Abstract
Rationale
Reduced physical activity (PA) in patients with COPD is associated with a poor prognosis. Increasing PA is a key therapeutic target, but thus far few strategies have been found effective in this patient group.
Objectives
To investigate the effectiveness of a 12-week semiautomated telecoaching intervention on PA in patients with COPD in a multicentre European randomised controlled trial.
Methods
343 patients from six centres, encompassing a wide spectrum of disease severity, were randomly allocated to either a usual care group (UCG) or a telecoaching intervention group (IG) between June and December 2014. This 12-week intervention included an exercise booklet and a step counter providing feedback both directly and via a dedicated smartphone application. The latter provided an individualised daily activity goal (steps) revised weekly and text messages as well as allowing occasional telephone contacts with investigators. PA was measured using accelerometry during 1 week preceding randomisation and during week 12. Secondary outcomes included exercise capacity and health status. Analyses were based on modified intention to treat.
Main results
Both groups were comparable at baseline in terms of factors influencing PA. At 12 weeks, the intervention yielded a between-group difference of mean, 95% CI (lower limit – upper limit; ll-ul) +1469, 95% CI (971 to 1965) steps/day and +10.4, 95% CI (6.1 to 14.7) min/day moderate PA; favouring the IG (all p≤0.001). The change in 6-min walk distance was significantly different (13.4, 95% CI (3.40 to 23.5) m, p<0.01), favouring the IG. In IG patients, an improvement could be observed in the functional state domain of the clinical COPD questionnaire (p=0.03) compared with UCG. Other health status outcomes did not differ.
Conclusions
The amount and intensity of PA can be significantly increased in patients with COPD using a 12-week semiautomated telecoaching intervention including a step counter and an application installed on a smartphone.
Trial registration number:
Keywords: Exercise, Pulmonary Rehabilitation
Key messages.
What is the key question?
What is the effectiveness of a 12-week semiautomated telecoaching intervention on objectively measured physical activity in patients with COPD included in a multicentre European randomised controlled trial?
What is the bottom line?
A 12-week semiautomated telecoaching intervention including a step counter and an application installed on a smartphone is effective in increasing the amount and intensity of physical activity in patients with COPD.
Why read on?
This article reports on an effective, innovative and promising coaching programme for people with COPD with a low burden on health professionals administering it.
Introduction
Based on overwhelming evidence, regular physical activity (PA) is at present seen as one of the most powerful health-promoting acts.1 Physical inactivity is the fourth leading cause of death, attributing to >5 million premature deaths worldwide.2 Also, 6–10% of major non-communicable disease burden is caused by inactivity.2 Despite this evidence, a large proportion of healthy elderly people do not meet PA recommendations.2 Patients with chronic disease show an even more pronounced inactive lifestyle.3 This is particularly true when exercise induces symptoms.
COPD is a leading cause of mortality and morbidity, resulting in a substantial economic and social burden.4 Patients with COPD are less active than age-matched controls.5 In this patient population, low levels of PA are an independent risk factor for mortality and hospitalisation.6 7 Generally, activity level decreases with increasing disease severity but inactivity is not simply a reflection of lung function impairment.8 9 Activity promotion is included as a key element in the recommendations for the management of patients with COPD, 4 although there are, thus far, few interventions of proven efficacy. In particular, both pulmonary rehabilitation programmes10 and pharmacotherapy,11 12 while of proven benefit in other respects, have led to small and inconsistent increases in PA.
PA is a complex health behaviour; consequently, effective behaviour change interventions need to reflect this. In chronically ill patients, larger improvements on PA have been observed in intervention studies where behaviour strategies (goal setting, contracting, feedback, consequences and/or cues) were included.13 Activity monitors can provide direct feedback, which has shown promise in COPD, in combination with goal setting.14 15 Implementing behaviour strategies can be done by face-to-face contacts between patients and clinicians. However, such interventions are relatively time-consuming and may depend on the skills and training of the clinicians involved, making it more difficult for them to be widely adopted. Electronic information and communication technologies offer the possibility of decreasing the burden on clinicians and patients, standardising interventions and making them available to people who live in remote areas or in areas where access to healthcare providers is limited (‘telecoaching’).16
One study in the USA has shown the effectiveness of a solely internet-based telecoaching programme on PA in patients with COPD, observing improvements in both health-related quality of life and in PA.17 Various health-related applications (‘m-health’), focusing on PA, are available on the market,18 but applications tailored for patients with COPD have not yet been developed nor tested. We hypothesised that a smartphone application, specifically designed for the intended population and allowing human contact if needed, across the whole spectrum of COPD, would offer insight into the potential effectiveness of such an intervention on the PA level.
We therefore assessed the effectiveness of a 12-week PA promotion telecoaching programme in addition to usual care in a multicentre trial including patients with COPD. This study forms part of the European IMI-JU PROactive project.
Methods
Study population
Patients with a physician-based diagnosis of COPD,4 age >40 with a smoking history of at least 10 pack-years, who were not actively participating in a pulmonary rehabilitation programme at the moment of inclusion (or did not plan to start), were enrolled at six centres across Europe (Leuven (Belgium), Athens (Greece), London and Edinburgh (the UK), Zurich (Switzerland) and Groningen (The Netherlands)); these centres were chosen in order to recruit patients with a range of severity of COPD. Patients were excluded if they had any comorbidity limiting a normal activity pattern, had another respiratory disease as primary diagnosis or were unable to understand or operate a smartphone device. Stable patients as well as patients having an acute exacerbation in the last month were included in this trial. Patients using walking aids or those on long-term oxygen treatment were as well included in the trial.
