Abstract
Re-emerging human adenovirus types 14 (Ad14) and 55 (Ad55) have caused severe respiratory diseases and even deaths during recent outbreaks. However, the seroprevalence of neutralizing antibodies (nAbs) in healthy adults, which may reflect previous circulation and help to predict potential outbreaks, remains unclear. In this study, we established micro-neutralizing (MN) assays on the basis of recombinant Ad14 and Ad55 reporter viruses, and we investigated serum nAbs in healthy blood donors from Southern China. We found that the overall seropositive rates were 24.8% and 22.4% for Ad14 and Ad55 nAbs, respectively. The seropositive rates were low in individuals younger than 20, and they gradually increased with age. Ad55-seropositive individuals tended to have high nAb titers (>1000), while low (72–200) and moderate (201–1000) nAb levels were frequently observed in Ad14-seropositive ones. Surprisingly, the seropositive rates and nAb levels were associated with the blood type but not the gender of the blood donors, with type AB individuals displaying higher seropositive rates and nAb levels. Interestingly, a significant positive correlation was observed between Ad14 and Ad55 seroprevalence, and higher titers of nAbs were detected in double-positive individuals compared to single-positive ones. These results clarified the human humoral immune responses against Ad14 and Ad55 and revealed a low level of herd immunity in some subpopulations, which emphasized the importance of monitoring these two highly virulent adenoviruses and reinforced the development of prophylactic vaccines.
Keywords: human adenovirus type 14, human adenovirus type 55, neutralizing antibody, seroprevalence, Southern China
INTRODUCTION
Human adenoviruses (AdVs) are non-enveloped, double-stranded DNA viruses belonging to Adenoviridae.1 To date, more than 69 AdV genotypes, which were classified into seven species (A to G), were identified according to their genetic, biophysical or biochemical characteristics.2 AdV infection causes a broad spectrum of diseases in humans, including pharyngoconjunctivitis, keratoconjunctivitis, gastroenteritis, myocarditis, acute hemorrhagic cystitis, meningoencephalitis and pneumonia, depending on the infection genotype.3 Although the symptoms are usually mild or self-limited in immuno-competent adults, outbreaks of acute respiratory diseases (ARDs), such as community-acquired pneumonia (CAP), have been documented in particular populations, including newborns, school students and military recruits.2 In immuno-comprised individuals such as patients with AIDS or individuals receiving transplants, AdV infection can lead to fatal pneumonia.4
Recently, two members of human AdV species B, type 14 (Ad14) and 55 (Ad55), were reported to cause severe CAP in military and civilian populations.5, 6, 7, 8, 9 Ad14 was first identified in an outbreak of ARDs in 1955 and then vanished for a long period.10 A newly emergent variant of Ad14, which was designated Ad14p1, has caused several outbreaks of acute febrile respiratory illness globally since 2006.11, 12, 13, 14, 15 High rates of severe illness and even deaths were reported following these outbreaks.13, 14, 15 Ad55, an inter-typic recombinant of Ad11 and Ad14, emerged since 2005 in Singapore and subsequently appeared in China, France and Israel.16, 17 As a highly virulent pathogen, Ad55 was associated with life-threatening pneumonia during recent outbreaks.18, 19 Their unpredictable high morbidity and mortality in otherwise healthy immuno-competent adults render Ad14 and Ad55 potential threats to public health.20 Although the genomic and virological characteristics of Ad14 and Ad55 have been investigated,21, 22 the prevalence of neutralizing antibodies (nAbs) in the general populations, which is helpful for understanding human anti-Ad14 and Ad55 immunity, remains unclear.
In this study, we established our micro-neutralizing (MN) assays on the basis of recombinant Ad14 and Ad55 reporter viruses, and we investigated the seroprevalence of nAbs against these two AdVs in healthy blood donors in Southern China. The distributions of nAbs in relation to different age, gender and blood type groups were also analyzed.
MATERIALS AND METHODS
Generation of recombinant Ad14 and Ad55 reporter viruses
Wild-type Ad14 (GenBank NO. JQ824845.1) and Ad55 (GenBank NO. KF911353.1) were kindly provided by Prof Qiwei Zhang (Southern Medical University, China) and Prof Chengfeng Qin (Beijing Institute of Microbiology and Epidemiology, China), respectively. In brief, wild-type Ad14 and Ad55 were propagated in A549 cells (ATCC, Manassas, VA, USA). The viral genomes were extracted and purified by sodium-dodecyl-sulfonate lysis (Sigma-Aldrich, St Louis, MO, USA) followed by phenol-chloroform extraction. The terminal regions of the Ad14 and Ad55 genomes were amplified by PCR and subcloned into pMD18T vectors (TaKaRa, Dalian, China) to obtain shuttle plasmids. The genomic plasmids pAd14 and pAd55 were then generated by homologous recombination between linearized shuttle plasmids and viral genomes in Escherichia coli BJ5183 competent cells (Agilent Technologies, Santa Clara, CA, USA). The fragments flanking the E3 region were subsequently amplified by PCR and subcloned into a pVAX1 plasmid (Thermo Fisher Scientific, Waltham, MA, USA) to obtain p14E3LR and p55E3LR. The E3 regions were then deleted by homologous recombination between E3LR fragments and linearized genomic plasmids to obtain pAd14ΔE3 and pAd55ΔE3. Simultaneously, the coding sequences for secreted alkaline phosphatase (SEAP) or enhanced green fluorescent protein (EGFP), which was flanked by a CMV promoter and a BGH poly(A) signal, were amplified by PCR and inserted into p14E3LR and p55E3LR to obtain shuttle reporter plasmids. Finally, pAd14-EGFP, pAd14-SEAP, pAd55-EGFP, and pAd55-SEAP were constructed by homologous recombination between linearized pAd14ΔE3 or pAd55ΔE3 and shuttle reporter plasmids. The genomes of the Ad14 and Ad55 reporter viruses were released by restricted enzyme digestion with AsiSI (New England Biolabs, Ipswich, MA, USA) and transfected into HEK293 cells (ATCC). The four reporter viruses Ad14-EGFP, Ad14-SEAP, Ad55-EGFP and Ad55-SEAP were then rescued and propagated. Purified viral stocks were obtained by cesium chloride gradient centrifugation, and the infectious virions were titrated as described.23, 24
Human sample collection
Serum samples from 1009 healthy blood donors were randomly collected at the Guangzhou Blood Centre, Guangzhou, China, from August 2015 to December 2015. Any factors that could lead to a biased analysis, such as the educational background and the careers of the donors, were not considered as the criteria during the collection of blood samples. The ages of the volunteers ranged from 18 to 57 years old. The percentages of males to females were 31.7% and 68.3%. The percentages of blood types A, B, AB and O were 24.4%, 41.2%, 11.5% and 22.9%, respectively. The research using human samples was approved by the Ethics Committee of Guangzhou Institutes of Biomedicine and Health (GIBH). Informed consent was obtained from each volunteer.
