI-AKI are shown in table 1. Patients with CI-AKI tended to be older and have a worse neurological deficit score, relatively lower eGFR, higher incidence of proteinuria, and higher prevalence of anemia than patients without CI-AKI. Only five patients received RRT because of CI-AKI.
Table 1.
Baseline clinical features in patients with and without CI-AKI
| Variables | CI-AKI |
p Value | |
|---|---|---|---|
| Yes (n=85) | No (n=1930) | ||
| Demographics | |||
| Age (years), mean±SD | 56.14±17.84 | 51.71±16.68 | 0.017 |
| Women, n (%) | 44 (51.8) | 951 (49.3) | 0.882 |
| Weight (kg), mean±SD | 57.39±10.77 | 57.78±15.73 | 0.819 |
| Medical history, n (%) | |||
| Smoking | 8 (9.4) | 144 (7.5) | 0.505 |
| Hypertension | 27 (31.8) | 581 (30.1) | 0.928 |
| Diabetes | 16 (18.8) | 264 (13.7) | 0.180 |
| Prior cerebrovascular disease | 4 (4.7) | 194 (10.1) | 0.105 |
| Type of stroke | |||
| Ischemic stroke, n (%) | 81 (95.3) | 1739 (90.1) | |
| Laboratory findings | |||
| Baseline SCr (mol/L), mean±SD | 103.02±35.88 | 97.05±30.90 | 0.084 |
| Baseline eGFR (mL/min/1.73 m2), mean±SD | 57.30±22.46 | 64.82±34.09 | 0.044 |
| Baseline eGFR <60 mL/min/1.73 m2, n (%) | 53 (62.4) | 961 (49.8) | 0.023 |
| Proteinuria, n (%) | 12 (14.1) | 53 (2.7) | <0.001 |
| Anemia, n (%) | 31 (36.5) | 143 (7.4) | <0.001 |
| Hypoalbuminemia, n (%) | 4 (4.7) | 120 (6.2) | 0.570 |
| Hyperuricemia, n (%) | 51 (60) | 1099 (56.9) | 0.557 |
| High LDL, n (%) | 8 (9.4) | 279 (14.5) | 0.193 |
| Hypercholesterolemia, n (%) | 9 (10.6) | 279 (14.5) | 0.319 |
| Hypertriglyceridemia, n (%) | 27 (31.8) | 665 (34.5) | 0.609 |
| Fasting glucose (mmol/L), mean±SD | 5.90±2.55 | 5.73±1.94 | 0.437 |
| Neurological deficit scoring | |||
| NIHSS, median | 15 (14.17) | 1 (1.3) | <0.001 |
| Medicine, n (%) | |||
| Hydration | 21 (24.7) | 580 (30.1) | 0.292 |
| Statins | 17 (20.0) | 328 (17.0) | 0.472 |
| ACEI/ARB | 37 (43.5) | 832 (43.1) | 0.939 |
| Diuretics | 66 (77.6) | 1624 (84.1) | 0.111 |
| Antiplatelet agents | 53 (62.4) | 1108 (57.4) | 0.367 |
| Anticoagulants | 31 (36.5) | 730 (37.8) | 0.801 |
| Procedural characteristics | |||
| Contrast type: Ultravist 370, n (%) | 79 (92.9) | 1859 (96.3) | 0.112 |
| Contrast volume (ml), mean±SD | 71.18±27.05 | 69.11±16.76 | 0.487 |
| Contrast exposure time > 30 min, n (%) | 36 (42.4) | 816 (42.3) | 0.989 |
| No endovascular therapy, n (%) | 63 (74.1) | 1459 (75.6%) | 0.760 |
ACEI, ACE inhibitor; ARB, angiotensin receptor blocker; CI-AKI, contrast-induced acute kidney injury; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein; NIHSS, National Institute of Health Stroke Scale; SCr, serum creatinine.
The proportion of subjects with proteinuria was 3.2%. The patients with proteinuria had a higher prevalence of diabetes (41.5% vs 13.0%, p<0.001) and a worse neurological deficit score (1 (1,4) vs 1 (1,3), p<0.001).