Design
The trial consisted of three visits—a screening visit (V1), a randomisation visit (V2) 1–2 weeks later and a final visit (V3), 12 weeks post randomisation. In this 1:1 randomised controlled trial, patients were allocated in either a control group (UCG) receiving usual care or in an intervention group (IG). The random sequence was generated with varying block sizes of 4, 6 or 8 and stratified by centre using a statistical software (STATA V.12.0, StataCorp, College Station, Texas, USA). After obtaining informed consent and when all inclusion criteria were met at V2, investigators obtained group allocation using an online system that ensured concealment of random allocation.
Usual care
Patients in both groups received a standard leaflet explaining the importance of PA in COPD as well as information about PA recommendations. This leaflet was discussed with all patients in a 5–10 min one-to-one discussion with the investigator during V2. The usual medical treatment was not altered throughout the study.
Intervention
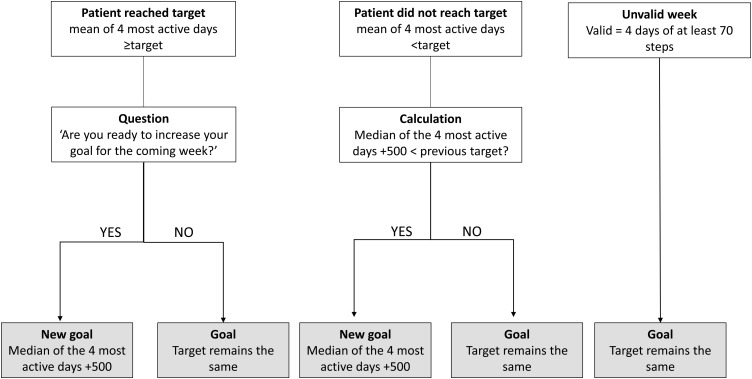
Patients in the IG received the usual care plus the telecoaching intervention. This intervention included several components: (1) a one-to-one interview with the investigator during V2 discussing motivation, barriers, favourite activities and strategies to become more active; (2) a step counter (Fitbug Air) providing direct feedback on the step count, on a 2 × 3 cm display; (3) smartphone with Fitbug application and a project-tailored coaching application. This application was specifically designed for use by patients with COPD in the present project. It provided automated coaching by displaying an activity goal (number of steps) and feedback on a daily basis. The feedback included a graphical representation of that day's performance and an educational tip. Patients' targets were automatically revised every Sunday, based on performance in the preceding week (figure 1). Investigators could alter or ‘lock’ the goals if needed, based on interaction with the patient; (4) a booklet containing home exercises; (5) weekly group text message with activity proposals sent by the investigator, taking into account the local weather forecast; and (6) telephone contacts triggered in the case of non-compliance with wearing the step counter, failure to transmit data or failure to progress. More information is provided in the online supplementary material.
Figure 1.
Flow chart for the calculation of the new goal (steps) during the intervention period.
thoraxjnl-2016-209026supp001.pdf (771.1KB, pdf)
Assessments
Physical activity
PA was objectively measured with the Dynaport Movemonitor (DAM, McRoberts BV, The Hague, the Netherlands) and Actigraph GT3x (ACT, Actigraph LLC Pensacola, Florida, USA) for two periods; 1 week preceding V2 and 1 week preceding V3. Patients were asked to wear the monitors whenever awake. These triaxial accelerometers, worn around the waist, have been thoroughly validated in COPD.19 20 Outputs from the activity monitors used for assessment were not available for patients. A valid PA measurement was defined as a minimum of two weekdays with at least eight hours of wearing time, with weekends excluded from the analysis21 (rationale in online supplementary material). The increase in the number of steps per day over 3 months was chosen as primary outcome. Time in at least moderate intense PA (MPA), walking time and movement intensity during walking were chosen as secondary PA outcomes. Sedentarism has been defined as a step count lower than 5000 steps,22 and for responder analysis a clinically significant increase in step count was defined as ≥1000 steps.23 Although we collected data from two monitors, we report data from the ACT in terms of step count and MPA (‘Freedson 2011’24), while walking time and intensity during walking are based on the DAM, more information about these decisions is included in the online supplementary material.
Other assessments
Postbronchodilator spirometry was performed following ATS-European Respiratory Society (ERS) recommendations25 during V1 and V3, data were related to reference values.26 The following clinical outcomes were collected at V2 and V3. (1) Functional exercise capacity measured as the best of two 6-min walk tests, following ATS-ERS guidelines;27 (2) isometric quadriceps force (QF) measured with the patient fixed in 90° hip and knee flexion;28 and (3) health status measured using the COPD assessment test (CAT) and the clinical COPD questionnaire (CCQ). Breathlessness was measured using the modified Medical Research Council (mMRC) questionnaire. Exacerbation history in the last 12 months, demographic data, medical history including comorbidities and anthropometric data were obtained and a physical examination was performed during V1.
Quality control, safety and time consumption
In two rounds (one during the initial phase and one after recruitment had been completed), investigators from each site visited in a random order another site to assess protocol deviations, verify data and ensure standardisation of procedures. Adverse events were collected during the course of the trial as well as by direct questioning during the final visit. All contacts with patients were logged in the electronic case report file by the investigators including the reason for and duration of the contact.
Statistics
Based on a between-group difference of 1500 steps/day, an SD of 3400 steps/day and a dropout rate of 20%, a need of 68 patients in each arm (total of 136) was calculated for obtaining 90% power using an α level of 0.05 (two-sided). The sample size calculation is based on repeated measurements between two groups and is detailed in the online supplementary material. All statistical analyses were performed using the SAS statistical package (V.9.3, SAS Institute, Cary, North Carolina, USA). All analyses were based on a statistical analysis plan a priori accepted by all authors. Data are presented as mean±SD or median (Q1–Q3). Statistical significance was set at p<0.05 for all these analyses. All randomised patients were encouraged to return for the final visit and considered for these modified intention-to-treat analyses. No imputation was made for missing data, interpreted as ‘missing at random’.