Micro-neutralizing (MN) assays
The titers of nAbs that acted against Ad14 and Ad55 were detected using MN assays as previously described.23, 24 In brief, the HEK293 cells were seeded into 96-well plates at 3 × 104 cells per well. One day later, serial dilutions of the human serums were heat-treated at 56 °C for 90 min and incubated with Ad14-SEAP or Ad55-SEAP at 4 × 106 viral particles per well for 1 h at 37 °C. The mixtures were subsequently added to the 96-well plates and incubated for 24 h at 37 °C. Finally, the supernatants were harvested, and the SEAP activity was detected using the Phospha-Light System according to the manufacturer’s instructions (Thermo Fisher Scientific). The relative light units (RLUs) were recorded and the titers were calculated as the dilutions that inhibited 50% RLU values. The values <72, 72–200, 201–1000 and >1000 were denoted as negative, low, moderate and high nAb titers, respectively. To validate the feasibility of SEAP activity-based MN assays, HEK293 cells were seeded into 96-well plates at 3 × 104 cells per well. Twenty-four hours later, serial dilutions of serum samples with low, moderate or high titers of Ad14 or Ad55 nAbs were incubated with Ad14-EGFP or Ad55-EGFP (4 × 106 viral particles per well), respectively. Subsequently, the mixtures were added to the plates. At 24 h after infection, EGFP-expressing cells were imaged using fluorescence microscopy.
Statistical analysis and figure development
Seroprevalence comparisons for different groups were conducted using a χ2-test. The trends in the seropositive rates throughout the groups were analyzed by using the Trend χ2-test. Comparisons of nAb titers among groups were performed by Mann–Whitney test. The trends in the nAb titers throughout the groups were analyzed by Kruskal–Wallis Test. All the statistical analyses were computed with SPSS version 13.0 (SPSS Inc., Chicago, IL, USA), and P values of less than 0.05 were considered statistically significant. The graphs were generated with GraphPad Prism version 5 (GraphPad Software, La Jolla, CA, USA) and Photoshop version 8 (Adobe Systems Incorporated, San Jose, CA, USA).
RESULTS
Establishment and validation of MN assays based on recombinant Ad14 and Ad55 reporter viruses
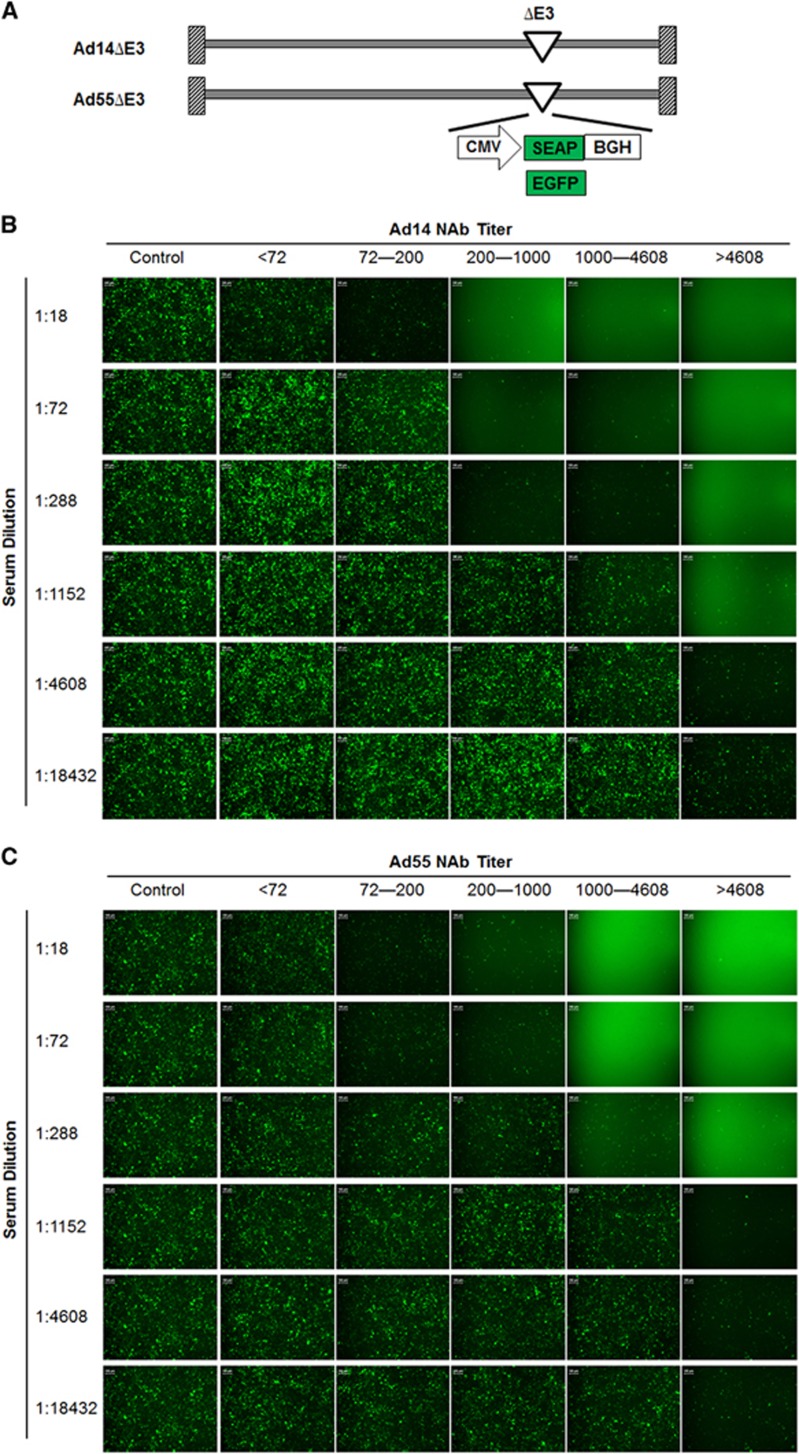
To generate replication-competent reporter viruses, the E3 regions of Ad14 and Ad55 were depleted, and the expression cassettes for EGFP- or SEAP were inserted (Figure 1A). The four reporter viruses Ad14-EGFP, Ad14-SEAP, Ad55-EGFP and Ad55-SEAP, were successfully obtained. The reporter genes were efficiently expressed in cells that were infected by these viruses (data not shown).
Figure 1.
