Abstract
Humoral responses are essential for the protective efficacy of most Ebola virus (EBOV) candidate vaccines; however, the in vivo development of protective anti-EBOV B-cell responses is poorly defined. Here, by using the virus-like particle (VLP) as a model antigen, we demonstrate that humoral responses are generated through follicular B-cell and T-cell-dependent mechanisms in a mouse model of EBOV infection. In addition, we show that the inclusion of the clinical-grade dsRNA adjuvant known as poly-ICLC in VLP vaccinations both augments and sustains germinal center B-cell reactions, antigen-specific B-cell frequencies and anti-EBOV serum titers. Finally, we used mice that were deficient in either B-cells or T-cell-dependent antibody production to distinguish the contributing roles of EBOV humoral responses. We demonstrate that while anti-EBOV antibody responses promote protection, VLP-vaccinated mice can survive EBOV infection in the absence of detectable anti-EBOV antibodies. Moreover, we found that adjuvant signaling could circumvent the complete requirement for B-cell immunity in protection against EBOV. Collectively, these studies may prove valuable for the characterization and future development of additional EBOV vaccine candidates.
Keywords: adjuvant, Ebola, germinal center B-cell, T-cell dependent antibody, vaccine, virus-like particle
INTRODUCTION
The 2013–2016 Ebola virus disease (EVD) epidemic in Western Africa, which involved more than 28 000 individuals and claimed more than 11 000 lives (World Health Organization, http://www.who.int/en/; Centers for Disease Control and Prevention, http://www.cdc.gov/), triggered the advancement of several medical countermeasures against the Ebola virus (EBOV), some of which had been in development for decades.1, 2, 3 Multiple vaccine platforms expressing or consisting of the EBOV trimeric glycoprotein (GP1,2) have been generated, and several are currently under clinical evaluation.4, 5, 6, 7, 8 However, there are fundamental gaps in our understanding of how candidate vaccines induce protective host responses, and a definitive immune correlate for vaccine efficacy has yet to be defined.9, 10
On the basis of studies using nonhuman primate (NHP) and rodent models of EVD, achieving both cellular and humoral responses against EBOV is thought to be critical for protection.11, 12 The roles of EBOV-specific CD4+ and CD8+ T-cell responses have been extensively characterized; nonetheless, they remain complex and appear to be largely vaccine platform-dependent. Adenovirus 5 and Venezuelan equine encephalitis virus-like replicon-based EBOV vaccine studies have demonstrated a critical role for CD8+ T-cell responses in protecting against NHP and murine EBOV infection.13, 14, 15 However, dispensable roles for EBOV-specific CD8+ T-cell responses have been supported by studies using recombinant vesicular stomatitis virus and adenovirus Hu5 vaccine platforms.16, 17, 18 Murine studies from our group that used EBOV viral-like particles (VLPs) have shown a similar importance for T-cells; however, adjuvants have impacted the establishment and relative contributions of these responses in protecting against EVD.19, 20, 21 Despite reported differences and alternative mechanisms for T-cell-mediated immunity between EBOV-based vaccine platforms, most converge on an obligate requirement for humoral responses.16, 17, 18, 21, 22 Further supporting this importance is the preclinical success of anti-EBOV antibodies (for example, ZMapp, ZMab) in treating EBOV infection.23, 24, 25
The production of antibodies following a direct interaction between B-cells and the cognate antigen can occur through T-cell-independent mechanisms (TI); however, the generation of high-affinity class-switched antibodies is dependent on follicular T-cell help (TFH). The formation of T-cell-dependent (TD) antibody responses can be shaped through several key events including (i) the induction of germinal center (GC) B-cell reactions; (ii) TFH quantity and quality; (iii) activation-induced cytidine deaminase (AICDA/AID)-mediated somatic hypermutation and isotype class-switching; and (iv) the selection and differentiation of GC B-cells to form the antigen-specific B-cell compartment.26, 27 However, in vivo studies that define how EBOV B-cell immunity is established, the relative contributions of TI and TD B-cell mechanisms and the direct requirement for B-cell responses to protect against EBOV infection have been limited to date.17, 28
Previously, we demonstrated that the vaccination of laboratory mice, guinea pigs, or NHPs with a VLP consisting of EBOV matrix protein (VP-40) and GP1,2 elicited complete protection against EBOV infection.29, 30, 31 We also discovered that the inclusion of the clinical-grade dsRNA polyinosinic-polycytidylic acid (poly-IC) derivative known as poly-IC poly-l-lysine carboxymethylcellulose (poly-ICLC) in VLP vaccine preparations increased EBOV GP1,2-specific antibody titers and durable protection from EVD in mice.19, 20 Here, we use VLP as a model system to examine the establishment and requirement for EBOV B-cell immunity in mice and the impact of poly-ICLC adjuvant signaling on VLP-mediated B-cell responses.