Between-group differences were analysed using an analysis of covariance (ANCOVA) model to adjust for potentially confounding factors judged to be distributed unevenly between groups. End-of-study variables were used as the dependent variable, the baseline variable as a covariate and the group factor as the explanatory variable whose effect is to be tested. Because randomisation was performed by centre, the adjustment by centre was tested. Goodness of fit was assessed by means of normality of residuals. ORs for achieving a 1000 steps/day increase in PA, defined as the minimal important difference,23 were calculated.
As an exploratory analysis, we investigated the intervention effect in different subgroups. Similar ANCOVA analyses as previously mentioned were performed, including the subgroup and interaction subgroup×group factor. The different subgroups were based on baseline data of mMRC (<2 vs ≥2), median split of 6-min walk distance (6MWD) (<450 vs ≥450 m), comorbidity (<2 vs ≥2), Global Initiative for Chronic Obstructive Lung Disease (GOLD) quadrants (A–B vs C–D) and baseline step count (<5000 vs ≥5000 steps/day).
Results
Study population
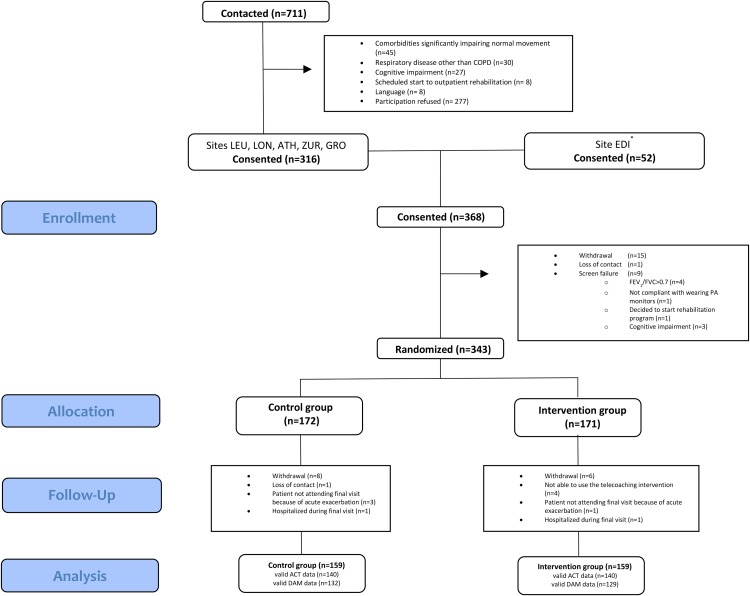
Of the 368 enrolled patients, 343 were randomised (figure 2), between June and December 2014. Baseline characteristics were comparable between groups with regard to factors influencing PA (see table 1). In total, 13 patients in the control and 12 in the IG dropped out (figure 2). Patients who dropped out had a lower body mass index (BMI) (p<0.01), worse QF (p<0.01) and a worse health status (p=0.02) compared with completers (see online supplementary material).
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) diagram. Valid data based on two weekdays with at least eight hours of wearing time during baseline and final visit. *No information on screening in site of Edinburgh. ACT, Actigraph; ATH, Athens; DAM, Dynaport Movemonitor; EDI, Edinburgh; GRO, Groningen; LEU, Leuven; LON, London; PA, physical activity; ZUR, Zurich.
Table 1.
Baseline characteristics
| Variable | UCG n=172 |
IG n=171 |
|---|---|---|
| Female/male* | 64 (37) / 108 (63) | 60 (35)/111 (65) |
| Age (years) | 67±8 | 66±8 |
| BMI (kg/m2) | 25.9±4.8 | 26.7±5.3 |
| Active smoker* | 51 (30) | 39 (23) |
| Recent severe AECOPD* | 9 (5) | 8 (5) |
| Comorbidity* | 91 (53) | 102 (60) |
| FEV1 (%pred) | 57±21 | 55±20 |
| 6MWD (m) | 448±106 | 438±107 |
| 6MWD (%pred) | 72±16 | 70±16 |
| QF (kg) | 31.6±9.5 | 31.0±10.9 |
| CAT score | 13 (8–19) | 13 (7–20) |
| GOLD quadrants (A/B/C/D)* | 66 (38)/17 (10)/37 (22)/52 (30) | 52 (30)/16 (9)/43 (25)/60 (35) |
| Step count (steps) | 5120±2932 | 4634±2697 |
| MPA (min) | 15 (5–34) | 12 (4–26) |
| MI (m/s2) | 1.86±0.36 | 1.82±0.30 |
| Walking time (min) | 72±36 | 69±34 |
| Sedentary* | 95 (58) | 104 (64) |
QF was not measured in 2 centres, QF was missing in 35 control and 32 intervention patients, 6MWD was missing in 2 intervention patients. Steps and MPA are measured by the ACT, MI and walking time are measured by DAM; being sedentary has been defined as a step count measured by ACT <5000 steps/day. A valid baseline PA measurement was present in 165 control and 162 intervention patients for outcomes measured by ACT (steps, MPA) and in 155 control and 156 intervention patients for outcomes measured by DAM (MI, walking time).
Data are expressed as mean±SD and median (Q1–Q3). *Data expressed as n (%).