The validation of micro-neutralizing assays based on Ad14 and Ad55 reporter viruses. (A) A diagram of the genomic structures for Ad14 and Ad55 reporter viruses. (B and C) Serial dilutions of Ad14 (B) or Ad55 (C) -seropositive serums with nAb titers <72, 72–200, 201–1000, 1001–4608 and >4608 were incubated with Ad14-EGFP or Ad55-EGFP, and then infected into HEK293 cells. EGFP-expressing cells were imaged by fluorescence microscopy at 24 h post infection. Serums from mice that were immunized with empty Ad5 vectors were used as negative controls. This figure is representative of two independent experiments.
To validate the feasibility of the MN assays based on Ad14-SEAP and Ad55-SEAP, serums with different nAb titers (<72, 72–200, 201–1000, 1001–4608 and >4608) were serially diluted, incubated with Ad14-EGFP or Ad55-EGFP and infected into HEK293 cells. The results showed that serums with nAb titers <72 displayed no detectable inhibition in relation to Ad14-EGFP or Ad55-EGFP infection for all the dilutions, while those with nAb titers >72 exhibited various neutralizing activities, which was consistent with the titers that were determined by SEAP activity (Figures 1B and 1C). In addition, the MN assays based on SEAP or EGFP reporter genes were much more sensitive than the ones based on cytopathic effects (CPEs) (Supplementary Figure S1). These results suggested that the MN assays based on Ad14-SEAP and Ad55-SEAP were feasible.
The overall seroprevalence of Ad14 and Ad55 nAbs in healthy blood donors from Southern China
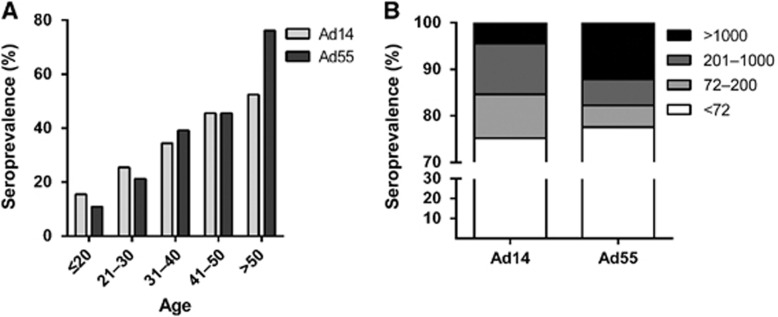
By using Ad14-SEAP and Ad55-SEAP, the nAbs in the serums from 1009 healthy blood donors were detected (Supplementary Tables S1 and S2). The overall seroprevalence levels of Ad14 and Ad55 nAbs were 24.8% (95% confidence interval, CI: 22.1%–27.5%) and 22.4% (95% CI: 19.8%–25.0%), respectively (Supplementary Tables S1 and S2). The trends in the Ad14 and Ad55-seropositive rates were similar in this cohort, and both increased with age (Trend Chi-square test, Ad14: P-value <0.001; Ad55: P-value <0.001; Figure 2A). Notably, the seropositive rates in individuals below 20 years of age were extremely low for both Ad14 and Ad55 (15.5% and 10.9%, respectively; Figure 2A), revealing that the infections by these two AdVs and the circulation of nAbs in young adults were relatively rare. The donors were categorized into four groups according to their nAb titers as follows: negative, <72; low, 72–200; moderate, 200–1000; and high, >1000. The distributions of the different nAb titers in the whole cohort were analyzed. Moderate and low nAb titers were frequently detected in Ad14-seropositive donors, while the majority of Ad55-seropositive ones had high nAb titers (Figure 2B), suggesting that Ad55 infection might elicit stronger nAb responses in comparison with Ad14 infection.