Role of proteinuria in CI-AKI
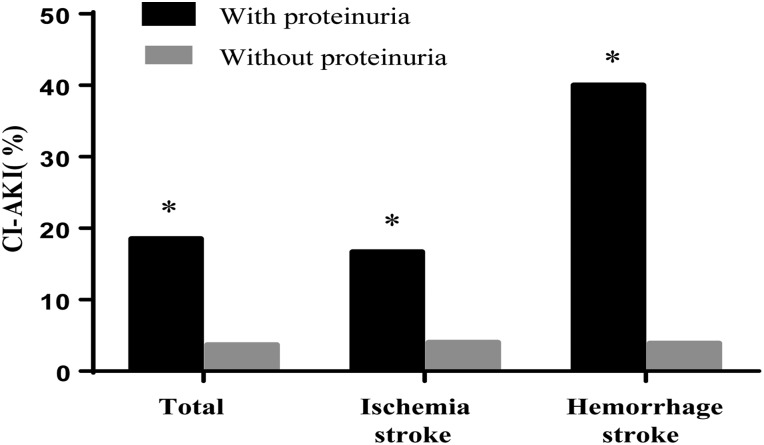
The CI-AKI incidence in patients with proteinuria was higher than that in patients without proteinuria (18.5% vs 3.7%, p<0.001; figure 1). Subgroup analysis showed that patients with proteinuria have a higher incidence of CI-AKI among both patients with ischemic stroke (16.7% vs 4.0%, p<0.001) and those with hemorrhagic stroke (40.0% vs 1.1%, p<0.001; figure 1). In the univariate logistic regression analysis, proteinuria was significantly associated with CI-AKI (OR=5.82, 95% CI 2.98 to 11.36, p<0.001). Advanced age, eGFR <60 mL/min/1.73 m2, anemia, and a high NIHSS score were also associated with CI-AKI (table 2). The multivariate logistic regression analysis showed that even after adjusting for potential confounding risk factors, patients with proteinuria still had a fivefold higher risk of CI-AKI than patients without proteinuria (OR=5.74, 95% CI 2.22 to 14.82, p<0.001; table 2). Other independent risk factors for CI-AKI were eGFR <60 mL/min/1.73 m2, anemia, and a high NIHSS score (table 2).
Figure 1.
Incidence of contrast-induced acute kidney injury (CI-AKI) among patients with or without proteinuria. *p<0.001 vs patients without proteinuria.
Table 2.
Univariate and multivariate logistic analysis of CI-AKI risk factors
| Variable | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Proteinuria | 5.82 | 2.98 to 11.36 | <0.001 | 5.74 | 2.23 to 14.83 | <0.001 |
| eGFR <60 mL/min/1.73 m2 | 1.67 | 1.07 to 2.61 | 0.025 | 2.74 | 1.48 to 5.10 | 0.001 |
| Anemia | 7.17 | 4.47 to 11.51 | <0.001 | 6.82 | 3.67 to 12.71 | <0.001 |
| NIHSS | 1.21 | 1.18 to 1.25 | <0.001 | 1.20 | 1.16 to 1.24 | <0.001 |
| Age >65 years | 2.51 | 1.57 to 4.02 | <0.001 | – | – | – |
CI-AKI, contrast-induced acute kidney injury; eGFR, estimated glomerular filtration rate; NIHSS, National Institute of Health Stroke Scale.
Role of proteinuria in mortality
The in-hospital mortality rate was 6.3% (127 patients) in this cohort of patients. Proteinuria did not increase in-hospital mortality (OR=1.25; 95% CI 0.49 to 3.17; p=0.639).
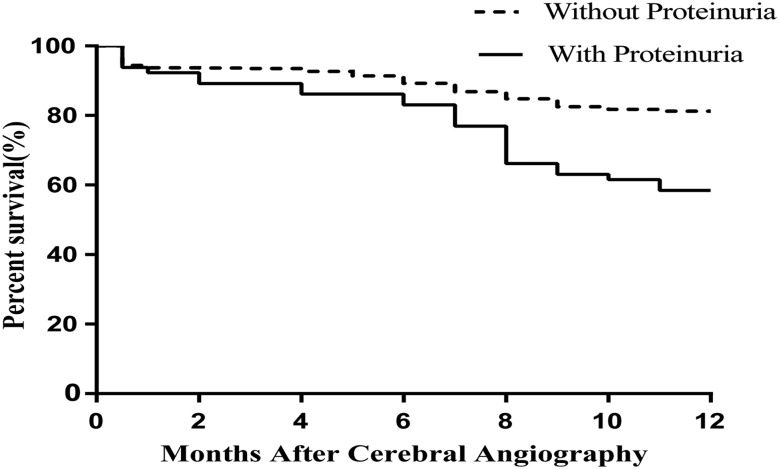
Throughout the 1-year follow-up period, 392 patients (19.5%) died. The Kaplan–Meier curve showed that the 1-year mortality of patients with proteinuria was significantly higher than that of patients without proteinuria (41.5% vs 18.7%, p<0.001; figure 2). Univariate Cox proportional regression analyses showed that proteinuria (HR=2.41, 95% CI 1.63 to 3.56, p<0.001) and NIHSS (HR=1.02, 95% CI 1.00 to 1.04, p<0.001) among the baseline characteristics were associated with 1-year mortality. In the multivariate Cox stepwise regression model, proteinuria was an independent risk factor for 1-year mortality (HR=2.30, 95% CI 1.55 to 3.41, p<0.001).