6MWD, 6-min walk distance; ACT, Actigraph; BMI, body mass index; comorbidity, presence of at least one comorbidity; CAT, COPD assessment test; DAM, Dynaport Movemonitor; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IG, intervention group; MI, movement intensity during walking; MPA, time in at least moderate intense activity; n, number of patients; PA, physical activity; QF, quadriceps force; recent severe AECOPD, an acute exacerbation of COPD requiring hospitalisation within 1 month prior to inclusion; UCG, usual care group.
Main PA analyses are based on 140 UCG and 140 IG patients with valid ACT measurements on both time points. Patients without valid PA data had slightly worse lung function (FEV1%pred) compared with those included in the PA analyses (see online supplementary material). The 280 patients included in the PA analyses wore the accelerometers for 4.7±0.6 weekdays with a wearing time of 806±120 min and 4.6±0.7 weekdays with a wearing time of 785±120 min, respectively, during the baseline and final measurement (no differences between groups). Daylight during the baseline measurement of these patients (745±150 min) as well as changes in daylight (−164±162 min) were comparable between groups. None of the considered confounders remained in the ANCOVA analyses. Patients in the IG (n=140) wore the Fitbug step counter for a median (Q1–Q3) of 91 (84–98) % of the days they were included in the coaching programme, representing 6.3 (5.8–6.8) days/week.
Physical activity
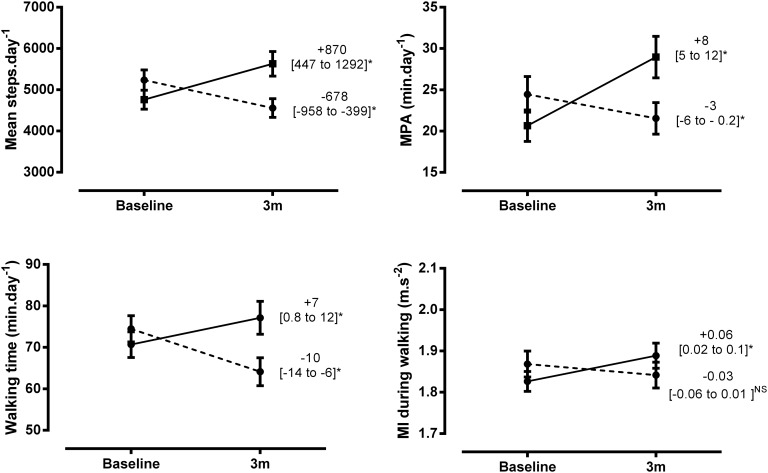
IG patients had an increase over controls of mean, 95% CI (lower limit – upper limit; ll-ul) 1469, 95% CI (973 to 1965) steps/day (29% from baseline) and 10.4, 95% CI (6.1 to 14.7) min/day MPA (44% from baseline), p≤0.001 for all. PA increased in the patients in the IG (median (Q1–Q3) 4305 (2841–5851) to 4767 (3080–7949) steps/day; 14 (5–26) to 18 (6–48) min MPA) and decreased in the UCG (4643 (2932–6955) to 4059 (2624–6332) steps/day; 15 (5–35) to 14 (3–32) min MPA). Results were comparable for both accelerometers used (see online supplementary material and figure 3). Including centre in the analyses did not change the results.
Figure 3.
Changes in physical activity by group. Patients in the intervention group presented in solid line, and patients in the control group presented as dotted line. Data are presented as mean, 95% (ll to ul); Steps and time in at least moderate physical activity (MPA) analyses are based on Actigraph measurement and include 140 control and 140 intervention patients, Walking time and movement intensity (MI) are based on Dynaport Movemonitor measurement and include 132 control and 129 intervention patients. *indicates significant within-group changes (p<0.05). NS, not significant.
Patients in the IG were 4.44, 95% CI (2.38 to 8.29) times more likely to have a 1000 steps increase (51 (36%) vs 16 (11%) patients, p<0.001). Patients with a lower score on the mMRC (p=0.001), those with a better 6MWD (p=0.001) and patients in GOLD A-B (p=0.05) at baseline showed a larger intervention effect. There was no difference based on the baseline PA (p=0.48) or having comorbidities (p=0.30) (see figure 4).
Figure 4.
Between-group differences in mean step count, adjusted for baseline data. Data are presented for the different subgroups, based on baseline characteristics; Global Initiative for Chronic Obstructive Lung Disease (GOLD) quadrants based on modified Medical Research Council (mMRC) to classify symptoms; number of patients mMRC (<2 vs ≥2; 166 vs 114), 6-min walk distance (6MWD) (≥450 vs <450 m; 140 vs 138), comorbidity (<2 vs ≥2; 238 vs 42), GOLD quadrants (A–B vs C–D; 129 vs 151), steps (≥5000 vs <5000; 115 vs 165); data represented as mean between-group effect with 95% CI. Numbers indicate the percentage of patients of the subgroup in the control/intervention group. Analyses are based on 278 patients for 6MWD and 280 for mMRC, comorbidity, GOLD and physical activity subgroups.
Other outcomes
The analyses of the other outcomes are based on all patients who completed the study (n=159 in both groups) with data on both baseline and final visit; more information is given in table 2. The change in functional capacity was small but significantly different between IG and UCG (13.4, 95% CI (3.4 to 23.5) m, p=0.01). An improvement could be observed in the functional state domain of the CCQ (p=0.03). Other domains of the CCQ, QF or CAT scores were not affected by the intervention (see table 2).
Table 2.