Figure 2.
Seroprevalence of Ad14 and Ad55 nAbs for healthy adults in Southern China. Serum samples from 1009 healthy blood donors were detected using MN assays based on Ad14-SEAP and Ad55-SEAP viruses. (A) The overall seropositive rates of Ad14 and Ad55 nAbs by age groups. The data were analyzed by χ2-test. (B) The distributions of serums with different nAb titers.
Titer distributions of Ad14 and Ad55 nAbs in different age groups
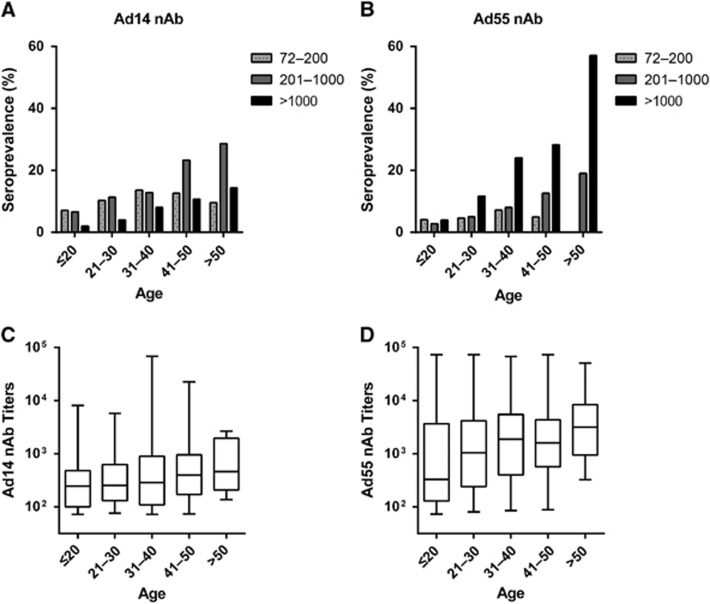
We then analyzed the distributions of nAb titers by age group. The frequency of serum samples with moderate or high nAb titers increased with age (Trend χ2-test, P-value <0.001 for moderate and high Ad14 and Ad55 nAbs, respectively), while the frequency for low nAb titers increased before 40 years old and decreased thereafter for both Ad14 and Ad55 (Figures 3A and 3B). Individuals with moderate nAb levels were dominant in Ad14-seropositive individuals older than 40 (Figure 3A), whereas the majority of Ad55-seropositive individuals older than 20 had high nAb titers (Figure 3B). Interestingly, almost 60% of volunteers older than 50 generated high levels of Ad55 nAb, and none of the individuals in this subgroup had a low titer of Ad55 nAb (Figure 3B). Thus, the overall seroprevalence levels for Ad14 and Ad55 nAbs were comparable, but their titer distributions within different age groups were dramatically different. Although no significant difference was observed for the Ad14 nAb levels in different age groups (Kruskal–Wallis test, P-value=0.07; Figure 3C), the Ad55 nAb levels definitely increased with age (Kruskal–Wallis test, P-value <0.05; Figure 3D), suggesting that nAb responses to Ad55 but not Ad14 infection tended to be enhanced when these individuals were re-infected by these strains.
Figure 3.
Titer distributions of Ad14 and Ad55 nAbs in age groups. The Ad14 (A and C) and Ad55 (B and D) seropositive donors were categorized into three subgroups according to their nAb titers as follows: low, 72–200; moderate, 201–1000; and high, >1000. The percentages of these subgroups in different age groups are shown (A and B), and the trends were analyzed with Trend χ2-test. The overall nAb levels in different age groups are also shown (C and D) and were analyzed by Kruskal–Wallis Test.
Seropositive rate and titer distributions of Ad14 and Ad55 nAbs in different gender and blood type groups
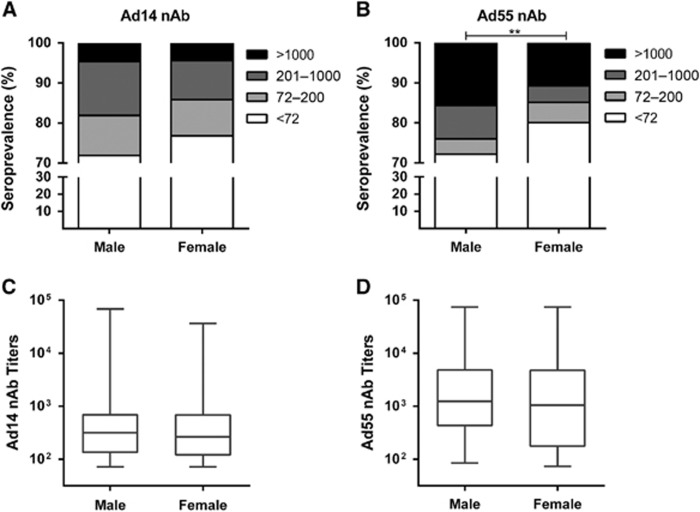
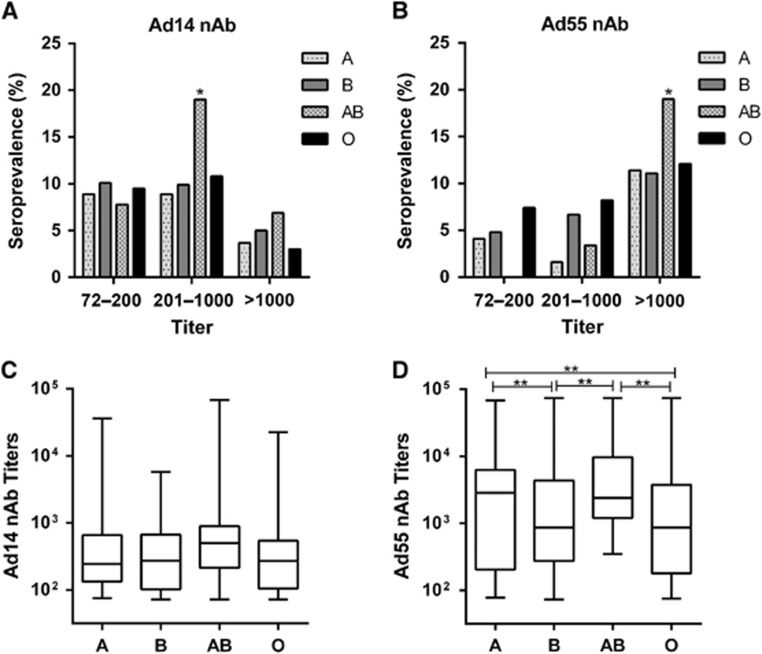
There was no significant difference for the Ad14-seropositive rates in the gender groups (χ2-test, P-value=0.093; Figure 4A), whereas a higher Ad55-seropositive rate was detected in males compared to females (χ2-test, P-value <0.01; Figure 4B). The titers of either Ad14 or Ad55 nAb were comparable in males and females (Mann–Whitney Test, P-value=0.552 and 0.600 for Ad14 and Ad55 nAb, respectively; Figures 4C and 4D), suggesting that the gender was not a major impacting factor on either the Ad14 or Ad55 nAb levels. Notably, the frequency of Ad14-seropositive samples, especially those with moderate nAb titers, was significantly higher in the AB blood type group than in the other blood type groups (χ2-test, P-value <0.05; Figure 5A). In addition, the percentage of Ad55-seropositive samples with high nAb