Abstract
Background
Children (<15 years) are vulnerable to TB disease following infection, but no systematic review or meta-analysis has quantified the effects of HIV-related immunosuppression or antiretroviral therapy (ART) on their TB incidence.
Objectives
Determine the impact of HIV infection and ART on risk of incident TB disease in children.
Methods
We searched MEDLINE and Embase for studies measuring HIV prevalence in paediatric TB cases (‘TB cohorts’) and paediatric HIV cohorts reporting TB incidence (‘HIV cohorts’). Study quality was assessed using the Newcastle-Ottawa tool. TB cohorts with controls were meta-analysed to determine the incidence rate ratio (IRR) for TB given HIV. HIV cohort data were meta-analysed to estimate the trend in log-IRR versus CD4%, relative incidence by immunological stage and ART-associated protection from TB.
Results
42 TB cohorts and 22 HIV cohorts were included. In the eight TB cohorts with controls, the IRR for TB was 7.9 (95% CI 4.5 to 13.7). HIV-infected children exhibited a reduction in IRR of 0.94 (95% credible interval: 0.83–1.07) per percentage point increase in CD4%. TB incidence was 5.0 (95% CI 4.0 to 6.0) times higher in children with severe compared with non-significant immunosuppression. TB incidence was lower in HIV-infected children on ART (HR: 0.30; 95% CI 0.21 to 0.39). Following initiation of ART, TB incidence declined rapidly over 12 months towards a HR of 0.10 (95% CI 0.04 to 0.25).
Conclusions
HIV is a potent risk factor for paediatric TB, and ART is strongly protective. In HIV-infected children, early diagnosis and ART initiation reduces TB risk.
Trial registration number
CRD42014014276.
Keywords: Tuberculosis
Key messages.
What is the key question?
What effects do HIV infection and antiretroviral therapy (ART) have on TB risk in children?
What is the bottom line?
HIV infection increases the incidence of TB in children by a factor of around 8, increasing with degree of immunosuppression; ART reduces TB risk by around 70%, with protection continuing to increase over 1–2 years.
Why read on?
TB incidence in HIV-infected children is very high, but no systematic review has quantified the effects of HIV or ART on TB risk in children.
Introduction
Children are at high risk of progression to TB disease following infection with Mycobacterium tuberculosis, particularly children in the first 2 years of life, who often develop non-pulmonary forms of TB.1 The variety of presentation, difficulty in obtaining samples for laboratory testing and paucibacillary nature of disease mean that confirming a TB diagnosis in children can be challenging. This adds to difficulties in understanding the natural history and epidemiology of disease. Recent indirect approaches to burden estimation have used mathematical modelling of exposure and disease progression risks to predict paediatric TB incidence.2 WHO estimated that in 2014, 1 million children developed TB globally.3
Although programmes to prevent mother-to-child transmission (PMTCT) of HIV have reduced new cases of vertically infected infants, coverage is incomplete; in 2013, an estimated 199 000 (170 000–230 000) children were newly infected with HIV.4 Children experience more rapid HIV disease progression than adults, making them highly susceptible to opportunistic infections.5 Antiretroviral therapy (ART) can restore immune function and has enormously reduced morbidity and mortality among HIV-infected children. In 2015, WHO guidelines were revised, recommending all HIV-infected children should initiate ART, irrespective of clinical disease stage or degree of immunosuppression.6 However, ART coverage among children lags behind adults, with only one-third of eligible children currently on ART compared with two-thirds of adults.4
In adults, HIV is a known potent risk factor for developing TB, with incident rate ratios (IRR) >5 when averaged across all levels of immunodeficiency.7 Evidence synthesis suggests an exponential increase in the IRR with decrease in CD4 T-cell counts.8 9 The effect of ART in reducing TB risk in adults living with HIV infection is well described.10 This quantitative understanding of the effects of HIV and ART on TB progression has been widely used by modellers, for example, in predicting the impact of HIV interventions on TB incidence.9 11 12 In contrast to adults, the impact of HIV infection and ART on TB progression in children is poorly quantified; no systematic reviews have been performed to evaluate this relationship. In the context of revised WHO treatment recommendations, our objective was therefore to systematically review the paediatric HIV/TB literature to quantify the effect of HIV and ART on TB risk in children.
Methods
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses statement13 (see online supplementary material). A protocol was registered with PROSPERO (identification number: CRD42014014276) in October 2014.
thoraxjnl-2016-209421supp001.pdf (6.6MB, pdf)
Cohort definitions
For all studies, the exposure/intervention was HIV infection, with or without ART. We sought studies that either: (i) reported HIV prevalence in children with TB (‘TB cohorts’) or (ii) reported TB disease incidence in cohorts of children with HIV (‘HIV cohorts’). For TB cohorts, the outcome was HIV prevalence, and the comparator was HIV prevalence in control groups of children without TB. For HIV cohorts, the outcome was TB incidence, and the comparison was between subgroups of the same cohort with different levels of immune suppression, or ART exposure (all HIV-infected).
Search strategy, selection criteria, data extraction
To be eligible, a study had to present empirical data on more than five cases of TB in children (aged <15 years). Studies were excluded if they were not generally representative of paediatric TB cases in that population at that time, for example, studies that only addressed one form of TB; that only comprised hospitalised TB cases; studies explicitly focused on migrant populations or autopsy studies. If the same cohort was published more than once, or individuals were described in more than one cohort, studies/children were only included once. Where explicit use of isoniazid preventive therapy (IPT) was stated, children receiving IPT were excluded. TB cohorts were excluded if the coverage of HIV testing was below 70% among TB cases. HIV cohorts were excluded if data were not reported that could be interpreted as an incidence (ie, number of events and person-time).
MEDLINE and Embase were systematically searched without language, publication or date restrictions using terms designed to capture children and TB and (HIV or ART) on 20 December 2014 (see online supplementary material for full search). A combination of MeSH and EMTREE headings were used with free-text terms to enhance the sensitivity of the search. We further searched the Cochrane Controlled Trials Register and AIDSinfo for relevant studies. All references in review articles found by our database search were included. All citations listed in Google Scholar of the five most cited review articles found by our database search were screened. One additional study in press at the time of our systematic review was brought to our attention after presenting draft results.14 Two investigators (PJD and JAS) independently assessed titles and abstracts for inclusion. Full texts were independently assessed for inclusion and study type by PJD and JAS with disagreements resolved by discussion. Two investigators extracted the data for TB cohorts in tandem; PJD extracted the data for HIV cohorts with 25% of data checked by JAS.
For both study designs, the following were recorded: first author, publication year, study years, study country, number of TB cases, age range, number of male children. For TB cohorts, if local TB-free controls were reported in the study, the HIV prevalence among control children (numerator and denominator) was extracted. Other data extracted from the TB cohorts included: the coverage of HIV testing, the numerator and denominator of HIV prevalence in TB cases and the UNAIDS paediatric HIV estimate for corresponding country-year (if available; mid-point and uncertainty range). For HIV cohorts, the following were recorded: TB incidence (with uncertainty range), ART coverage at enrolment, estimate of protective effect of ART against TB (if present) and TB incidence in any ART-related or immune-related stratum (with uncertainty range). Co-trimoxazole use was recorded where described. TB incidence by age band was extracted if present. Some studies recorded immune status using the WHO immunological classification6 (not-significant, mild, advanced and severe immunosuppression), whereas others used alternative CD4% or CD4 count categories, which differed between studies. Mid-points and CIs (assumed Poisson exact) were used to infer event numbers and person-time when aggregation was necessary. Some studies excluded incident TB within the first few months on ART, stating that they sought to exclude immune reconstitution inflammatory syndrome reactions and only determine true TB incidence.
Quality of individual studies was assessed using an adapted Newcastle-Ottawa quality assessment tool.15 The version for case-control studies was used for TB cohorts; the version for cohort studies for HIV cohorts. For HIV cohorts, the quality for each study as input to each meta-analysis was assessed separately (see online supplementary material pp. 18–21) and reported as low/moderate/high quality (depending on whether few/some/most criteria were met) on domains of selection, comparability and either outcome (for cohort studies) or exposure (for case-control studies).
Statistical analyses
For TB cohorts, we undertook a random-effects meta-analysis of the OR for HIV prevalence given TB among those studies reporting HIV prevalence in controls. This OR can be interpreted as an IRR for developing TB disease if HIV positive7 (see online supplementary material p. 7). We produced funnel plots to assess evidence of publication bias.
For TB cohorts where UNAIDS estimates of national HIV prevalence in the age group aged <15 years were available for the same year and country as the study, we undertook a Bayesian meta-analysis using both the UNAIDS data and control data. This analysis modelled the relationship between UNAIDS HIV prevalence and HIV prevalence in study controls from studies where both were available, and used this relationship to predict individual study ORs where controls were not available. For comparison, a Bayesian version of the meta-analysis of studies with controls was conducted (see online supplementary material pp. 8–11).
For HIV cohorts reporting TB incidence by immune stage, we undertook a random-effects meta-analysis of the IRR for each stratum relative to the ‘not-significant’ WHO immune stage. For HIV cohorts reporting incidence by >1 CD4% category (using the mid-point of the CD4% category in which the incidence was reported), or an analysis of the relation between CD4% and IRR for TB, we undertook a Bayesian meta-analysis to estimate the gradient of logarithmic IRR with respect to CD4% (see online supplementary material pp. 13–15). We averaged the IRR implied by this point estimate over CD4% between 0% and 50% to compare with our IRR from the TB cohort analysis. For HIV cohorts reporting an estimate of the protection against TB from ART as a HR, we performed a random-effects meta-analysis. For HIV cohorts reporting TB incidence by >1 category of time-since-ART-initiation, we fitted a non-linear mixed-effects model to estimate the temporal dependence of ART protection, using the mid-point of the time window in which the incidence was reported to model incidence (see online supplementary material pp. 15–16).