Secondary outcomes
| Within-group | Between-group |
||||
|---|---|---|---|---|---|
| Variable | Baseline | 3 m | meanΔ 95% CI (ll to ul) | meanΔ 95% CI (ll to ul)* | p Value* |
| 6MWD (m) | |||||
| UCG | 450±106 | 449±118 | −0.81 (−7.7 to 6.1) | 13.4 (3.40 to 23.5) | 0.009 |
| IG | 444±106 | 457±108 | 12.7 (5.4 to 20.1) | ||
| QF (kg) | |||||
| UCG | 32.0±9.7 | 31.7±9.3 | −0.356 (−1.59 to 0.88) | 0.340 (−1.38 to 2.06) | 0.697 |
| IG | 31.7±11.0 | 31.8±10.3 | 0.102 (−1.35 to 1.55) | ||
| CAT (points) | |||||
| UCG | 13 (8–18) | 13 (9–20) | 1.09 (0.032 to 2.15) | −0.57 (−1.88 to 0.74) | 0.391 |
| IG | 13 (7–20) | 14 (9–19) | 0.62 (−0.346 to 1.59) | ||
| CCQmental state | |||||
| UCG | 1 (0–2) | 1 (0–2) | 0.198 (−0.116 to 0.51) | −0.133 (−0.326 to 0.061) | 0.178 |
| IG | 1 (0–2.5) | 1 (0–2.5) | −0.031 (−0.320 to 0.257) | ||
| CCQfunctional state | |||||
| UCG | 1.5 (0.75–2.5) | 1.75 (0.75–2.75) | 0.64 (0.060 to 1.23) | −0.203 (−0.382 to −0.024) | 0.026 |
| IG | 1.5 (1–2.75) | 1.5 (1–2.75) | −0.069 (−0.639 to 0.50) | ||
| CCQsymptoms | |||||
| UCG | 1.75 (1.5–2.75) | 2 (1.25–2.75) | 0.94 (0.281 to 1.59) | −0.085 (−0.263 to 0.094) | 0.352 |
| IG | 1.75 (1.25–2.5) | 1.75 (1.25–2.5) | 0.97 (0.378 to 1.56) | ||
Data are presented as mean±SD, median (Q1-Q3), within-group and between-group differences are presented as mean Δ 95% CI (ll to ul). Analyses are based on 299 (6MWD, 94% of completers), 243 (QF, 76% of completers), 317 (CAT, 100% of completers) and 316 (CCQ, 99% of completers) patients.
*Adjusted for baseline outcome.
6MWD, 6-min walk distance; CAT, COPD assessment test; CCQ, clinical COPD questionnaire; IG, intervention group; QF, quadriceps force; UCG, usual care group.
Adverse events and contact time
In total, 48 patients (30%) in the control and 43 patients (27%) in the IG experienced at least one exacerbation (p=0.54) during the trial, 5 of which led to hospitalisation. Lung function variables during the final visit were not different from baseline variables in either group.
Eleven musculoskeletal events were described in the IG compared with two in the UCG (p=0.01), none causing study discontinuation. Of those in the IG, only one event required treatment (knee inflammation). One patient experiencing back pain was advised to lower his PA in the acute situation. Another patient with back pain was observed for 1 day in hospital, without any treatment being initiated. Three events were judged as possibly related to the intervention by the investigators (two patients with back pain, one patient with a rib fracture), one event (back pain) was judged as unlikely related. Other adverse events, all not related to the study protocol, were cardiovascular problems (n=2), diagnosis of a toe melanoma (n=1), urinary problems (n=2), and gastrointestinal problems (n=3).
Patients in the IG who completed the trial were contacted for a median (Q1–Q3) of 6 (4–9) times (range 0–25 times), with a total time consumption for the investigator of 50 (30–95) min per patient (range 0–375 min). Seventy-five per cent of contacts were initiated by the application (‘flags’), the minority (25%) were patient driven. More details are available in the online supplementary material.
Discussion
Our study demonstrates the effectiveness of a 12-week semiautomated telecoaching intervention in increasing PA in a broad spectrum of COPD. Patients with a better exercise capacity and those with less symptoms had a more favourable response.
Strengths and weakness of the study
To the best of our knowledge, this is the first multicentre trial showing the effectiveness of a telecoaching intervention in patients with COPD using triaxial accelerometry as an outcome measure. The paper reports on the effect of the intervention on patients included in six centres across Europe, including primary, tertiary care and rehabilitation centres, which increases the generalisability of the results. This study included people with a wide spectrum of disease severity and a wide range of PA levels without excluding patients who were ‘active’ at the start of the programme nor those not able to leave the house, as can be observed by the representation of all mMRC scores. Because of these liberal inclusion criteria, the present trial is likely to be relevant to patients seen in routine clinical practice. By providing a smartphone, the inclusion did not depend on any previous experience, even in this elderly population. Only four patients dropped out of the study because they were not able to work with the smartphone application. It could be argued that the intervention would have attracted the more educated COPD population, leading to a possible problem of external validity. This would nevertheless not have changed the interpretation of the present results. The feasibility of using smartphones for wireless transmission (via 4G) of data in this population is in line with previous studies.29 30 The algorithm used to revise the targets every week was based on having at least four days of step counter data. Compliance was not an issue as patients wore the step counter >6 days out of 7. Whether the intervention translates to long-term maintenance of higher PA levels has not yet been investigated.