titers was also higher in this group (χ2-test, P-value <0.05; Figure 5B). In addition, none of the volunteers with blood type AB had low Ad55 nAbs, and significantly fewer blood type A or AB donors generated moderate Ad55 nAbs (Figure 5B), which might imply that once the individuals with blood type AB were infected by Ad55, high titers of nAbs would be elicited. Consistent with this point, although the AB blood type individuals only had slightly higher levels of Ad14 nAb compared to other blood type groups (Kruskal–Wallis test, P-value=0.086; Figure 5C), the titer of Ad55 nAb in this group was significantly higher than that of the blood type B or O groups (Mann–Whitney test, P-value <0.05; Figure 5D). These results suggested that the blood type may influence Ad14 and Ad55 infection and the generation of nAb responses to these two AdVs.
Figure 4.
Seroprevalence and titer distributions of Ad14 and Ad55 nAbs in gender groups. The seropositive rates of Ad14 (A) and Ad55 (B) nAbs in different gender groups are shown. The difference in the overall seropositive rates between males and females was analyzed with χ2-test, and a P-value <0.05 was considered statistically significant. **P-value<0.01. The overall Ad14 (C) and Ad55 (D) nAb levels in the gender groups are shown, and the difference between males and females was analyzed by Mann–Whitney test.
Figure 5.
Seroprevalence and titer distributions of Ad14 and Ad55 nAbs in the blood type groups. The frequency of Ad14 (A) and Ad55 (B)-seropositive samples with different nAb titers in the blood type groups are shown. The difference in the frequencies between blood type groups was analyzed by χ2-test. A P-value <0.05 was considered to be statistically significant, *P-value <0.05. The overall Ad14 (C) and Ad55 (D) nAb levels in the blood type groups are shown, and the difference between the groups was analyzed by Mann–Whitney test. A P-value <0.05 was considered statistically significant, **P-value<0.01.
Seroprevalence of Ad14 nAb in Ad55-seropositive donors was higher than that in Ad55-seronegative ones, and vice versa
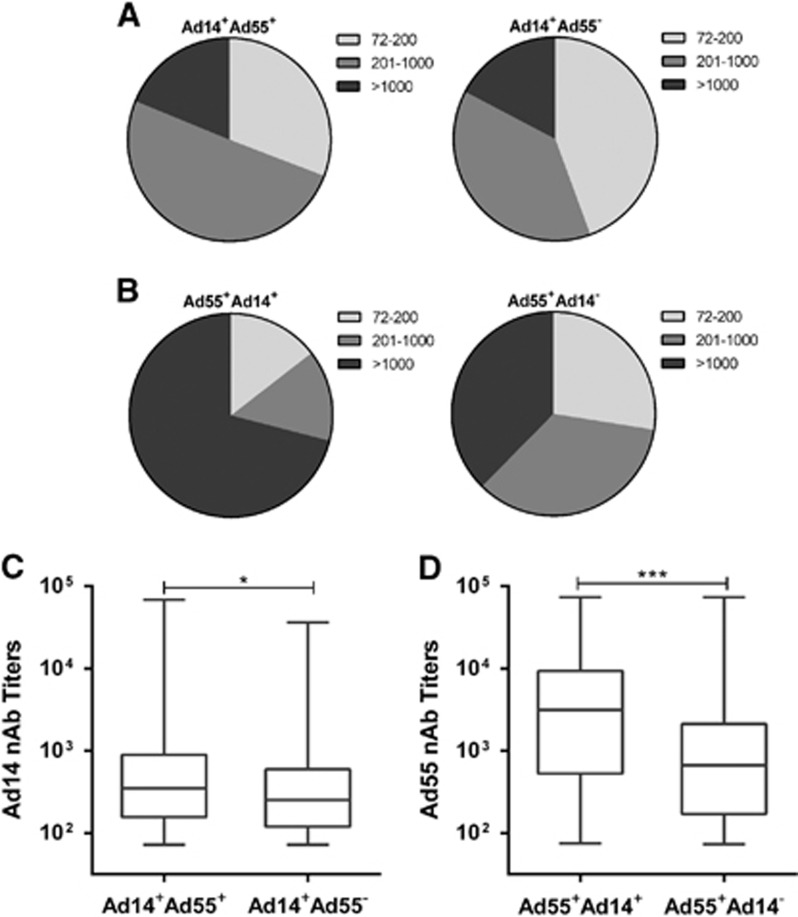
Next, we analyzed the frequency of Ad14-seropositive donors in Ad55-seropositive or seronegative individuals. The Ad14-seropositive rate was significantly higher in Ad55-seropositive donors than in Ad55-seronegative ones (Mann–Whitney test, P-value <0.01; Table 1). Similar trends were detected for the Ad55-seropositive rate in Ad14-seropositive or seronegative donors (P-value <0.01; Table 1). The frequency of blood donors with moderate Ad14 nAb levels was slightly higher in Ad55-seropositive compared to Ad55-seronegative donors (Figure 6A). However, in Ad14-seropositive donors, many more individuals had high titers of Ad55 nAb in comparison with Ad14-seronegative ones (χ2-test, P-value <0.001; Figure 6B). Interestingly, the titers of Ad14 nAb in Ad55-seropositive donors were significantly higher than they were in Ad55-seronegative ones (Mann–Whitney test, P-value <0.05; Figure 6C). In addition, the Ad55 nAb titers in Ad14-seropositive donors were much higher than they were in Ad14-seronegative donors (P-value <0.001; Figure 6D). All these results suggested that Ad55-seropositive donors tended to be Ad14-seropositive and vice versa, and double-positive serums had higher nAb titers than single-positive ones.