Bayesian techniques were employed where greater flexibility was required over standard software implementations to use all the data. All statistical analyses were undertaken using R statistical software (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org); random-effects meta-analysis was performed using the rma command in the metafor package implementing the restricted maximum-likelihood estimator.16
Results
Search results
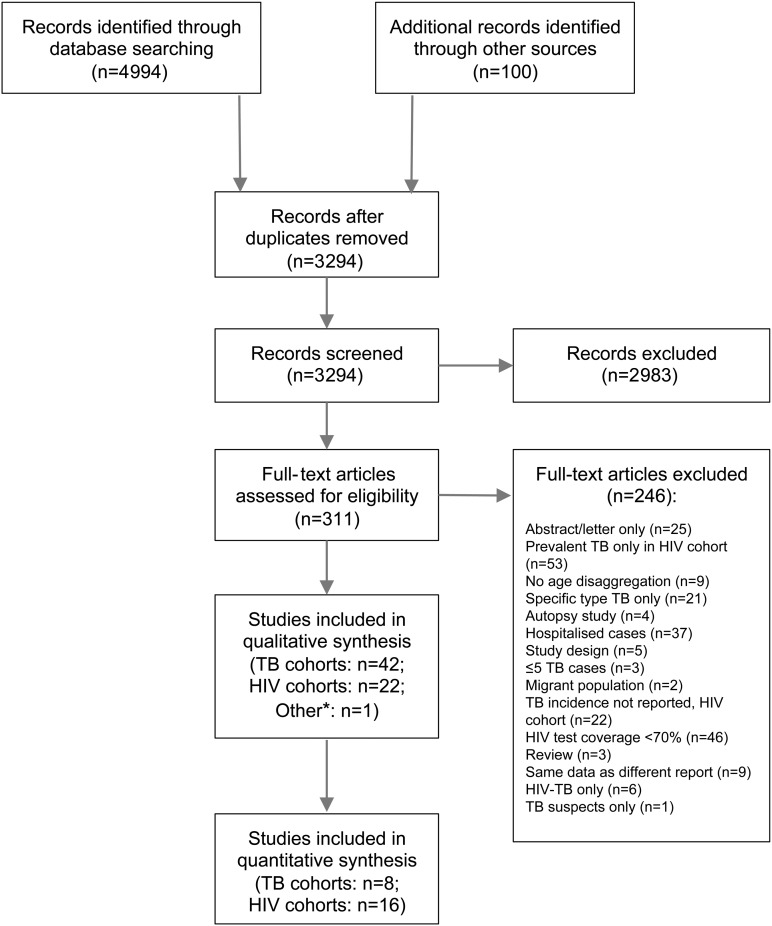
The systematic review process is presented in figure 1. Of the 311 full-text articles screened, 65 were included in this review. The most common reasons for exclusion were HIV test coverage <70% for TB cohorts (n=46) and TB incidence not reported for HIV cohorts (n=22). Of the 65 included studies, 42 were classified as TB cohorts (see table 1), 22 were classified as HIV cohorts (see table 2) and 1 study included both a TB and HIV cohort.17 Thirty-one of the 42 TB cohorts (74%) and 17 of the 22 HIV cohorts (77%) were from sub-Saharan Africa.
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-Analyses flow chart for systematic review (*one study included both a TB and HIV cohort17).
Table 1.
TB cohorts
| First author, year | Country | Years of study | Study description | Control HIV tested | Control with HIV | Children with TB | TB cases tested for HIV | TB cases with HIV | TB cases male | Age range | Quality assessment* (selection/comparability/exposure) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ade, 201318 | Benin | 2009–2011 | Cross-sectional study reviewing of all children treated for TB in one city over a 3-year period | na | na | 182 | 167 | 49 | 88 | 0–14 | C/-/C |
| Berggren Palme, 200419 | Ethiopia | 1995–1997 | Prospective study recruiting all children investigated for TB at one hospital over a 13-month period | na | na | 355 | 355 | 54 | ns | 0–14 | B/-/C |
| Berggren Palme, 200120 | Ethiopia | 1995–1997 | Case-control study, prospectively recruiting all children diagnosed with TB over a 1-year period at one children’s hospital, with a control group recruited concurrently among children undergoing elective surgery | 122 | 2 | 377 | 377 | 47 | 186 | 0–14 | B/A/A |
| Bhat, 199321 | Zambia | 1991–1991 | Case-control study, prospectively recruiting all children diagnosed with TB over a 9-month period at one hospital, with a control group recruited from outpatients clinics and among surgical inpatients | 134 | 18 | 116 | 96 | 36 | 58 | 0–14 | B/C/A |
| Bobossi-Serengbe, 200522 | Central African Republic | 1998–2000 | Retrospective analysis of all children treated for TB over a 2-year period in one hospital clinic | na | na | 284 | 284 | 211 | 153 | 1–14 | C/-/C |
| Campos-Herrero Navas, 199723 | Gran Canaria | 1986–1994 | Retrospective analysis of all children treated for TB over a 9-year period on the island | na | na | 49 | 49 | 1 | ns | 0–14 | B/-/C |
| Cathebras, 199824 | Central African Republic | 1997–1998 | Prospective study in which all patients (adults and children) treated for TB over a 3-month period at one hospital were tested for HIV | na | na | 37 | 37 | 4 | ns | 0–14 | B/-/C |
| Chintu, 199325 | Zambia | 1990–1991 | Case-control study of all children treated for TB over an 18-month period in one teaching hospital. Controls were recruited from the emergency department or inpatient surgical wards | 242 | 26 | 265 | 237 | 88 | 125 | 0–15 | B/C/A |
| Chintu, 199826 | Zambia | 1992–1993 | Case-control study of all patients (adults and children) presenting with diarrhoea over an 8-month period at one teaching hospital. Cases were HIV-infected with controls HIV-uninfected. Paediatric analysis restricted to children under 5 years | na | na | 4 | 4 | 3 | ns | 1–5 | C/-/C |
| Chintu, 199527 | Zambia | 1990–1991 | Prospective study of all children under 5 years admitted to the ward of a teaching hospital. Comparisons made between children with HIV and children without HIV, with respect to proportion with TB | 160 | 15 | 61 | 61 | 42 | ns | 0–5 | B/C/A |
| Daniel, 200728 | Nigeria | 1999–2003 | Retrospective study of all children diagnosed with TB at one TB centre over a 5-year period | na | na | 76 | 76 | 8 | 46 | 1–14 | A/-/A |
| Edwards, 200729 | Democratic Republic of Congo | 2002–2003 | Retrospective study of all children treated for TB at one hospital over a 1 year period | na | na | 110 | 91 | 42 | ns | 0–15 | B/-/C |
| Espinal, 199630 | Dominican Republic | 1991–1994 | Prospective study of all children aged 18–59 months treated for TB at two institutions over a 3-year period | na | na | 204 | 189 | 11 | 92 | 1–5 | B/-/C |
| Feldacker, 201231 | Malawi | 2008–2010 | Retrospective study of all patients (adults and children) treated for TB at one TB treatment centre over a 3-year period | na | na | 364 | 338 | 148 | 168 | 0–14 | C/-/C |
| Gava, 201332 | Brazil | 1997–2006 | Retrospective review of routinely collected data for all children treated for TB in one state over a 10-year period | na | na | 356 | 356 | 3 | 183 | 0–14 | C/-/C |
| Geoghagen, 200433 | Jamaica | 1999–2002 | Retrospective review of all children 0–12 years treated for TB at one hospital over a 4-year period | na | na | 26 | 24 | 11 | 16 | 0–12 | C/-/C |
| Henegar, 201334 | Democratic Republic of Congo | 2006–2007 | Prospective study of all patients (adults and children) starting treatment for TB at 14 clinics over a 17-month period | na | na | 830 | 701 | 59 | 398 | 0–14 | C/-/C |
| Hesseling, 200935 | South Africa | 2004–2006 | Prospective laboratory data collected from 3 hospitals over a 3-year period, used to estimate the incidence of TB in infants (<12 months) with HIV and without HIV | na | na | 245 | 175 | 53 | 133 | 0–1 | C/-/C |
| Hussain, 200736 | India | 2003–2004 | Prospective study of all children admitted to one hospital for the treatment of TB, over a 2-year period | na | na | 270 | 270 | 23 | 154 | 0–15 | C/-/C |
| Iriso, 200537 | Uganda | 2003 | Cross-sectional study of all children aged 2–60 months, investigated for TB at one hospital over a 12-week period | na | na | 126 | 126 | 62 | 63 | 0–5 | B/-/C |
| Jain, 201338 | India | 2010–2012 | Prospective study of all children <5 years investigated for suspected TB, at one hospital over a 20-month period | na | na | 26 | 21 | 6 | 15 | 0–5 | C/-/C |
| Jensen, 201239 | Spain | 1997–2008 | Case-control study. Details of all HIV-infected children (<17 years) hospitalised anywhere in the country over a 12-year period were extracted from a central database. 4 HIV-uninfected controls, matched on age and gender, were extracted for each case. Rates of mycobacterial diseases were compared between cases and controls | na | na | 30 | 30 | 20 | ns | 0–17 | C/-/C |
| Llerena, 201040 | Colombia | 2001–2009 | Cross-sectional study of data from one laboratory of all children with culture-confirmed TB diagnosed over an 8-year period | na | na | 128 | 128 | 7 | 62 | 0–14 | C/-/C |
| Luo, 199441 | Zambia | 1991–1992 | Prospective study of all children treated for TB at one hospital over an 8-month period. Controls were children with traumatic injuries, selected from the emergency department or from the surgical wards | 167 | 16 | 120 | 110 | 67 | 70 | 0–14 | B/C/A |
| Madhi, 199942 | South Africa | 1996–1997 | Prospective study of all children (2 months to 12 years) treated for TB at hospitals attached to an academic department of paediatrics over a 5-month period | na | na | 130 | 130 | 52 | 85 | 0–12 | C/-/C |
| Mehta, 201143 | Tanzania | 2005–2007 | Randomised controlled trial of multivitamin supplementation in children with TB. All children (6 weeks to 5 years) treated for TB at one clinic were included and randomised over a 30-month period | na | na | 255 | 255 | 87 | 139 | 0–5 | B/-/C |