Some remarks should be made concerning the interpretation of our results. Based on the present study, we cannot state whether all component parts of this intervention were essential. Providing a step counter in combination with goal setting could potentially be equally effective.15 The present application however, using a more complex technology interface, allowed clinicians to have insight into the compliance of patients and intervene if patients were unable to cope with the preset goals. Another advantage of such interventions is the standardisation of the intervention, avoiding bias related to the investigator providing the coaching. This makes the intervention, on the one hand, feasible to investigate in a multicentre trial and, on the other hand, it is potentially applicable to routine clinical practice without specific expertise requirements. One could argue that the different parts of the intervention, or the combination of these, rather than the medium used to provide these (smartphone) were essential. In this case, the present intervention could be provided by any kind of modern communication (eg, internet-mediated via any interface). The present authors are convinced that the intervention can be adapted to the preference of communication given by the patient or patient population. It could as well be debated that the contact with the investigator rather the telecoaching could have resulted in the present results. However, this is unlikely as adding a behavioural intervention based on face-to-face contacts in patients following a rehabilitation programme did not result in an enhanced PA level.31
It is of interest that in both the IG and the UCG group the non-completers had a more severe disease judged by quadriceps strength, BMI and CAT score; moreover, those unable to undertake valid PA monitoring had a lower FEV1. This mirrors previous findings that these patients are less likely to complete an intervention.32 We acknowledge therefore that some patients with the most severe disease may need a more personal approach such as participation in a pulmonary rehabilitation programme before they engage in PA coaching programmes. The IG patients reported more musculoskeletal problems compared with the UCG. This information should be interpreted with some caution as the frequency of contact with patients in the IG was higher; patients in the UCG only reported adverse events at the end of the trial. Knowing that almost none of these events required any treatment, it may be that mild adverse events were therefore under-reported in the UCG. This bias could have been minimised by providing a diary to patients in the control group. On the other hand, minor transient musculoskeletal problems are not unexpected when sedentary people start an activity programme. PA is highly dependent on the season of measurement.33 Therefore, a limitation of the present trial is the narrow inclusion between June and December 2014. This can be seen as by the mean decrease in daylight over time, a proxy for season. This likely explains the decrease in PA seen in the control group. Although this influences the within-group results, it does not change the between-group comparisons as the groups were randomly assigned and therefore comparable (including change in daylight). Our results show that patients can be motivated to be active, even during periods of worse weather conditions. Lastly, neither patients nor investigators were blinded to treatment allocation. However, by using the objective PA measurement, the lack of blinding of investigator will not have influenced the primary end point. Nevertheless, we do acknowledge the lack of blinding could have minimally influenced the 6MWD results.
Comparison with other studies
Several studies have used step counters to provide real-time feedback on PA in combination with goal setting in comparable patient groups.14 15 17 The weighted mean effect of these studies was 1259 steps/day improvement over controls. One study, which used a pedometer intervention and monthly face-to-face meetings, showed a significantly larger effect.15 In that study, exercise capacity was relatively well preserved (463 m 6MWD), perhaps providing more window for improvement as there was a larger functional reserve.34 It is clear that coaching programmes with the specific aim of increasing PA lead to larger improvements in amount of PA compared with rehabilitation,35 and pharmacology.11 12 In the present study, patients in the IG were four times more likely to increase their PA by >1000 steps. This increment was defined a priori because it has been defined as the minimal important difference23 and because of its relation with the decreased risk for COPD-related hospitalisation or mortality.36 The present intervention was effective irrespective of patients' baseline PA being above or below 5000 steps. Shifting to more active PA provides additional health benefits in healthy elderly and special populations.3 This supports the concept that aiming for an increase in PA level to lift patients above the ‘sedentary lifestyle index’ of 5000 steps,22 or the ‘active’ threshold of 7500 steps,3 is important. The objective of this intervention was to enhance PA by empowering patients and motivating them to engage in more daily activity. It comes as no surprise that the intervention, while successful in enhancing PA, had only a minimal impact on exercise capacity and muscle strength. This observation is in line with others15 and is likely due to the relatively low intensity of PA, insufficient to enhance skeletal muscle function. Indeed, the time in MPA only increased by 10 min/day. This may yield health benefits but is not an adequate exercise training stimulus to incur in training effects in the peripheral muscle. Our findings are likely important from a healthcare perspective since the present intervention could easily be combined with a formal exercise training intervention or other interventions with a more direct impact on exercise capacity potentiating the effects of both interventions.
Future perspectives
The present intervention should be seen as a promising tool as an increased number of patients own a smartphone (mobile broadband is the most dynamic Information and communications technology (ICT) market segment with a 12-fold increase in users worldwide between 2007 and 201537). In the present study, patients were equipped with a smartphone. The intervention will become more clinically available and cost-effective if patients own their own smartphone device. It can be envisaged that this intervention can be embedded in a broader healthcare intervention using a smartphone (m-health). In any case, the coaching tools available on the market should be adapted for users with chronic diseases. Given the limited effect of this intervention on exercise capacity, the impact of delivering this telecoaching intervention in the context of a pulmonary rehabilitation programme or further optimised pharmacotherapy could be a future investigation.
Conclusion
In conclusion, the present study shows the effectiveness of a 12-week semiautomated telecoaching programme (application installed on a smartphone device) and a step counter providing direct feedback on total daily number of steps, walking time, time in at least moderate PA and movement intensity during walking in patients with COPD across the whole disease spectrum.
Acknowledgments
The authors acknowledge Novartis for their kind contribution and input in the protocol development, Anne Elie Carsin (CREAL, Barcelona) for the data verification and all patients involved in the design of the intervention and the participants of the study.