Table 1. Correlation of Ad14 and Ad55 nAb seropositive rates.
| Ad14-seropositivea | Ad14-seronegativeb | Ad14 positive rate (%)c | |
|---|---|---|---|
| Ad55-seropositivea | 117 | 109 | 51.8d |
| Ad55-seronegativeb | 133 | 650 | 17.0 |
| Ad55 positive rate (%)e | 46.8d | 14.4 |
The absolute number of serums containing Ad14 or Ad55 nAbs.
The absolute number of serums without Ad14 or Ad55 nAbs.
The rates of Ad14-seropositive serums in Ad55-seropositive or negative ones.
Statistics were performed by χ2-test, P<0.01.
The rates of Ad55-seropositive serums in Ad14-seropositive or negative ones.
Figure 6.
Profiling of the seropositive rates and nAb levels in single- and double-positive blood donors. (A) The distributions of Ad14-seropositive donors with different nAb titers in Ad55-seropositive or seronegative groups. (B) The distributions of Ad55-seropositive donors with different nAb titers in Ad14-seropositive or seronegative groups. The difference between the groups was analyzed by χ2-test. The overall Ad14 (C) and Ad55 (D) nAb levels in single- or double-positive groups are also shown. The comparison between groups was performed by Mann–Whitney test, and a P-value <0.05 was considered statistically significant. *P<0.05; ***P<0.001.
DISCUSSION
Recently, two highly virulent AdVs called Ad14 and Ad55 have attracted increasing attention; these viruses caused severe clinical consequences in immuno-competent adults over a series of outbreaks.2 Clarifying the circulation of Ad14 and Ad55 nAbs in general populations is essential for understanding the herd immunity. Here, we investigated the seroprevalence of Ad14 and Ad55 nAbs in a large cohort from Southern China, and we found that the seropositive rates of Ad14 and Ad55 were much lower than that of Ad5 according to our previous results.24 This finding may imply that the general populations, especially those younger than 40, were threatened by these two AdVs. Therefore, Ad14 and Ad55 should be taken into account when patients with severe ARDs are hospitalized.2
In this study, we adopted EGFP- or SEAP-expressing reporter viruses based on clinical isolates of Ad14 and Ad55, and we established sensitive and high-throughput MN assays for assessing the nAbs against these two AdVs (Figure 1). These assays possessed at least two advantages over the methods that were described previously.25 Because the nAbs against AdV could be raised against hexon as well as fiber proteins, the chimeric Ad3 that carried the Ad14 hexon as well as Ad3 fiber proteins could only detect Ad14 nAbs that were specific for hexon.26 In addition, MN assays based on SEAP-expressing AdVs were reported to be more sensitive and objective than previously used CPE-based methods.27 Consistent results were obtained when SEAP and EGFP reporter viruses were applied (Figure 1), reflecting the feasibility of these MN assays. Given that nAbs have shown beneficial effects for treating patients infected with H5N1 influenza virus or Ebola virus,28, 29 these MN assays could be utilized to screen serums containing high levels of nAbs or monoclonal nAbs against Ad14 and Ad55, to treat patients with severe illnesses caused by these two AdVs (unpublished data).