| Miranda, 201144 | Brazil | 2000–2006 | All cases of paediatric TB recorded on a state register over a 6-year period were included. Matching with the state AIDS database then performed | na | na | 411 | 411 | 27 | 191 | 0–14 | C/-/C |
| Mukadi, 199745 | Côte d’Ivoire | 1994–1995 | Prospective study of all children (0–9 years) diagnosed with TB in two TB centres and two hospitals over a 21-month period | 161 | 0 | 161 | 160 | 31 | 84 | 0–9 | A/A/A |
| Berggren Palme, 200246 | Ethiopia | 1995–1997 | Prospective study of all children diagnosed with TB at the outpatient clinic or from the inpatient wards at one paediatric hospital over a 13-month period | na | na | 517 | 517 | 58 | 259 | 0–14 | B/-/C |
| Panigatti, 201447 | India | 2009–2010 | Prospective study of all children (0–12 years) diagnosed with TB at one hospital over an 18-month period, treated using a DOTS regimen | na | na | 93 | 93 | 7 | 45 | 0–12 | C/-/C |
| Rachow, 201248 | Tanzania | 2008–2010 | Prospective study of all children diagnosed with TB at one research facility over a 31-month period | na | na | 164 | 164 | 84 | 86 | 0–14 | C/-/C |
| Ramos, 201049 | Ethiopia | 2007 | Retrospective data collection from TB registers and treatment cards of all patients (adults and children) treated for TB over a 10-year period at one private hospital† | na | na | 187 | 158 | 149 | ns | 0–14 | C/-/C |
| Rose, 201250 | Tanzania | 2008–2010 | Prospective study of all children with suspected TB at one district hospital over a 27-month period | 93 | 22 | 33 | 33 | 18 | 24 | 0–14 | A/C/A |
| Sassan-Morokro, 199451 | Côte d’Ivoire | 1989–1990 | Prospective study of all children diagnosed with TB at two outpatient treatment centres over an 18-month period | na | na | 289 | 289 | 34 | ns | 1–14 | B/-/C |
| Schaaf, 201452 | South Africa | 2009–2011 | Retrospective study of all children (<13 years) with culture-confirmed TB at one teaching hospital over a 2-year period | na | na | 340 | 288 | 63 | 177 | 0–13 | C/-/C |
| Seddon, 201253 | South Africa | 2007–2009 | Retrospective study of all children (<13 years) with culture-confirmed TB at one teaching hospital over a 2-year period | na | na | 294 | 217 | 63 | 156 | 0–13 | C/-/C |
| Shahab, 200454 | India | 1999–2000 | Prospective study of all children (<12 years) treated for TB in the outpatient and inpatient departments of one tertiary hospital over a 17-month period | na | na | 250 | 250 | 5 | 174 | 0–12 | C/-/C |
| Thomas, 201455 | South Africa | 2009–2010 | Prospective study of all children (6 months to 12 years) with possible probable or confirmed TB recruited from outpatient and inpatient settings of one district hospital over a 17-month period | na | na | 33 | 26 | 17 | 19 | 0–12 | B/-/C |
| Yassin, 201156 | Ethiopia | 2009 | Cross-sectional study of all children (1–15 years) with TB symptoms and a TB source case who were investigated at two health centres and one hospital. TB-exposed but asymptomatic and unexposed children were also recruited as controls. | 153 | 3 | 164 | 141 | 14 | 105 | 1–15 | A/C/B |
| Kwara, 201657 | Ghana | 2012–2014 | Prospective pharmacokinetic study of all children (3 months to 14 years) starting treatment for TB disease at one teaching hospital over a 2-year period | na | na | 62 | 62 | 28 | 32 | 0–14 | C/-/C |
| López-Varela, 201558 | Mozambique | 2011–2012 | Prospective study from one heath district where all children (<3 years) with presumptive TB were recruited over a 12-month period | na | na | 32 | 32 | 18 | 15 | 0–3 | B/-/C |
| Perfura Yone, 201259 | Cameroon | 2005–2010 | Retrospective study all children (<15 years) diagnosed with TB and treated as inpatients at one hospital over a 5½-year period | na | na | 101 | 101 | 25 | 50 | 0–15 | B/-/C |
*Adapted Newcastle-Ottawa score for case-control studies with some questions (and the comparability domain) not applicable to studies without controls: A=high quality; B=moderate quality; C=low quality; see online supplementary material pp. 18–19, 24–25.
†Data from years with high enough HIV testing rates used; age range obtained where stated, for eligibility otherwise.
na, not applicable; ns, not stated.
Table 2.
HIV cohorts
| First author, year | Country | Years of study | Study description | Number of TB cases | Number in cohort | Patient-years | TB incidence, per cent per year (95% CI) | ART at enrolment (%) | Number male | Age range | Quality, TB incidence (selection/outcomes)* | Quality, immunosuppression (selection/comparability/outcomes)* | Quality, CD4% (selection/comparability/outcomes)* | Quality, time-on-ART (selection/comparability/outcomes)* | Quality, ART protection (selection/comparability/outcomes)* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abuogi, 201360 | Kenya | 2009–2010 | Prospective cohort study of HIV-infected children (6 weeks to 14 years, some on ART and some not) followed-up for the incidence of clinically diagnosed TB. Study at two urban clinics with children recruited over a 2-year period | 10 | 686 | 720 | 1.39 (0.75 to 2.58) | 74 | 322 | 0–14 | B/A | B/C/A | na | na | na |
| Alarcón, 201261 | Brazil | 2002–2007 | Prospective cohort study of HIV-infected children (<21 years) at 17 sites in Latin America; children recruited over a 5-year period and followed for the incidence of a number of opportunistic infections, including TB | 7 | 731 | 2523 | 0.28 (0.07 to 0.48) | 67 | 325 | 0–22 | B/B | na | na | na | na |
| Auld, 201462 | Côte d'Ivoire | 2004–2008 | A nationally representative retrospective cohort study of children (<15 years) starting ART over a 5-year period at 29 facilities | 56 | 1960 | 4190 | 1.34 (1.03 to 1.74) | 100 | 1058 | 0–14 | C/C | na | C/A/C | B/C/C | na |
| Bakeera-Kitaka, 201163 | Uganda | 2003–2006 | Retrospective cohort study of children and adolescents starting ART over a 3½-year period at one specialist clinic | 311 | 1806 | 1690 | 18.4 (17.1 to 20.3) | 100 | 932 | ns | C/C | na | na | B/C/C | B/A/C |
| Braitstein, 200964 | Kenya | 2001–2007 | Retrospective observational study of all children (<13 years) enrolled in a network of HIV clinics over a 5-year period | 765 | 6301 | 4368 | 17.6 (16.3 to 18.8) | 19 | 3144 | 0–13 | C/C | na | na | B/C/C | B/A/C |
| Brennan, 201465 | South Africa | 2004–2011 | Retrospective cohort of all children (<19 years) on ART enrolled over a 7-year period at 12 urban clinics | 113 | 3329 | 2828 | 4.0 (3.3 to 4.8) | 100 | 1608 | 0–18 | C/C | na | C/A/C | B/A/C | na |
| Ciaranello, 201466 | East Africa | 2002–2008 | All HIV-infected infants (<12 months) enrolled over a 6-year period at 7 sites across 3 countries | 128 | 847 | 738 | 17.4 (14.5 to 20.6) | 0 | 418 | <1 | C/C | na | C/C/C | na | na |
| Crook, 201614 | Uganda, Zimbabwe | 2007–2012 | Randomised controlled trial assessing different monitoring strategies for providing ART. All HIV-infected children (3 months to 17 years) enrolled from 4 centres in 2 countries over an 18-month period | 69 | 969 | 3632 | 1.9 (1.5 to 2.4) | 100 | 488 | 0–17 | B/B | na | B/A/B | A/C/B | na |
| Curtis, 201267 | Eight countries | 2002–2010 | Data from 25 HIV treatment centres in 8 countries collected prospectively over an 8-year period. Adults and children included if starting ART at one of the centres. Data from children (<15 years) disaggregated from adults | 140 | 3946 | 1687 | 8.3 (8.1 to 8.6) | 100 | ns | 0–15 | C/C | na | na | B/C/C | na |
| Dankne, 200168 | USA | 1988–1998 | Data from 13 studies over a period of 10 years with children (<21 years) included. From pre-ART period. Wide range of opportunistic infections assessed | 27 | 3331 | 6750 | 0.4 (0.3 to 0.6) | 0 | 1852 | 0–21 | C/C | na | C/C/C | na | na |