Footnotes
Twitter: Follow Nicholas Hopkinson @COPDdoc, The PROactive consortium @PROactiveCOPD, Thierry Troosters @Troosters, Heleen Demeyer @Demeyer_H, Judith Garcia-Aymerich @judithgarciaaym and Elena Gimeno-Santos @EleGim
Collaborators: Mr Papp PROactive study group: Thierry Troosters, Wim Janssens, Paul Van den Brande, Heleen Demeyer, Maarten Spruyt, Matthias Loeckx, Miek Hornikx (Leuven), Michael I Polkey, Nicholas S. Hopkinson, Rebecca Tanner, Yogini Raste, Sara Buttery (London), Ioannis Vogiatzis, Zafeiris Louvaris (Athens), Roberto Rabinovich, Claire Yerramasu, Noah Rubio (Edinburgh), Thys Van der Molen, Corina de Jong, Helma Oosterom (Groningen), Milo Puhan, Anja Frei, Gilbert Buesching, Alexandra Strassman, Martin Frey, Alexander Turk, Stephan Keusch, Alice Zürcher (Zurich), Judith Garcia-Aymerich, Ignasi Serra, Elena Gimeno-Santos (CREAL, Barcelona). PROactive consortium: The PROactive Consortium members are as follows. Nathalie Ivanoff: Almirall, Barcelona, Spain; Niklas Karlsson and Solange Corriol-Rohou: AstraZeneca AB, Mölndal, Sweden; Ian Jarrod: British Lung Foundation, London, UK; Damijen Erzen: Boehringer Ingelheim, Nieder-Ingelheim, Germany; Mario Scuri: Chiesi Farmaceutici S.A. Parma, Italy; Paul McBride: Choice Healthcare Solutions, Hitchin, UK; Nadia Kamel: European Respiratory Society, Lausanne, Switzerland; Margaret Tabberer: GlaxoSmithKline, Uxbridge, UK; Fabienne Dobbels,: Katholieke Universiteit Leuven, Leuven, Belgium; Pim de Boer: Netherlands Asthma Foundation, Amersfoort, The Netherlands; Enkeleida Nikai: UCB, Brussels, Belgium; Bill MacNee: University of Edinburgh, Edinburgh, UK.
Contributors: HD, ZL, AF, RR, CdJ, IV, JGA, MIP, MS, RT, CY, and TT contributed to the study protocol and development of the intervention. HD, ZL, AF, RR, CdJ, ML, SB, NR, MS, RT, CY, HO, GB, AS, and IV contributed to the data collection. HD, MP, JGA, IS, and TT contributed to the data analyses and interpretation of the data. HD, NH, MIP, JGA, MP, and TT contributed to the writing of the manuscript. HD, ZL, AF, RR, CdJ, EGS, ML, SB, NR, TVM, NH, IV, MIP, JGA, MP, and TT critically reviewed the manuscript. TT is the guarantor of the study. All authors had full access to all the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: The PROactive project is funded by the Innovative Medicines Initiative Joint Undertaking (IMU JU) #115011. The Leuven study group was supported by the Flemish Research Foundation (grant # G.0871.13). HD is the recipient of a joint ERS/SEPAR Fellowship (LTRF 2015). ZL is the recipient of an ERS fellowship (LTRF 2016). The Zurich study group was supported by an additional grant of the Lung League Aargau (non-profit organisation) as well as by Swisscom AG who provided 30 sim cards and data usage of up to 1 GB per month. MIP's contribution to this work was supported by the NIHR Respiratory Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College, London UK who part fund his salary.
Disclaimer: The views expressed in this publication are those of the authors and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health. The sponsors did not have any influence on the design, conduct and analysis of the study. TT affirms that the manuscript is an honest, accurate and transparent account of the study being reported. No important aspects of the study have been omitted and the study has been conducted as planned.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: This study was approved by the local ethics committee at each centre (Commissie medische ethiek van de universitaire ziekenhuizen KU Leuven (Leuven, S-55919); Medische ethische toetsingscommissie universitair medisch centrum Groningen (Groningen, Metc 2013.362); RES Committee London—South East (London and Edinburgh, 13/LO/1660); Scientific Council of the ‘Sotiria’ General Hospital for Chest Diseases (Athens, 27852/7-10-13); Kantonale Ethikkommission Zürich and Ethikkommission Nordwest- und Zentralschweiz (Zurich, KEK-ZH-Nr. 2013-0469 and EKNZ2014-192, respectively)).
Data sharing statement: Full data set and statistical coding are available with the corresponding author.
Contributor Information
Collaborators: on behalf of the Mr Papp PROactive study group and the PROactive consortium, Wim Janssens, Paul Van den Brande, Maarten Spruyt, Miek Hornikx, Rebecca Tanner, Yogini Raste, Claire Yerramasu, Helma Oosterom, Gilbert Buesching, Alexandra Strassman, Martin Frey, Alexander Turk, Stephan Keusch, Alice Zürcher, Ignasi Serra, Nathalie Ivanoff, Niklas Karlsson, Solange Corriol-Rohou, Ian Jarrod, Damijen Erzen, Mario Scuri, Paul McBride, Nadia Kamel, Margaret Tabberer, Fabienne Dobbels, Pim de Boer, Enkeleida Nikai, and Bill MacNee
References
- 1.Kraus WE, Bittner V, Appel L, et al. The National Physical Activity Plan: a call to action from the American Heart Association: a science advisory from the American Heart Association. Circulation 2015;131:1932–40. 10.1161/CIR.0000000000000203 [DOI] [PubMed] [Google Scholar]
- 2.Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219–29. 10.1016/S0140-6736(12)61031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act 2011;8:80 10.1186/1479-5868-8-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. (accessed 28 Jul 2016).