For the purpose of genetic vector development, numerous studies have investigated the pre-existing nAbs against human and chimpanzee AdVs.24, 26, 27, 30, 31, 32, 33, 34 Regarding Ad14 and Ad55, although several case reports or epidemiological studies have been described,5, 6, 7, 8, 9, 11, 12, 13, 14, 35 the surveillance of their pre-existing nAbs was seldom performed. One recent study described seropositive rates of Ad55 nAbs in pediatric patients, and another study analyzed the Ad14 nAbs in a small population.25, 36 However, the seroprevalence of Ad14 and Ad55 nAbs in healthy adults remained to be explored, and it relied on randomly collected large-scale cohorts. Moreover, detailed analyses within age, gender or blood type groups could not be performed due to the limited samples in these two studies. Here, we investigated the seroprevalance of Ad14 and Ad55 nAbs based on a randomly collected cohort including up to 1009 healthy blood donors, and we found that the seropositive rates in young adults were low and increased with age (Figure 2), which was in accordance with the findings observed in other AdVs.32 The seropositive rates and nAb titers in different subgroups were successfully analyzed. We also found that Ad14-seropositive donors tended to generate moderate nAb titers while Ad55-seropositive ones were prone to having high nAb titers, especially in individuals older than 20 (Figure 3). These findings added to our knowledge of human immunity against Ad14 and Ad55 infections.
Similar to the results of other studies,33, 37 the seroprevalence of Ad14 and Ad55 nAbs was independent of the donor’s gender (Figure 4). However, our results showed that the blood type significantly impacted the susceptibility and nAb responses of individuals who were exposed to Ad14 or Ad55 infection (Figure 5). Although a relationship between the blood type and AdV infection was not described previously, the histo-blood group antigens were involved in the infection of hepatitis B virus and norovirus.38, 39, 40 We showed that individuals with blood type AB displayed the highest seropositive rates for Ad14 and Ad55 nAbs (Figure 5), indicating that this group of people might be more susceptible to infection by Ad14 or Ad55. In addition, the nAb titers in AB blood type donors were higher than that of other groups, revealing that stronger immune responses were induced by Ad14 or Ad55 infection in these individuals.
We also showed that Ad14-seropositive donors tended to have higher Ad55-seropositive rates and nAb titers than Ad14-seronegative ones, and vice versa (Figure 6), indicating that there was a positive correlation between Ad14 and Ad55 seroprevalence. The nAb titers in double-positive donors were significantly higher than they were in Ad14 or Ad55 single-positive ones (Figure 6). These results may be attributed to fiber protein, which is one of the major capsid antigens of AdVs. As a recombinant of Ad11 and Ad14, Ad55 shares a similar hexon protein with Ad11 and a fiber protein with Ad14.21 Although many studies reported that hexon-specific antibodies were predominantly elicited in individuals who were infected by other AdVs,41, 42 fiber-specific nAbs were also frequently detected.26, 43, 44 If fiber-specific nAbs were generated in individuals who were infected by either Ad14 or Ad55, it may neutralize both variants and thus contribute to the cross-neutralizing activity of double-positive serums. However, these speculations should be confirmed in future studies.
In summary, we established convenient MN assays to survey the effects of nAbs against Ad14 and Ad55, and we investigated the seroprevalence of these two AdVs in a general population from Southern China. We also provided the distributions of nAbs by age, gender and blood type groups. These results emphasized the importance of monitoring these two AdVs in general populations and developing vaccines for disease prevention.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NO 31200693 and 31470892), the Guangzhou Health Care and Cooperative Innovation Major Project (NO 201508020252), the Pearl River S&T Nova Program of Guangzhou (NO 201506010076), and a grant from the CAS Youth Innovation Promotion Association to Liqiang Feng (NO 2014328). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We also thanked Prof Qiwei Zhang (Southern Medical University, China) and Prof Chengfeng Qin (Beijing Institute of Microbiology and Epidemiology, China) for their kind gift of the Ad14 and Ad55 viruses.
Footnotes
Supplementary Information for this article can be found on the Emerging Microbes & Infections website (http://www.nature.com/emi)
Supplementary Material
References
- Davison AJ, Benko M, Harrach B. Genetic content and evolution of adenoviruses. J Gen Virol 2003; 84: 2895–2908. [DOI] [PubMed] [Google Scholar]
- Tan D, Zhu H, Fu Y et al. Severe community-acquired pneumonia caused by human adenovirus in immunocompetent adults: a multicenter case series. PLoS One 2016; 11: e0151199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz AN, Ottolini M. The role of adenovirus in respiratory tract infections. Curr Infect Dis Rep 2010; 12: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebruegge M, Curtis N. Adenovirus infection in the immunocompromised host. Adv Exp Med Biol 2010; 659: 153–174. [DOI] [PubMed] [Google Scholar]
- Chmielewicz B, Benzler J, Pauli G et al. Respiratory disease caused by a species B2 adenovirus in a military camp in Turkey. J Med Virol 2005; 77: 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PF, Schmidt MA, Lu X et al. A community-based outbreak of severe respiratory illness caused by human adenovirus serotype 14. J Infect Dis 2009; 199: 1427–1434. [DOI] [PubMed] [Google Scholar]
- Jin Y, Zhang RF, Xie ZP et al. Prevalence of adenovirus in children with acute respiratory tract infection in Lanzhou, China. Virol J 2013; 10: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QB, Tong YG, Wo Y et al. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009-2012. Influenza Other Respir Viruses 2014; 8: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Zhu Z, Huang F et al. Adenoviruses associated with acute respiratory diseases reported in Beijing from 2011 to 2013. PLoS One 2015; 10: e0121375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderveen J, Kok G. Isolation and typing of adenoviruses recovered from military recruits with acute respiratory disease in the Netherlands. Am J Hyg 1957; 65: 119–129. [DOI] [PubMed] [Google Scholar]