| De Beaudrap, 201369 | Burkina Faso/Côte d'Ivoire | 2006–2007/2000–2004 | Pooled data from a clinical trial (recruited over 1 year) and observational cohort (recruited over 4 years). Children (15 months to 15 years) enrolled at initiation of ART and followed for 2 years | 10 | 188 | 355 | 2.9 (1.4 to 5.1) | 100 | 66 | ns | C/A | na | C/C/A | B/C/A | na |
| Edmonds, 200970 | Democratic Republic of Congo | 2004–2008 | Prospective study of children (<18 years) enrolled from one hospital clinic over a 3½-year period. All children ART-naïve at baseline with some starting ART during follow-up | 81 | 364 | 596 | 13.6 (10.8 to 16.9) | 0 | 172 | 0–18 | B/B | B/C/B | na | A/C/B | A/A/B |
| Gray, 201471 | South Africa | 2005–2011 | Control (placebo) arm of a randomised controlled trial of isoniazid preventive therapy. Children (over 8 weeks) recruited over a 4-year period from three sites | 7 | 82 | 240 | 2.9 (1.2 to 6.0) | 100 | 42 | ns | A/B | na | na | na | na |
| Kouakoussui, 200472 | Côte d'Ivoire | 2000–2003 | All children recruited from one clinic and followed-up for incident TB. All children in the study initiated ART at some point in the study to provide pre-ART and post-ART TB incidences | 7 | 282 | 206 | 3.4 (1.5 to 6.7) | 65 | 153 | 1–16 | C/B | na | C/C/C | na | na |
| Li, 201373 | Tanzania | 2004–2011 | Prospective study of all children (<15 years) from 28 clinics recruited over a 7-year period. ART started based on local guidelines over follow-up | 376 | 5040 | 7221 | 5.2 (4.7 to 5.8) | 51 | 2460 | 0–14 | B/C | B/A/C | na | A/A/C | A/A/C |
| Martinson, 200974 | South Africa | 1994–2006 | Retrospective cohort of children (<15 years) recruited from four clinics over a 12-year period. Incidence determined for the period pre-ART and post-ART | 281 | 1132 | 1713 | 12.3 (9.8 to 12.6) | 2 | 563 | 0–14 | B/C | na | na | na | A/A/C |
| Prasitsuebsai, 201475 | SE Asia | 1993–2009 | Retrospective and prospective cohort of HIV-infected children (≤18 years) from 14 clinics in 5 countries in SE Asia. Recruited over a 16-year period. Wide range of OIs assessed. Period covered before ART, during monotherapy or dual-therapy and on HAART | 477 | 2280 | 1826 | 21.5 (19.4 to 23.7) | 7 | 1136 | 0–18 | C/C | na | na | na | na |
| Thomas, 200076 | USA (New York City) | 1989–1995 | Retrospective study in HIV-exposed and HIV-infected children enrolled in a longitudinal study. TB cases determined by review of clinical records and by linkage to local TB registry. Registry data assessed for a 6-year period | 45 | 1426 | 7384 | 0.61 (0.44 to 0.82) | 0 | 475 | 0–12 | B/B | na | na | na | na |
| Walters, 200877 | South Africa | 2003–2005 | Chart review of all children (<13 years) starting HAART at one hospital over a 3-year period and followed-up until the end of the study period for incident TB. Cases defined as pre-HAART (if in the 9 months prior to HAART initiation) or post-HAART | 10 | 290 | 546 | 25.1 (21.1 to 29.6) | 0 | 142 | 0–14 | C/A | na | na | na | na |
| Walters, 201478 | South Africa | 2003–2010 | Retrospective cohort of all children (<2 years) starting HAART at one hospital over an 8-year period. Children divided into those developing TB-IRIS (developing TB with 3 months of starting HAART) and those with incident TB (developing TB after 3 months) | 55 | 341 | 855 | 25.3 (22.0 to 28.9) | 0 | 161 | <2 | B/C | na | na | A/C/C | na |
| Yirdaw, 201479 | Ethiopia | 2007–2010 | Retrospective cohort study of the electronic records of all patients (adults and children) starting ART over a 3-year period in five hospitals in one region of Ethiopia. Patients followed until the end of the study period and the interventions of ART and IPT evaluated. Incidence stratified by age enabling extraction children (<15 years) | 23 | 475 | 1099 | 2.1 (1.3 to 3.1) | ns | ns | 0–15 | B/C | na | na | na | A/C/C |
| Zar, 200780 | South Africa | 2003–2004 | Double-blind randomised controlled trial evaluating the impact of IPT on TB incidence in HIV-infected children (aged older than 8 weeks). Children recruited from two sites with the trial stopped after 17 months. Data on TB incidence taken from placebo arm | 13 | 131 | 56 | 23.4 (12.4 to 50.0) | 8 | 74 | 0–14 | C/C | na | na | na | na |
*Adapted Newcastle-Ottawa tool for cohort studies: A=high quality; B=moderate quality; C=low quality; see online supplementary material pp. 19–21, 26–27.
ART, antiretroviral therapy; HAART, highly-active antiretroviral therapy; IPT, isoniazid preventive therapy; IRIS, immune reconstitution inflammatory syndrome; OIs, opportunistic infections; na, not applicable; ns, not stated.
Of the TB cohorts, 8 included HIV prevalence in controls without TB and could be used in the random-effects meta-analysis; 35 had relevant UNAIDS estimates and could be included in the Bayesian meta-analysis, including all 8 studies with controls.
Of the HIV cohorts, 7 could be included in the meta-analysis of CD4% influence, 3 could be included in the analysis of the influence of immunological staging, 10 were included in the analysis of time on ART and 6 were included in the pooled estimate of ART efficacy against TB. Only three HIV cohorts reported co-trimoxazole use.14 67 80
HIV prevalence in TB cohorts ranged between 5% and 94% (see online supplementary material p. 5). TB incidence in HIV cohorts ranged from 0.3 to 25.3 per 100 person-years (see online supplementary material p. 6). Insufficient data were available in either group of cohorts to stratify results by age.
Quality assessment
TB cohorts were of low and moderate quality for our analysis (table 1). Studies without controls could not be classed higher than moderate quality on selection and low quality on exposure. Controls were often drawn from clinic populations in the same context. In around half of studies, TB was defined only by a decision to treat.
HIV cohorts were typically of low quality for our analyses (table 2). Most studies were clinic-based and not necessarily community-representative; prevalent TB was not always ruled out at baseline; there were often shortcomings in follow-up or case definitions for TB.
Meta-analyses for TB cohorts
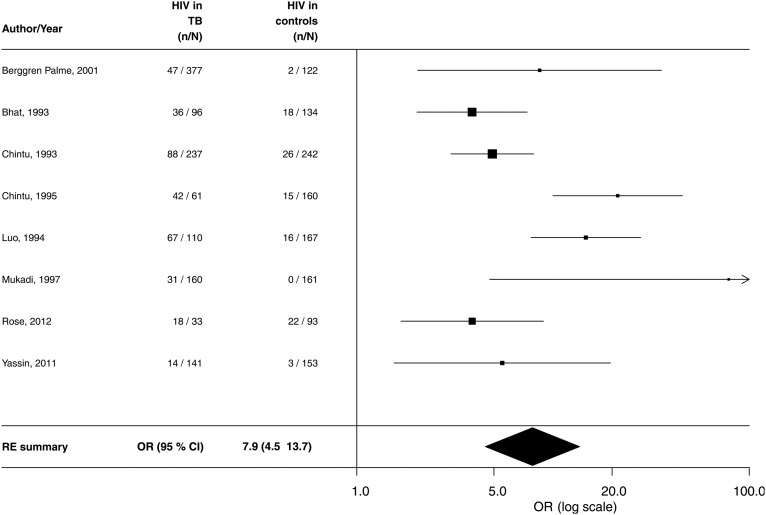
The random-effects meta-analysis of TB cohorts for the odds of having HIV in TB cases included eight case-control studies (figure 2). In total, 1215 TB cases and 1232 non-TB controls were included. The pooled estimate for the OR was 7.9 (95% CI 4.5 to 13.7), and the I-squared heterogeneity statistic (I2) was 69.8% (see figure 2). A funnel plot corresponding to this analysis is presented in online supplementary material p. 8.
Figure 2.
Forest plot for meta-analysis of HIV risk in children aged <15 years with prevalent TB—studies with controls (I2=69.8%). RE, random effects.
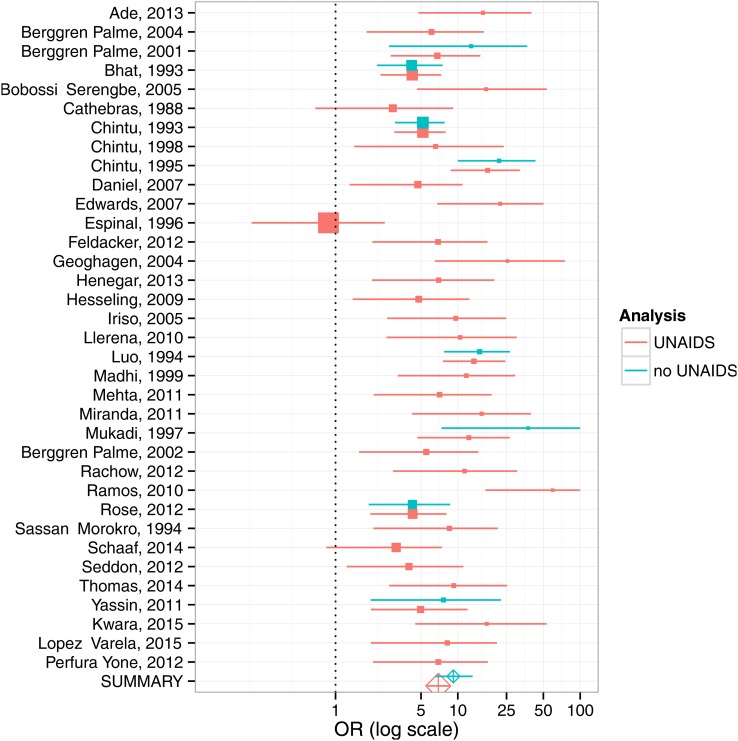
The Bayesian meta-analysis included 35 studies (including all 8 with controls) and produced a pooled estimate of the OR for HIV among children with TB of 7.0 (95% credible interval (CrI): 5.7–8.5), see figure 3. This analysis also found the paediatric HIV prevalence in controls to be substantially higher than national UNAIDS estimates of HIV prevalence in the age group aged <15 years, with an OR of 7.3 (95% CrI: 5.9 to 8.8). For studies that lacked explicit control groups (indicated in red in figure 3), this analysis also predicted what the ORs would have been for HIV prevalence in TB cases versus HIV prevalence in a putative control group of comparable children without TB. The pooled estimates of effect for the analyses with and without the UNAIDS data were similar (see online supplementary material p. 11).