Figure 2.
Kaplan–Meier curve for 1-year survival according to proteinuria (p<0.001).
Discussion
In this retrospective observation study, the incidence of CI-AKI was 4.2% among patients with stroke undergoing cerebral angiography. We found that proteinuria is an independent risk factor for CI-AKI in patients with stroke undergoing cerebral angiography. Patients with proteinuria had a fivefold higher risk of CI-AKI than patients without proteinuria. In addition to proteinuria, eGFR <60 mL/min/1.73 m2, anemia, and a high NIHSS score were also independent risk factors for CI-AKI. We also found that among the baseline characteristics, proteinuria was an independent risk factor for 1-year mortality.
Recently, a multicenter retrospective population-based cohort study in China showed that CI-AKI accounted for 13.1% of hospital-acquired AKI.19 The incidence of CI-AKI in our study is significantly lower than the incidence of CI-AKI among patients with cardiovascular disease undergoing coronary arteriography (range 7.8–15.7%)7 9 19 20 but is consistent with other studies of patients with stroke undergoing cerebral angiography (range 1.5–5.0%).21–23 Several factors may explain the difference in incidence of CI-AKI between cerebral angiography and coronary arteriography. First, the patients undergoing coronary arteriography present poor hemodynamics, and some patients may even have cardiogenic shock. In our study, we excluded those patients with hemodynamic instability, such as hypovolemia, hypotension, heart failure, and cardiogenic shock. Second, the comorbidities of patients with cardiovascular disease and those with cerebrovascular disease differ. The patients in our study presented with a lower proportion of diabetes and hyperlipidemia. In addition, the contrast volume in our study was less than that used in the previous study for coronary arteriography.7 9 19 20
Previous studies have shown that CI-AKI increases in-hospital mortality and long-term mortality rates. Because there is no effective treatment for CI-AKI, it is essential to identify factors related to early CI-AKI and prevent its development. Although previous studies revealed some CI-AKI indicators (eg, renal failure, anemia, advanced age, and diabetes), most of these studies were performed in patients with cardiovascular disease.7–9 Ray et al analyzed the differences between patients with and without CI-AKI among patients with subarachnoid hemorrhage. They found that only age >75 years was associated with the development of CI-AKI.22 Prasad et al23 found that the proportion of patients with diabetes was higher among those who developed CI-AKI in patients undergoing a neuroendovascular procedure. Another study showed that among patients with acute ischemic stroke, patients with CI-AKI had a higher baseline serum creatinine level.21 However, multivariate regression analysis was not used to identify the risk factors for CI-AKI in these studies.
Our study showed that proteinuria, eGFR <60 mL/min/1.73 m2, anemia, and a high NIHSS score are independent risk factors in patients undergoing cerebral angiography. Some established risk factors for CI-AKI in coronary arteriography, such as age and diabetes, are not risk factors for CI-AKI in our study, owing to the lower proportion of elderly patients and patients with diabetes included. It has been proved that anemia is a risk factor for CI-AKI in patients undergoing coronary arteriography. Local renal hypoxia can be more aggravated in patients with low hemoglobin after exposure to contrast media.24 This may explain why anemia has a strong correlation with the development of CI-AKI in patients undergoing cerebral angiography.
The KDIGO guidelines define and stage CKDs according to GFR and proteinuria.10 Although previous studies showed that renal failure is an independent risk factor for CI-AKI, few studies have investigated the role of proteinuria in CI-AKI.
Normally, only a small amount of albumin is filtered across the glomeruli, and nearly all filtered albumin is reabsorbed by apical receptors in proximal tubular cells. In healthy individuals, proteinuria cannot be detected, but when the glomerular filtration barrier or proximal tubular cells are injured, proteinuria occurs. Furthermore, proteinuria, which is a marker of kidney injury, also participates in the injury process. An overload of albumin in proximal tubules may upregulate NF-κB, which increases the expression of multiple inflammatory mediators, such as interleukin 18 and monocyte chemoattractant protein-1.25 26 The increase in these inflammatory mediators leads to tubulointerstitial inflammation. Proteinuria also increases reactive oxygen species, resulting in proximal tubule inflammation.27 Additionally, proteinuria may induce endoplasmic reticulum stress,28 eventually leading to the apoptosis of proximal tubule cells. For these reasons, patients with proteinuria have reduced physiological adaptability and are less able to tolerate nephrotoxic insults, such as contrast medium.