- Metzgar D, Osuna M, Kajon AE et al. Abrupt emergence of diverse species B adenoviruses at US military recruit training centers. J Infect Dis 2007; 196: 1465–1473. [DOI] [PubMed] [Google Scholar]
- Huang G, Yu D, Zhu Z et al. Outbreak of febrile respiratory illness associated with human adenovirus type 14p1 in Gansu Province, China. Influenza Other Respir Viruses 2013; 7: 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajon AE, Lu X, Erdman DD et al. Molecular epidemiology and brief history of emerging adenovirus 14-associated respiratory disease in the United States. J Infect Dis 2010; 202: 93–103. [DOI] [PubMed] [Google Scholar]
- Carr MJ, Kajon AE, Lu X et al. Deaths associated with human adenovirus-14p1 infections, Europe, 2009-2010. Emerg Infect Dis 2011; 17: 1402–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, An J, Xie Z et al. Genome and bioinformatic analysis of a HAdV-B14p1 virus isolated from a baby with pneumonia in Beijing, China. PLoS One 2013; 8: e60345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama M, Amitai Z, Nutman A et al. Outbreak of adenovirus type 55 infection in Israel. J Clin Virol 2016; 78: 31–35. [DOI] [PubMed] [Google Scholar]
- Lafolie J, Mirand A, Salmona M et al. Severe pneumonia associated with adenovirus type 55 infection, France, 2014. Emerg Infect Dis 2016; 22: 2012–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MP, Seto J, Jones MS et al. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J Clin Microbiol 2010; 48: 991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Huang G-H, Pu Z-H et al. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest 2014; 145: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildgen O, Gu L, Qu J et al. Sustained viremia and high viral load in respiratory tract secretions are predictors for death in immunocompetent adults with adenovirus pneumonia. Plos One 2016; 11: e0160777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Nian QG, Zhang Y et al. In vitro characterization of human adenovirus type 55 in comparison with its parental adenoviruses, types 11 and 14. PLoS One 2014; 9: e100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke JR, Yong SL, Cook JL. Low-level expression of the E1B 20-kilodalton protein by adenovirus 14p1 enhances viral immunopathogenesis. J Virol 2016; 90: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aste-Amezaga M, Bett AJ, Wang F et al. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum Gene Ther 2004; 15: 293–304. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang Y, Feng L et al. Epidemiology of adenovirus type 5 neutralizing antibodies in healthy people and AIDS patients in Guangzhou, southern China. Vaccine 2011; 29: 3837–3841. [DOI] [PubMed] [Google Scholar]
- Su XB, Tian XG, Jiang ZX et al. Human adenovirus serotype 3 vector packaged by a rare serotype 14 hexon. Plos One 2016; 11: e0156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Dong J, Wang C et al. Characteristics of neutralizing antibodies to adenovirus capsid proteins in human and animal sera. Virology 2013; 437: 118–123. [DOI] [PubMed] [Google Scholar]
- Zhang S, Huang W, Zhou X et al. Seroprevalence of neutralizing antibodies to human adenoviruses type-5 and type-26 and chimpanzee adenovirus type-68 in healthy Chinese adults. J Med Virol 2013; 85: 1077–1084. [DOI] [PubMed] [Google Scholar]
- Mupapa K, Massamba M, Kibadi K et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. J Infect Dis 1999; 179: S18–S23. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Zhong NS, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med 2007; 357: 1450–1451. [DOI] [PubMed] [Google Scholar]
- Mast TC, Kierstead L, Gupta SB et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 2010; 28: 950–957. [DOI] [PubMed] [Google Scholar]
- Abbink P, Lemckert AA, Ewald BA et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol 2007; 81: 4654–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner AR, Vogels R, Kaspers J et al. Age dependence of adenovirus-specific neutralizing antibody titers in individuals from sub-Saharan Africa. J Clin Microbiol 2006; 44: 3781–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xing M, Zhang C et al. Neutralizing antibody responses to enterovirus and adenovirus in healthy adults in China. Emerg Microbes Infect 2014; 3: e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Hennequi M, Elvachev A et al. The sero-prevalence of anti-adenovirus 5 neutralizing antibodies is independent of a chronic hepatitis B carrier state in China. Arch Virol 2015; 160: 1125–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajon AE, Dickson LM, Metzgar D et al. Outbreak of febrile respiratory illness associated with adenovirus 11a infection in a Singapore military training cAMP. J Clin Microbiol 2010; 48: 1438–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Jiang Z, Ma Q et al. Prevalence of neutralizing antibodies to common respiratory viruses in intravenous immunoglobulin and in healthy donors in southern China. J Thorac Dis 2016; 8: 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W-X, Zou X-H, Jiang S-Y et al. Prevalence of serum neutralizing antibodies to adenovirus type 5 (Ad5) and 41 (Ad41) in children is associated with age and sanitary conditions. Vaccine 2016; 34: 5579–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato H. Norovirus and histo-blood group antigens. Jpn J Infect Dis 2011; 64: 95–103. [PubMed] [Google Scholar]
- Branch DR. Blood groups and susceptibility to virus infection: new developments. Curr Opin Hematol 2010; 17: 558–564. [DOI] [PubMed] [Google Scholar]
- Wang D-S, Chen D-L, Ren C et al. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer 2012; 131: 461–468. [DOI] [PubMed] [Google Scholar]
- Roberts DM, Nanda A, Havenga MJ et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 2006; 441: 239–243. [DOI] [PubMed] [Google Scholar]
- Qiu H, Li X, Tian X et al. Serotype-specific neutralizing antibody epitopes of human adenovirus type 3 (HAdV-3) and HAdV-7 reside in multiple hexon hypervariable regions. J Virol 2012; 86: 7964–7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RR, Lynch DM, Iampietro MJ et al. Adenovirus serotype 5 neutralizing antibodies target both hexon and fiber following vaccination and natural infection. J Virol 2012; 86: 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Gall JG, Nason M et al. Differential specificity and immunogenicity of adenovirus type 5 neutralizing antibodies elicited by natural infection or immunization. J Virol 2010; 84: 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.