Figure 3.
Forest plot for Bayesian meta-analysis of HIV risk in children aged <15 years with prevalent TB. Where studies lacked their own controls, UNAIDS national HIV prevalence data were used to model HIV prevalence in controls based on those studies with both controls and UNAIDS estimates (red). Meta-analyses for studies with controls only are shown in blue; meta-analyses for studies using UNAIDS estimates of paediatric HIV prevalence are shown in red.
Meta-analyses for HIV cohorts
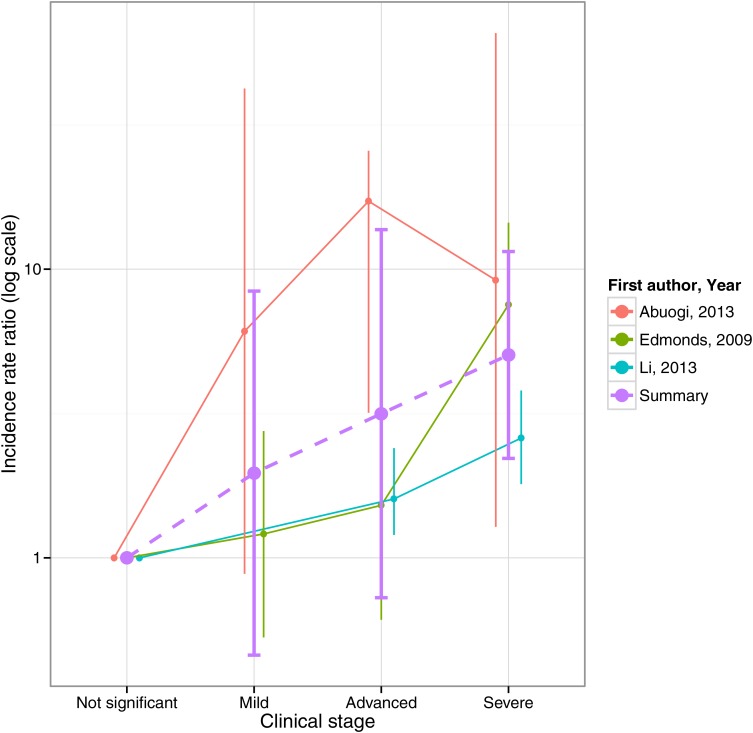
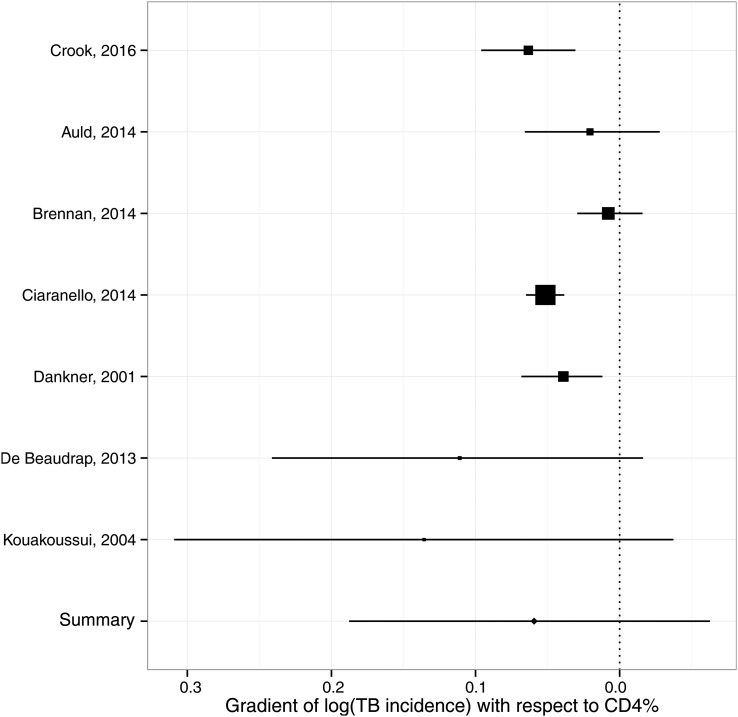
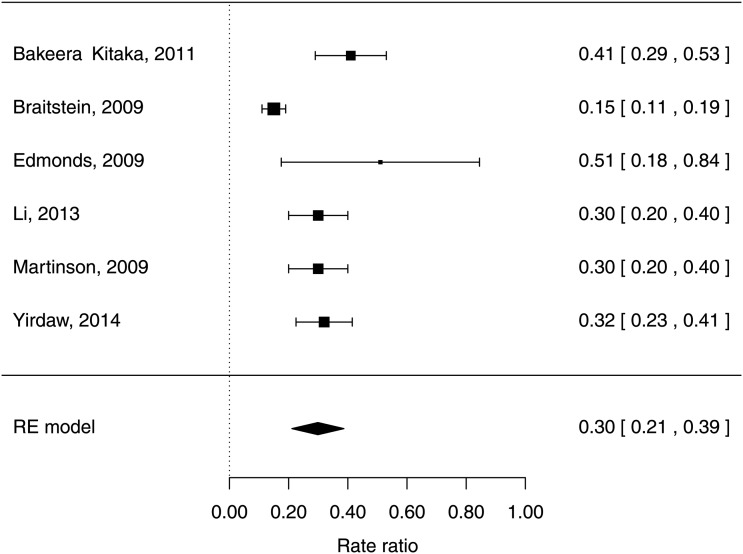
For HIV cohorts, the random-effects meta-analysis of TB incidence in children with severe compared with non-significant immunosuppression according to the WHO categorisation gave an IRR of 5.0 (95% CI 4.0 to 6.0) with I2=87.1% (see figure 4). The Bayesian meta-analysis of the gradient of the logarithmic IRR with respect to CD4% yielded a pooled estimate of −0.063 (95% CrI: −0.188 to +0.063), corresponding to a reduction in IRR of 0.94 (95% CrI: 0.83 to 1.07) per one percentage-point increase in CD4% (see figure 5). This point estimate implies a mean IRR of 7.1 over CD4% ranging between 0% and 50%. The random-effects meta-analysis of protection from ART yielded a pooled HR estimate of 0.30 (95% CI 0.21 to 0.39) with I2=79% (see figure 6).
Figure 4.
Relative TB incidence in children aged <15 years with HIV by WHO immunological staging (I2=87.1%).
Figure 5.
Forest plot for meta-analysis of relation between incidence rate ratio for TB incidence and CD4% in children aged <15 years.
Figure 6.
Forest plot of protection on antiretroviral therapy against TB incidence in children <15 years with HIV infection (I2=79.0%). RE, random effects.
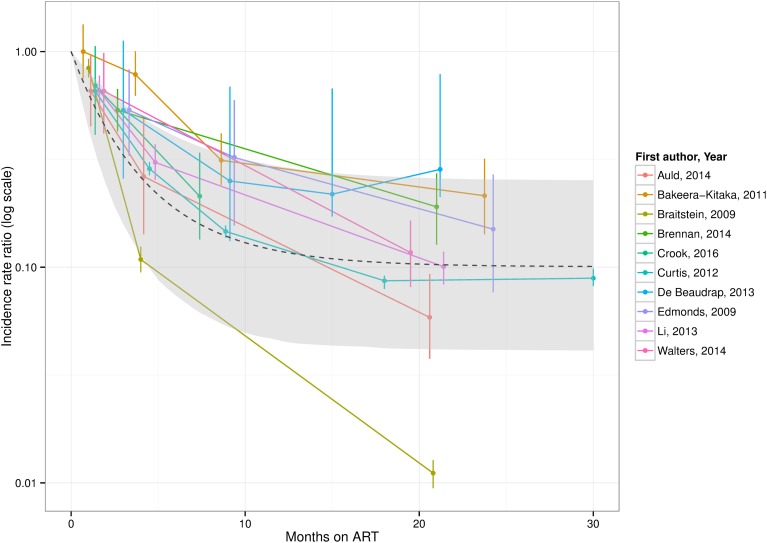
The non-linear mixed-effects regression for the protection from ART by time-since-initiation estimated a rapid initial decline in incidence over the first year, reaching a plateau as protection from ART fully establishes over around 2 years at a pooled asymptotic HR of 0.10 (95% CI 0.04 to 0.25), giving a mean HR over the first 30 months on ART of approximately 0.25 (see figure 7).
Figure 7.
Meta-regression of protection from TB incidence in children <15 years by time-on-antiretroviral therapy (ART), and realigned incidence estimates from studies.
Discussion
In this systematic review, we identified cohorts of children with TB and cohorts of children with HIV. We observed a high but variable prevalence of HIV infection in the TB cohorts, and a very high TB incidence in the HIV cohorts. Accounting for background risk of TB, we found, as in adults, HIV was important risk factor for TB and that TB risk increases with immunosuppression. ART was strongly protective against TB, but took 2 years for full potential of protection against TB to be realised.