Numerous studies have shown that proteinuria aggravates renal outcomes and increases the rate of cardiovascular events and long-term mortality in patients with CKD.11–14 Multiple studies have found that proteinuria is a marker of chronic renal insults and may also serve as a surrogate marker of acute damage. Proteinuria has been confirmed as an indicator of AKI independent of eGFR.15–17 In addition, Han et al17 also found that proteinuria increases the risk of AKI and also the risk of 3-year mortality in patients admitted to the intensive care unit. Recently, three studies found that proteinuria is also a risk factor for CI-AKI.20 29 30 However, these studies did not include patients with cerebrovascular disease and were of relatively lower power. Similarly, our study indicated that proteinuria increased the risk of CI-AKI. It is noteworthy that proteinuria is also an independent indicator of 1-year mortality in our study. Proteinuria appeared to be useful for evaluating the risk of CI-AKI and 1-year mortality in patients with stroke. Special attention should be paid to the development of CI-AKI in patients with stroke and proteinuria.
Study limitations
Our study has several limitations. First, this is a retrospective observational study conducted in a single center, and we excluded patients without urine analysis data and who were lost to the 1-year follow-up. Therefore, there may be some bias in patient selection. It would be best to validate our results in a multicenter prospective study. Second, most patients underwent routine urine analysis rather than an assessment of the ACR, which would more accurately reflect the degree of kidney damage. But routine urine analysis is an easy and reliable method for clinicians to judge whether patients have proteinuria, and has been used to predict AKI risk in many published reports. Additionally, because the proportion of subjects with proteinuria was low, we were unable to analyze the dose–effect relationship between proteinuria and outcomes.
Conclusions
In conclusion, this study revealed the epidemiology of CI-AKI in patients with stroke undergoing cerebral angiography and showed that proteinuria is an independent indicator of CI-AKI and 1-year mortality. Other risk factors for CI-AKI include eGFR <60 mL/min/1.73 m2, anemia, and a high NIHSS score. Therefore, the detection of proteinuria is essential for the CI-AKI risk stratification of patients with stroke and more attention should be paid to the development of CI-AKI in patients with stroke with proteinuria.
Footnotes
Contributors: Conceived and designed the experiments: YT, LZ and XL. Performed the experiments: YT, WD, ZL, YC, HL, LM, LX, SL, RL and WS. Analyzed the data: YT and WD. Wrote the paper: YT.
Funding: This work was supported by the National Natural Science Foundation (grant number 81170683, 81200544); and the National Clinical Key Specialty Construction Preparatory Projects, China.
Competing interests: None declared.
Ethics approval: The ethics research committee at Guangdong General Hospital approved this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Susantitaphong P, Cruz DN, Cerda J, et al. . World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013;8:1482–93. 10.2215/CJN.00710113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turney JH. Acute renal failure—a dangerous condition. JAMA 1996;275:1516–17. [PubMed] [Google Scholar]
- 3.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Di 2002;39:930–6. 10.1053/ajkd.2002.32766 [DOI] [PubMed] [Google Scholar]
- 4.McCullough PA, Wolyn R, Rocher LL, et al. . Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med 1997;103:368–75. 10.1016/S0002-9343(97)00150-2 [DOI] [PubMed] [Google Scholar]
- 5.Weisbord SD, Chen H, Stone RA, et al. . Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol 2006;17:2871–7. 10.1681/ASN.2006030301 [DOI] [PubMed] [Google Scholar]
- 6.Neyra JA, Shah S, Mooney R, et al. . Contrast-induced acute kidney injury following coronary angiography: a cohort study of hospitalized patients with or without chronic kidney disease. Nephrol Dial Transplant 2013;28:1463–71. 10.1093/ndt/gft082 [DOI] [PubMed] [Google Scholar]
- 7.Mehran R, Aymong ED, Nikolsky E, et al. . A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention. J Am Coll Cardiol 2004;44:1393–9. [DOI] [PubMed] [Google Scholar]
- 8.Stacul F, van der Molen AJ, Reimer P, et al. . Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011;21:2527–41. 10.1007/s00330-011-2225-0 [DOI] [PubMed] [Google Scholar]