- 5.Van Remoortel H, Hornikx M, Demeyer H, et al. Daily physical activity in subjects with newly diagnosed COPD. Thorax 2013;68:962–3. 10.1136/thoraxjnl-2013-203534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gimeno-Santos E, Frei A, Steurer-Stey C, et al. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax 2014;69:731–9. 10.1136/thoraxjnl-2013-204763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaes AW, Garcia-Aymerich J, Marott JL, et al. Changes in physical activity and all-cause mortality in COPD. Eur Respir J 2014;44:1199–209. 10.1183/09031936.00023214 [DOI] [PubMed] [Google Scholar]
- 8.Shrikrishna D, Patel M, Tanner RJ, et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J 2012;40:1115–22. 10.1183/09031936.00170111 [DOI] [PubMed] [Google Scholar]
- 9.Watz H, Waschki B, Meyer T, et al. Physical activity in patients with COPD. Eur Respir J 2009;33:262–72. 10.1183/09031936.00024608 [DOI] [PubMed] [Google Scholar]
- 10.Cindy Ng LW, Mackney J, Jenkins S, et al. Does exercise training change physical activity in people with COPD? A systematic review and meta-analysis. Chron Respir Dis 2012;9:17–26. 10.1177/1479972311430335 [DOI] [PubMed] [Google Scholar]
- 11.Troosters T, Bourbeau J, Maltais F, et al. Enhancing exercise tolerance and physical activity in COPD with combined pharmacological and non-pharmacological interventions: PHYSACTO randomised, placebo-controlled study design. BMJ Open 2016;6:e010106 10.1136/bmjopen-2015-010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watz H, Mailänder C, Baier M, et al. Effects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: a randomised, placebo-controlled, crossover study (The MOVE Study). BMC Pulm Med 2016;16:95 10.1186/s12890-016-0256-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conn VS, Hafdahl AR, Brown SA, et al. Meta-analysis of patient education interventions to increase physical activity among chronically ill adults. Patient Educ Couns 2008;70:157–72. 10.1016/j.pec.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altenburg WA, ten Hacken NH, Bossenbroek L, et al. Short- and long-term effects of a physical activity counselling programme in COPD: a randomized controlled trial. Respir Med 2015;109:112–21. 10.1016/j.rmed.2014.10.020 [DOI] [PubMed] [Google Scholar]
- 15.Mendoza L, Horta P, Espinoza J, et al. Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J 2015;45:347–54. 10.1183/09031936.00084514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field MJ, Institute of Medicine CoECAoT. Telemedicine: a guide to assessing telecommunications in health care. Washington DC: National Academy Press, 1996. [PubMed] [Google Scholar]
- 17.Moy ML, Collins RJ, Martinez CH, et al. An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD: a randomized controlled trial. Chest 2015;148:128–37. 10.1378/chest.14-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middelweerd A, Mollee JS, van der Wal CN, et al. Apps to promote physical activity among adults: a review and content analysis. Int J Behav Nutr Phys Act 2014;11:97 10.1186/s12966-014-0097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinovich RA, Louvaris Z, Raste Y, et al. Validity of physical activity monitors during daily life in patients with COPD. Eur Respir J 2013;42:1205–15. 10.1183/09031936.00134312 [DOI] [PubMed] [Google Scholar]
- 20.Van Remoortel H, Raste Y, Louvaris Z, et al. Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PLoS ONE 2012;7:e39198 10.1371/journal.pone.0039198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demeyer H, Burtin C, Van Remoortel H, et al. Standardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation program. Chest 2014;146:318–27. 10.1378/chest.13-1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tudor-Locke C, Craig CL, Thyfault JP, et al. A step-defined sedentary lifestyle index: <5000 steps/day. Appl Physiol Nutr Metab 2013;38:100–14. 10.1139/apnm-2012-0235 [DOI] [PubMed] [Google Scholar]
- 23.Demeyer H, Burtin C, Hornikx M, et al. The minimal important difference in physical activity in patients with COPD. PLoS ONE 2016;11:e0154587 10.1371/journal.pone.0154587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport 2011;14:411–16. 10.1016/j.jsams.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 25.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 26.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows—report working party standardization of lung-function tests European-Community for Steel and Coal—official statement of the European Respiratory Society. Eur Respir J 1993;6(Suppl 16):5–40. 10.1183/09041950.005s1693 [DOI] [PubMed] [Google Scholar]
- 27.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428–46. 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 28.Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014;189:e15–62. 10.1164/rccm.201402-0373ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen HQ, Gill DP, Wolpin S, et al. Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. Int J Chron Obstruct Pulmon Dis 2009;4:301–13. 10.2147/COPD.S6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabak M, Vollenbroek-Hutten MM, van der Valk PD, et al. A telerehabilitation intervention for patients with Chronic Obstructive Pulmonary Disease: a randomized controlled pilot trial. Clin Rehabil 2014;28:582–91. 10.1177/0269215513512495 [DOI] [PubMed] [Google Scholar]
- 31.Burtin C, Langer D, van Remoortel H, et al. Physical activity counselling during pulmonary rehabilitation in patients with COPD: a randomised controlled trial. PLoS ONE 2015;10:e0144989 10.1371/journal.pone.0144989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 2016;71: 988–95. 10.1136/thoraxjnl-2016-208460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alahmari AD, Mackay AJ, Patel AR, et al. Influence of weather and atmospheric pollution on physical activity in patients with COPD. Respir Res 2015;16:71 10.1186/s12931-015-0229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langer D, Demeyer H, Troosters T, et al. The importance of physical activity for COPD health. ERS Monogr 2015;69:224–39. [Google Scholar]
- 35.Spruit MA, Pitta F, McAuley E, et al. Pulmonary rehabilitation and physical activity in patients with COPD. Am J Respir Crit Care Med 2015;192:924–33. [DOI] [PubMed] [Google Scholar]
- 36.Durheim MT, Smith PJ, Babyak MA, et al. Six-minute-walk distance and accelerometry predict outcomes in chronic obstructive pulmonary disease independent of Global Initiative for Chronic Obstructive Lung Disease 2011 Group. Ann Am Thorac Soc 2015;12:349–56. 10.1513/AnnalsATS.201408-365OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ICT facts and figures, the world in 2015. accessed on 2–7–2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2016-209026supp001.pdf (771.1KB, pdf)