In adults, IRRs for TB given HIV around six have been estimated for populations with generalised HIV epidemics,7 corresponding to most newly diagnosed adult TB cases having HIV infection. Our IRR for children is comparable, but lower HIV prevalence is seen in children with TB due to a lower overall paediatric HIV prevalence. In adults, meta-analyses of cohorts of HIV patients suggest exponentially increasing IRR for TB with declining CD4 cell count,9 and current CD4 cell counts on ART strongly predict TB incidence.81 A systematic review and meta-analysis of the protective effect of ART against TB found a HR of 0.35 (0.28–0.44) across all baseline CD4 cell counts,10 comparable to our result for children. Long-term follow-up of adults on ART suggests 4–5 years for protection against TB to become fully established.82 Our analysis suggests that protection against TB in children on ART establishes more rapidly (1–2 years), consistent with faster CD4 reconstitution among children83 compared with adults.84 85
Our findings have implications for patient care, guidelines, TB programmes and mathematical modelling. For clinicians, TB risk with HIV and ART informs patient treatment and appropriate family counselling. For TB programme officers, understanding risks informs resource allocation, and service configuration for HIV testing and treatment. These findings can inform evidence-based guideline development; timely given the recent revision of the WHO ART guidelines advocating universal treatment for children with HIV, irrespective of clinical and immunological stage. Finally, these data inform models of burden estimates and for evaluating the impact of interventions, in relation to HIV and ART.
Our review has limitations. Although our search strategy was comprehensive, studies were excluded if not representative of children with TB in that context. The cohorts identified were generally of low quality for our analyses, the most common shortcomings being around population representativeness and TB case-definitions. The majority of studies were from sub-Saharan Africa, where 90% of all HIV-infected children live, and the findings may not generalise to other regions. The studies spanned a long time period, and may not be representative of the current era. Statistical heterogeneity was high for summary statistics from both TB cohorts and especially HIV cohorts, reflecting diverse study settings and dates, and unreported clinical and methodological heterogeneity. However, measures of heterogeneity were comparable with those from similar studies.86 There were a number of limitations of our meta-analyses of TB cohort data. Controls were often not from the general population. This may partially explain the differences between HIV prevalence in study controls and UNAIDS paediatric HIV prevalence estimates. However, UNAIDS reports country-level estimates for children aged <15 years, which are not likely to reflect the HIV prevalence in younger children local to these studies. Difficulties in diagnosing TB in children with HIV may have led to differential detection of cases by HIV status, affecting the IRR from case-control TB cohorts. Moreover, estimates of the increased risk of TB progression will be confounded by higher exposure due to sharing households with HIV-infected parents, themselves at increased risk of TB. This makes it difficult to differentiate the direct biological impact of HIV from indirect effects. Implicitly, our analyses assume the same relationship between HIV, ART and TB holds regardless of population TB or HIV prevalence, which may not be the case across the very wide range of prevalences in the studies included.
A number of limitations apply to our meta-analyses of HIV data. CD4% categories were disparately reported, and we used category mid-points for analyses of CD4% and time-on-ART. Many studies allowed for unmasking of prevalent TB at ART initiation by excluding a certain period after initiation from comparisons, but this varied between studies. However, excluding all children diagnosed with TB soon after initiating ART may underestimate early incident TB in children who are still highly immunosuppressed. Only three HIV cohorts reported co-trimoxazole use,14 67 80 but as guidelines have varied over time and by setting, co-trimoxazole use may have been under-reported. Confounding by indication was not considered in estimates of ART protection (except for Edmonds et al70), and choice of covariates varied between studies. Our direct meta-analysis of the protective effect of ART was not able to include how long children had been on ART, and will average over the different distribution of treatment durations between studies.
We were not able to analyse age effects on TB, as data were seldom reported with multiple age stratifications. Our analysis of protection by time-on-ART may be confounded by age. Young children have particularly high TB progression risk, in part due to immature cell-mediated immune responses.1 The impact of age on efficacy of ART is also complex, as early ART initiation, at a better baseline immune status, leads to better immune reconstitution (although adherence can be challenging at the youngest ages due to a paucity of palatable formulations).83 87 88 The studies in our review were conducted over years when ART treatment guidelines, availability and formulations were evolving. The relevance of our findings to current universal treatment recommendations needs careful consideration; individual patient data meta-analysis would be highly informative on the impact of host characteristics on risk of TB, in particular the age of the child and age at which ART was initiated. As children are started on ART at higher CD4 values, the protective benefit of ART against TB may be lower.
Despite these limitations, internal consistency between results from different analyses increases the confidence in our conclusions. Our IRR from TB cohorts is comparable with the estimate of ‘severe’ compared with ‘not significant’ immune staging, recognising that HIV may confer an increased risk of TB even before immunosuppression is clinically evident. The mean IRR over a range of CD4% between 0% and 50% was 7.1 using the risk gradient point-estimate from our CD4 analysis—close to our IRR estimate for all HIV from TB cohorts. The mean protection against TB over the first 30 months on ART was 0.25—comparable to our meta-analysis of estimates of protection (although these analyses ultimately stem from the same data). The decline in TB rates by time-on-ART occurred on a similar timescale to the analysis presented by Li et al,73 although to a lower final value. Comparing our HIV and ART risk results suggests that children commencing ART are at higher risk of TB than HIV-uninfected children initially, but our results are too uncertain to specify whether long-term TB risks ART return to those of an HIV-uninfected child. For adults, TB risks established on long-term ART remain elevated over those of HIV-uninfected individuals.82
It is important to place ART in the context of other public health interventions. Our results suggest early diagnosis of infants and young children with HIV, followed by immediate ART, will reduce the pool of highly susceptible children and consequent progression to TB disease, providing further impetus to scale-up coverage among paediatric ART programmes. High HIV prevalence in the TB cohorts and high TB incidence in the HIV cohorts suggest that all children diagnosed with TB should be tested for HIV infection and all children with HIV should be regularly screened for TB disease. Scale-up of PMTCT will lead to fewer children becoming infected with HIV and is likely to be one of the most effective public health strategies to reduce paediatric HIV/TB; similarly, prompt diagnosis of TB in HIV-infected adults will reduce the risk of TB transmission to their HIV-infected children. TB control in adults more generally,89 and infection control measures, will reduce the risk of TB transmission to vulnerable children. IPT appears similarly effective at reducing TB incidence in HIV-infected children (around 70% reduction in incidence),71 80 and evidence suggests that use in conjunction with ART is more protective than either intervention alone.90 Evidence for the benefit of co-trimoxazole in preventing incident TB in children is emerging;14 since it is now recommended long-term, co-trimoxazole prophylaxis may also help to reduce TB incidence among HIV-infected children on ART. Improving nutritional status among HIV-infected children may also be beneficial, as there is a high risk of TB in the context of malnutrition. Although individually these interventions are likely less effective than ART, their combination can be expected to protect the biggest number of children from developing TB.
Conclusions
Our results indicate that HIV infection is a potent risk factor for TB in children, with a gradient of risk by indices of immunosuppression. ART is strongly protective against TB in children with HIV infection, taking up to 2 years for protection to become fully established, underscoring the importance of prompt ART initiation.
Footnotes
Twitter: Follow Claire Beecroft @mscihta
Contributors: Conceived and designed the study; undertook the review and data abstraction; drafted article: PJD, JAS. Designed the search strategy: CB, PJD, JAS. Statistical analysis: PJD. Commented on and revised article: all authors.
Funding: Study funded by the STEP TB UNITAID grant to TB Alliance. The funders had no role in the design or execution of the study, nor the decision to publish. BK is funded by a programme grant from the MRC. AJP is funded by the Wellcome Trust (108065/Z/15/Z).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data used in the analyses are available either in the main article or the online supplementary material.
References
- 1.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004;8:392–402. [PubMed] [Google Scholar]
- 2.Dodd PJ, Gardiner E, Coghlan R, et al. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health 2014;2:e453–9. 10.1016/S2214-109X(14)70245-1 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global tuberculosis report 2015. Geneva, 2015. [Google Scholar]
- 4.UNAIDS. 2014 progress report on the global plan. Geneva, 2014. [Google Scholar]
- 5.Tobin NH, Aldrovandi GM. Immunology of pediatric HIV infection. Immunol Rev 2013;254:143–69. 10.1111/imr.12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Guidelines on when to start antiretroviral therapy and pre-exposure prophylaxis for HIV. Geneva: WHO, 2015. [PubMed] [Google Scholar]
- 7.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 2003;163:1009 10.1001/archinte.163.9.1009 [DOI] [PubMed] [Google Scholar]
- 8.Williams BG, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science 2003;301:1535–7. 10.1126/science.1086845 [DOI] [PubMed] [Google Scholar]
- 9.Williams BG, Granich R, De Cock KM, et al. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci USA 2010;107:19485–9. 10.1073/pnas.1005660107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suthar AB, Lawn SD, del Amo J, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med 2012;9:e1001270 10.1371/journal.pmed.1001270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pretorius C, Menzies NA, Chindelevitch L, et al. The potential effects of changing HIV treatment policy on tuberculosis outcomes in South Africa: results from three tuberculosis-HIV transmission models. AIDS 2014;28(Suppl 1):S25–34. 10.1097/QAD.0000000000000085 [DOI] [PubMed] [Google Scholar]
- 12.Williams BG, Granich R, Chauhan LS, et al. The impact of HIV/AIDS on the control of tuberculosis in India. Proc Natl Acad Sci USA 2005;102:9619–24. 10.1073/pnas.0501615102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 14.Crook AM, Turkova A, Musiime V, et al. Tuberculosis incidence is high in HIV-infected African children but is reduced by co-trimoxazole and time on antiretroviral therapy. BMC Med 2016;14:50 10.1186/s12916-016-0593-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O'connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 1 Jan 2008).