- 9.Inohara T, Kohsaka S, Abe T, et al. . Development and validation of a pre-percutaneous coronary intervention risk model of contrast-induced acute kidney injury with an integer scoring system. Am J Cardiol 2015;115:1636–42. 10.1016/j.amjcard.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 10.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 11.Tangri N, Stevens LA, Griffith J, et al. . A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011;305:1553–9. 10.1001/jama.2011.451 [DOI] [PubMed] [Google Scholar]
- 12.Halbesma N, Jansen DF, Heymans MW, et al. . Development and validation of a general population renal risk score. Clin J Am Soc Nephrol 2011;6:1731–8. 10.2215/CJN.08590910 [DOI] [PubMed] [Google Scholar]
- 13.Gerstein HC, Mann JF, Yi Q, et al. . Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001;286:421–6. 10.1001/jama.286.4.421 [DOI] [PubMed] [Google Scholar]
- 14.Matsushita K, van der Velde M, Astor BC, et al. . Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James MT, Hemmelgarn BR, Wiebe N, et al. . Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet 2010;376:2096–103. 10.1016/S0140-6736(10)61271-8 [DOI] [PubMed] [Google Scholar]
- 16.Grams ME, Astor BC, Bash LD, et al. . Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol 2010;21:1757–64. 10.1681/ASN.2010010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SS, Ahn SY, Ryu J, et al. . Proteinuria and hematuria are associated with acute kidney injury and mortality in critically ill patients: a retrospective observational study. BMC Nephrol 2014;15:93 10.1186/1471-2369-15-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Nie S, Liu Z, et al. . Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol 2015;10:1510–18. 10.2215/CJN.02140215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He F, Zhang J, Lu ZQ, et al. . Risk factors and outcomes of acute kidney injury after intracoronary stent implantation. World J Emerg Med 2012;3:197–201. 10.5847/wjem.j.issn.1920-8642.2012.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loh Y, McArthur DL, Vespa P, et al. . The risk of acute radiocontrast-mediated kidney injury following endovascular therapy for acute ischemic stroke is low. AJNR Am J Neuroradiol 2010;31:1584–7. 10.3174/ajnr.A2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray B, Rickert KL, Welch BG, et al. . Development of contrast-induced nephropathy in subarachnoid hemorrhage: a single center perspective. Neurocrit Care 2013;19:150–6. 10.1007/s12028-013-9850-1 [DOI] [PubMed] [Google Scholar]
- 23.Prasad V, Gandhi D, Stokum C, et al. . Incidence of contrast material-induced nephropathy after neuroendovascular procedures. Radiology 2014;273:853–8. 10.1148/radiol.14131104 [DOI] [PubMed] [Google Scholar]
- 24.Li WH, Li DY, Han F, et al. . Impact of anemia on contrast-induced nephropathy (CIN) in patients undergoing percutaneous coronary interventions. Int Urol Nephrol 2013;45:1065–70. 10.1007/s11255-012-0340-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Rangan GK, Tay YC, et al. . Induction of monocyte chemoattractant protein-1 by albumin is mediated by nuclear factor kappaB in proximal tubule cells. J Am Soc Nephrol 1999;10:1204–13. [DOI] [PubMed] [Google Scholar]
- 26.Tang S, Leung JC, Abe K, et al. . Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J Clin Invest 2003;111:515–27. 10.1172/JCI16079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu D, Xu M, Ding LH, et al. . Activation of the Nlrp3 inflammasome by mitochondrial reactive oxygen species: a novel mechanism of albumin-induced tubulointerstitial inflammation. Int J Biochem Cell Biol 2014;57:7–19. 10.1016/j.biocel.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohse T, Inagi R, Tanaka T, et al. . Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int 2006;70:1447–55. 10.1038/sj.ki.5001704 [DOI] [PubMed] [Google Scholar]
- 29.Piskinpasa S, Altun B, Akoglu H, et al. . An uninvestigated risk factor for contrast-induced nephropathy in chronic kidney disease: proteinuria. Ren Fail 2013;35:62–5. 10.3109/0886022X.2012.741646 [DOI] [PubMed] [Google Scholar]
- 30.Yang JQ, Ran P, Chen JY, et al. . Development of contrast-induced acute kidney injury after elective contrast media exposure in patients with type 2 diabetes mellitus: effect of albuminuria. PLoS ONE 2014;9:e106454 10.1371/journal.pone.0106454 [DOI] [PMC free article] [PubMed] [Google Scholar]