- 16.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft 2010;36:1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 17.Jaganath D, Zalwango S, Okware B, et al. Contact investigation for active tuberculosis among child contacts in Uganda. Clin Infect Dis 2013;57:1685–92. 10.1093/cid/cit645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ade S, Harries AD, Trébucq A, et al. The burden and outcomes of childhood tuberculosis in Cotonou, Benin. Public Health Action 2013;3:15–19. 10.5588/pha.12.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berggren Palme I, Gudetta B, Bruchfeld J, et al. Detection of Mycobacterium tuberculosis in gastric aspirate and sputum collected from Ethiopian HIV-positive and HIV-negative children in a mixed in- and outpatient setting. Acta Paediatr 2004;93:311–15. 10.1111/j.1651-2227.2004.tb02953.x [DOI] [PubMed] [Google Scholar]
- 20.Berggren Palme I, Gudetta B, Degefu H, et al. A controlled estimate of the risk of HIV infection in Ethiopian children with tuberculosis. Epidemiol Infect 2001;127:517–25. 10.1017/S0950268801006215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhat GJ, Diwan VK, Chintu C, et al. HIV, BCG and TB in children: a case control study in Lusaka, Zambia. J Trop Pediatr 1993;39:219–23. 10.1093/tropej/39.4.219 [DOI] [PubMed] [Google Scholar]
- 22.Bobossi-Serengbe G, Tembeti PJ, Mobima T, et al. [Tuberculosis and HIV co-infection among children hospitalized in Bangui (Central African Republic)]. Arch Pediatr 2005;12:1215–20. 10.1016/j.arcped.2005.04.083 [DOI] [PubMed] [Google Scholar]
- 23.Campos-Herrero Navas MI, Rodriguez Villalobos H, Perera Gonzalez A, et al. [Tuberculosis en pediatric patients in the island of Gran Canaria]. An Esp Pediatr 1997;46:561–4. [PubMed] [Google Scholar]
- 24.Cathebras P, Vohito JA, Yete ML, et al. [Tuberculosis and human immunodeficiency virus infection in the Central African Republic]. Med Trop (Mars) 1988;48:401–7. [PubMed] [Google Scholar]
- 25.Chintu C, Bhat G, Luo C, et al. Seroprevalence of human immunodeficiency virus type 1 infection in Zambian children with tuberculosis. Pediatr Infect Dis J 1993;12:499–504. 10.1097/00006454-199306000-00008 [DOI] [PubMed] [Google Scholar]
- 26.Chintu C, Dupont HL, Kaile T, et al. Human immunodeficiency virus-associated diarrhea and wasting in Zambia: selected risk factors and clinical associations. Am J Trop Med Hyg 1998;59:38–41. [DOI] [PubMed] [Google Scholar]
- 27.Chintu C, Luo C, Bhat G, et al. Impact of the human immunodeficiency virus type-1 on common pediatric illnesses in Zambia. J Trop Pediatr 1995;41:348–53. 10.1093/tropej/41.6.348 [DOI] [PubMed] [Google Scholar]
- 28.Daniel OJ, Ogunfowora OB, Oladapo OT. HIV sero-prevalence among children diagnosed with TB in Nigeria. Trop Doct 2007;37:268–9. 10.1258/004947507782333080 [DOI] [PubMed] [Google Scholar]
- 29.Edwards DJ, Kitetele F, Van Rie A. Agreement between clinical scoring systems used for the diagnosis of pediatric tuberculosis in the HIV era. Int J Tuberc Lung Dis 2007;11:263–9. [PubMed] [Google Scholar]
- 30.Espinal MA, Reingold AL, Perez G, et al. Human immunodeficiency virus infection in children with tuberculosis in Santo Domingo, Dominican Republic: prevalence, clinical findings, and response to antituberculosis treatment. J Acquir Immune Defic Syndr Hum Retrovirol 1996;13:155-9. [DOI] [PubMed] [Google Scholar]
- 31.Feldacker C, Tweya H, Keiser O, et al. Characteristics of adults and children diagnosed with tuberculosis in Lilongwe, Malawi: findings from an integrated HIV/TB clinic. Trop Med Int Health 2012;17:1108–16. 10.1111/j.1365-3156.2012.03041.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gava C, Malacarne J, Rios DPG, et al. Tuberculosis in indigenous children in the Brazilian Amazon. Rev Saude Publica 2013;47:77–85. 10.1590/S0034-89102013000100011 [DOI] [PubMed] [Google Scholar]
- 33.Geoghagen M, Farr JA, Hambleton I, et al. Tuberculosis and HIV co-infections in Jamaican children. West Indian Med J 2004;53:339–45. [PubMed] [Google Scholar]
- 34.Henegar C, Behets F, Vanden Driessche K, et al. Impact of HIV on clinical presentation and outcomes of tuberculosis treatment at primary care level. Int J Tuberc Lung Dis 2013;17:1411–13. 10.5588/ijtld.13.0151 [DOI] [PubMed] [Google Scholar]
- 35.Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis 2009;48:108-14. [DOI] [PubMed] [Google Scholar]
- 36.Hussain T, Sinha S, Talan S, et al. Seroprevalence of HIV infection among paediatric tuberculosis patients in Agra, India: a hospital-based study. Tuberculosis (Edinb) 2007;87:7–11. 10.1016/j.tube.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 37.Iriso R, Mudido PM, Karamagi C, et al. The diagnosis of childhood tuberculosis in an HIV-endemic setting and the use of induced sputum. Int J Tuberc Lung Dis 2005;9:716–26. [PubMed] [Google Scholar]
- 38.Jain SK, Ordonez A, Kinikar A, et al. Pediatric tuberculosis in young children in India: a prospective study. Biomed Res Int 2013;2013:783698 10.1155/2013/783698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen J, Álvaro-Meca A, Micheloud D, et al. Reduction in mycobacterial disease among HIV-infected children in the highly active antiretroviral therapy era (1997-2008). Pediatr Infect Dis J 2012;31:278–83. 10.1097/INF.0b013e318239e268 [DOI] [PubMed] [Google Scholar]
- 40.Llerena C, Fadul SE, Garzón MC, et al. [Drug-resistant Mycobacterium tuberculosis in children under 15 years]. Biomedica 2010;30:362–70. 10.7705/biomedica.v30i3.270 [DOI] [PubMed] [Google Scholar]
- 41.Luo C, Chintu C, Bhat G, et al. Human immunodeficiency virus type-1 infection in Zambian children with tuberculosis: changing seroprevalence and evaluation of a thioacetazone-free regimen. Tuber Lung Dis 1994;75:110–15. 10.1016/0962-8479(94)90039-6 [DOI] [PubMed] [Google Scholar]
- 42.Madhi SA, Gray GE, Huebner RE, et al. Correlation between CD4+ lymphocyte counts, concurrent antigen skin test and tuberculin skin test reactivity in human immunodeficiency virus type 1-infected and -uninfected children with tuberculosis. Pediatr Infect Dis J 1999;18:800–5. 10.1097/00006454-199909000-00011 [DOI] [PubMed] [Google Scholar]
- 43.Mehta S, Mugusi FM, Bosch RJ, et al. A randomized trial of multivitamin supplementation in children with tuberculosis in Tanzania. Nutr J 2011;10:120 10.1186/1475-2891-10-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miranda AE, Dietze R, Maciel EL, et al. Tuberculosis and AIDS co-morbidity in children: linkage of databases from Espirito Santo State, Brazil. J Trop Pediatr 2011;57:296–8. 10.1093/tropej/fmq087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukadi YD, Wiktor SZ, Coulibaly IM, et al. Impact of HIV infection on the development, clinical presentation, and outcome of tuberculosis among children in Abidjan, Cote d'Ivoire. AIDS 1997;11:1151–8. 10.1097/00002030-199709000-00011 [DOI] [PubMed] [Google Scholar]
- 46.Berggren Palme I, Gudetta B, Bruchfeld J, et al. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr Infect Dis J 2002;21:1053–61. 10.1097/01.inf.0000036090.75121.f3 [DOI] [PubMed] [Google Scholar]
- 47.Panigatti P, Ratageri VH, Shivanand I, et al. Profile and outcome of childhood tuberculosis treated with DOTS—an observational study. Indian J Pediatr 2014;81:9–14. 10.1007/s12098-013-1175-8 [DOI] [PubMed] [Google Scholar]
- 48.Rachow A, Clowes P, Saathoff E, et al. Increased and expedited case detection by Xpert MTB/RIF assay in childhood tuberculosis: a prospective cohort study. Clin Infect Dis 2012;54:1388-96. [DOI] [PubMed] [Google Scholar]
- 49.Ramos JM, Reyes F, Tesfamariam A. Childhood and adult tuberculosis in a rural hospital in Southeast Ethiopia: a ten-year retrospective study. BMC Public Health 2010;10:215 10.1186/1471-2458-10-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose MV, Kimaro G, Nissen TN, et al. QuantiFERON-TB gold in-tube performance for diagnosing active tuberculosis in children and adults in a high burden setting. PLoS ONE 2012;7:e37851 10.1371/journal.pone.0037851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sassan-Morokro M, De Cock KM, Ackah A, et al. Tuberculosis and HIV infection in children in Abidjan, Cote d'Ivoire. Trans R Soc Trop Med Hyg 1994;88:178–81. 10.1016/0035-9203(94)90285-2 [DOI] [PubMed] [Google Scholar]
- 52.Schaaf HS, Hesseling AC, Rautenbach C, et al. Trends in childhood drug-resistant tuberculosis in South Africa: a window into the wider epidemic? Int J Tuberc Lung Dis 2014;18:770–3. 10.5588/ijtld.13.0069 [DOI] [PubMed] [Google Scholar]
- 53.Seddon JA, Hesseling AC, Marais BJ, et al. The evolving epidemic of drug-resistant tuberculosis among children in Cape Town, South Africa. Int J Tuberc Lung Dis 2012;16:928–33. 10.5588/ijtld.11.0679 [DOI] [PubMed] [Google Scholar]
- 54.Shahab T, Zoha MS, Malik MA, et al. Prevalence of human immunodeficiency virus infection in children with tuberculosis. Indian Pediatr 2004;41:595–9. 10.1016/j.arcmed.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 55.Thomas TA, Heysell SK, Moodley P, et al. Intensified specimen collection to improve tuberculosis diagnosis in children from Rural South Africa, an observational study. BMC Infect Dis 2014;14:11 10.1186/1471-2334-14-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yassin MA, Petrucci R, Garie KT, et al. Can interferon-gamma or interferon-gamma-induced-protein-10 differentiate tuberculosis infection and disease in children of high endemic areas? PLoS ONE 2011;6:e23733 10.1371/journal.pone.0023733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwara A, Enimil A, Gillani FS, et al. Pharmacokinetics of first-line antituberculosis drugs using WHO revised dosage in children with tuberculosis with and without HIV coinfection. J Pediatr Infect Dis Soc 2016;5:356-65. 10.1093/jpids/piv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.López-Varela E, Augusto O, Gondo K, et al. Incidence of tuberculosis in children under the age of three in Manhiça, rural southern Mozambique Pediatr Infect Dis J 2015;34:686-92. [DOI] [PubMed] [Google Scholar]
- 59.Perfura Yone EW, Mbarga AE, Kuaban C. Impact de l'infection à VIH sur la tuberculose de l'enfant à Yaoundé, Cameroun. Rev Mal Respir 2012;29:1095–103. 10.1016/j.rmr.2012.05.017 [DOI] [PubMed] [Google Scholar]
- 60.Abuogi LL, Mwachari C, Leslie HH, et al. Impact of expanded antiretroviral use on incidence and prevalence of tuberculosis in children with HIV in Kenya. Int J Tuberc Lung Dis 2013;17:1291–7. 10.5588/ijtld.12.0740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alarcón JO, Freimanis-Hance L, Krauss M, et al. Opportunistic and other infections in HIV-infected children in Latin America compared to a similar cohort in the United States. AIDS Res Hum Retroviruses 2012;28:282–8. 10.1089/AID.2011.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Auld AF, Tuho MZ, Ekra KA, et al. Tuberculosis in human immunodeficiency virus-infected children starting antiretroviral therapy in Cote d'Ivoire. Int J Tuberc Lung Dis 2014;18:381–7. 10.5588/ijtld.13.0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bakeera-Kitaka S, Conesa-Botella A, Dhabangi A, et al. Tuberculosis in human immunodeficiency virus infected Ugandan children starting on antiretroviral therapy. Int J Tuberc Lung Dis 2011;15:1082–6. 10.5588/ijtld.10.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braitstein P, Nyandiko W, Vreeman R, et al. The clinical burden of tuberculosis among human immunodeficiency virus-infected children in Western Kenya and the impact of combination antiretroviral treatment. Pediatr Infect Dis J 2009;28:626–32. 10.1097/INF.0b013e31819665c5 [DOI] [PubMed] [Google Scholar]
- 65.Brennan AT, Maskew M, Schnippel K, et al. Risk factors associated with TB in children receiving ART in a South African multicenter HIV cohort. Top Antivir Med 2014;22:484–5. [Google Scholar]
- 66.Ciaranello A, Lu Z, Ayaya S, et al. Incidence of World Health Organization stage 3 and 4 events, tuberculosis and mortality in untreated, HIV-infected children enrolling in care before 1 year of age: an IeDEA (International Epidemiologic Databases To Evaluate AIDS) east Africa regional analysis. Pediatr Infect Dis J 2014;33:623–9. 10.1097/INF.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Curtis AJ, Marshall CS, Spelman T, et al. Incidence of WHO stage 3 and 4 conditions following initiation of anti-retroviral therapy in resource limited settings. PLoS ONE 2012;7:e52019 10.1371/journal.pone.0052019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dankner WM, Lindsey JC, Levin MJ, Pediatric ACTGPT. Correlates of opportunistic infections in children infected with the human immunodeficiency virus managed before highly active antiretroviral therapy. Pediatr Infect Dis J 2001;20:40–8. 10.1097/00006454-200101000-00008 [DOI] [PubMed] [Google Scholar]
- 69.De Beaudrap P, Boullé C, Lewden C, et al. Morbidity after antiretroviral therapy initiation in HIV-1-infected children in West Africa: temporal trends and relation to CD4 count. Pediatr Infect Dis J 2013;32:354–60. 10.1097/INF.0b013e318278b222 [DOI] [PubMed] [Google Scholar]
- 70.Edmonds A, Lusiama J, Napravnik S, et al. Anti-retroviral therapy reduces incident tuberculosis in HIV-infected children. Int J Epidemiol 2009;38:1612–21. 10.1093/ije/dyp208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gray DM, Workman LJ, Lombard CJ, et al. Isoniazid preventive therapy in HIV-infected children on antiretroviral therapy: a pilot study. Int J Tuberc Lung Dis 2014;18:322–7. 10.5588/ijtld.13.0354 [DOI] [PubMed] [Google Scholar]
- 72.Kouakoussui A, Fassinou P, Anaky MF, et al. Respiratory manifestations in HIV-infected children pre- and post-HAART in Abidjan, the Ivory Coast. Paediatr Respir Rev 2004;5:311–15. 10.1016/j.prrv.2004.07.008 [DOI] [PubMed] [Google Scholar]
- 73.Li N, Manji KP, Spiegelman D, et al. Incident tuberculosis and risk factors among HIV-infected children in Tanzania. AIDS 2013;27:1273–81. 10.1097/QAD.0b013e32835ecb24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinson NA, Moultrie H, van Niekerk R, et al. HAART and risk of tuberculosis in HIV-infected South African children: a multi-site retrospective cohort. Int J Tuberc Lung Dis 2009;13:862–7. [PMC free article] [PubMed] [Google Scholar]
- 75.Prasitsuebsai W, Kariminia A, Puthanakit T, et al. Impact of antiretroviral therapy on opportunistic infections of HIV-infected children in the therapeutic research, education and AIDS training Asia pediatric HIV observational database. Pediatr Infect Dis J 2014;33:747–52. 10.1097/INF.0000000000000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas P, Bornschlegel K, Singh TP, et al. Tuberculosis in human immunodeficiency virus-infected and human immunodeficiency virus-exposed children in New York City. The New York City Pediatric Spectrum of HIV Disease Consortium. Pediatr Infect Dis J 2000;19:700–6. 10.1097/00006454-200008000-00006 [DOI] [PubMed] [Google Scholar]
- 77.Walters E, Cotton MF, Rabie H, et al. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti-retroviral therapy. BMC Pediatr 2008;8:1 10.1186/1471-2431-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walters E, Duvenhage J, Draper HR, et al. Severe manifestations of extrapulmonary tuberculosis in HIV-infected children initiating antiretroviral therapy before 2 years of age. Arch Dis Child 2014;99:998–1003. 10.1136/archdischild-2013-305509 [DOI] [PubMed] [Google Scholar]
- 79.Yirdaw KD, Jerene D, Gashu Z, et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoS ONE 2014;9:e104557 10.1371/journal.pone.0104557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ 2007;334:136 10.1136/bmj.39000.486400.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lawn SD, Myer L, Edwards D, et al. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 2009;23:1717–25. 10.1097/QAD.0b013e32832d3b6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta A, Wood R, Kaplan R, et al. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS ONE 2012;7:e34156 10.1371/journal.pone.0034156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Picat MQ, Lewis J, Musiime V, et al. Predicting patterns of long-term CD4 reconstitution in HIV-infected children starting antiretroviral therapy in sub-Saharan Africa: a cohort-based modelling study. PLoS Med 2013;10:e1001542 10.1371/journal.pmed.1001542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.García F, de Lazzari E, Plana M, et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. J Acquir Immune Defic Syndr 2004;36:702–13. 10.1097/00126334-200406010-00007 [DOI] [PubMed] [Google Scholar]
- 85.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis 2007;44:441–6. 10.1086/510746 [DOI] [PubMed] [Google Scholar]
- 86.Jenkins HE, Yuen CM, Rodriguez CA, et al. Mortality among children diagnosed with tuberculosis: Systematic review and meta-analysis. Lancet Infect Dis. Published Online First: 7 Dec 2016. doi:10.1016/S1473-3099(16)30474-1. [DOI] [PMC free article] [PubMed]
- 87.Bamford A, Turkova A, Lyall H, et al. Paediatric European Network for Treatment of AIDS (PENTA) guidelines for treatment of paediatric HIV-1 infection 2015: optimizing health in preparation for adult life. HIV Med Published Online First: 3 Feb 2015. doi:10.1111/hiv.12217 10.1111/hiv.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis J, Walker AS, Castro H, et al. Age and CD4 count at initiation of antiretroviral therapy in HIV-infected children: effects on long-term T-cell reconstitution. J Infect Dis 2012;205:548–56. 10.1093/infdis/jir787 [DOI] [PubMed] [Google Scholar]
- 89.Denkinger CM, Kampmann B, Ahmed S, et al. Modeling the impact of novel diagnostic tests on pediatric and extrapulmonary tuberculosis. BMC Infect Dis 2014;14:477 10.1186/1471-2334-14-477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frigati LJ, Kranzer K, Cotton MF, et al. The impact of isoniazid preventive therapy and antiretroviral therapy on tuberculosis in children infected with HIV in a high tuberculosis incidence setting. Thorax 2011;66:496–501. 10.1136/thx.2010.156752 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2016-209421supp001.pdf (6.6MB, pdf)