MATERIALS AND METHODS
Reagents
Poly-ICLC (Hiltonol) was provided by Oncovir (Washington, DC, USA). Ebola VLPs were produced as previously described.20, 30 In brief, 293T cells were transfected with Ebola Zaire (Kikwit) virus glycoprotein and VP-40. VLP supernatants were collect at 72 h post-transfection, they were sucrose gradient-purified, and their total protein content was determined using a bicinchoninic acid protein assay. To ensure sterility, the VLPs were irradiated at 1e6 rad, and they contained less than 25 EU/mL endotoxin and less than 10 colony-forming units (CFU) of bacteria per vaccination. The particular lot of VLP within this study was previously used for EBOV vaccine studies and has been extensively characterized.19, 32 The GP content for these studies was determined by Western blot and fixed at a 10 μg GP dose for the vaccinations. The VLPs were maintained at −80 °C and diluted in sterile saline and/or combined with poly-ICLC prior to vaccination.
Mouse strain and vaccinations
C57BL/6 (NCI Charles River Strain Code 027, Jackson Stock No. 000664), CD40-deficient (Jackson Stock No. 002928), μMT (Jackson Stock No. 002288) and AID-deficient mice (kind gifts from Drs Pat Gearhart, Robert Maul, NIAIA, Baltimore and Rafael Casellas, NIH, Bethesda) were each vaccinated intramuscularly with VLP (10 μg GP1,2 content) or VLP (10 μg GP1,2 content) plus 10 μg of poly-ICLC at 3-week intervals (day 0, day 21).
Enzyme-linked immunosorbent assays
ELISAs were performed as previously described.19 In brief, blood was collected from the vaccinated mice at the indicated time points in Vacutainer serum-separating tubes. ELISA plates were coated with recombinant mammalian cell-expressed EBOV GP1,2 at 2 μg/mL in phosphate-buffered saline (PBS; Corning Life Sciences, Corning, NY, USA). The sera were diluted by half-log dilutions starting at 1:100, and then incubated for 1 h on GP1,2-coated plates. The plates were washed and then incubated with the indicated secondary horseradish peroxidase (HRP) antibody. ELISAs were developed using 3,3′,5,5′-tetramethylbenzidine (TMB) substrate/stop solution and measured on a Tecan plate reader. The absorbance cut-off was determined as the background+0.2 O.D.
Flow cytometry
Single-cell suspensions of draining lymph nodes and spleens were collected at the indicated time points. The cells were washed using FACS buffer (PBS, 0.5% BSA and 2 mM EDTA; Corning, Sigma, St Louis, MO, USA), lysed with red blood cell (RBC) buffer (Sigma), and subsequently counter-stained. The B-cell staining included B220 (Becton-Dickinson Biosciences (BD), Franklin Lanes, NJ, USA Clone RA3-6B2), IgM (BD Clone R6-60.2), IgD (eBioscience, San Diego, CA, USA Clone 11-26C), CD38 (BD Clone 90), CD95 (BD Clone Jo-2), and T & B Cell Activation Antigen (BD Clone GL-7). T follicular helper cell staining was performed by a primary incubation with CXCR5 (BD Clone 2G8) followed by secondary incubation using goat anti-rat (H+L)-biotin (Jackson ImmunoResearch, West Grove, PA, USA 112-067-003). Subsequently, the cells were counter-stained with streptavidin (BD 557598), CD3 (BD Clone 500A2), CD4 (BD Clone RM4-5), PD-1 (eBioscience Clone RMP1-30), and ICOS (BD Clone 7E.17G9). All the samples were Fc-blocked (anti-CD16/CD32, BD) and stained to evaluate their viability (live/dead aqua, Invitrogen, Carlsbad, CA, USA) before counter-staining. The data were collected on a BD FACSCanto II or BD FACSAria II and analyzed using FlowJo (Treestar, Ashland, OR, USA).
Neutralization assays
Neutralizing antibody titers from the serum samples were determined using recombinant vesicular stomatitis Indiana virus (rVSV) particles that coexpressed EBOV GP1,2 and enhanced green fluorescent protein (eGFP) (a kind gift from Kartik Chandran, Albert Einstein College of Medicine). In brief, serum dilutions beginning at 1:10 were incubated at 37 °C for 1 h with rVSV and 5% v/v Hemo-lo guinea pig complement (Cedarlane Laboratories, Burlington, NC, USA). rVSV/serum complexes were then incubated with 1 × 105 FreeStyle 293F cells (ThermoFisher Scientific, Waltham, MA, USA) at 1 × 106 cells/mL for 18–20 h at 37 °C. The infection percentage, as determined by eGFP expression, was measured using fluorescence-activated cell sorting (FACS). The data were collected on a BD Canto II and LSR II. The neutralization was calculated by normalizing the infection percentages to the infections performed in the presence of control sera from non-vaccinated laboratory mice.
B-cell ELISPOTs
ELISPOTs were performed according to the MabTech (Cincinnati, OH, USA) protocol. In brief, cryopreserved splenocyte populations were thawed, washed 2 × with complete medium, and adjusted to 2 × 106 cells/mL. Splenocyte suspensions (0.1 mL) were added to recombinant EBOV GP1,2-coated ELISPOT plates and then incubated overnight at 37 °C with 95/5 oxygen/CO2. The plates were then washed, incubated with biotin-IgG secondary antibody (MabTech) followed by streptavidin-HRP (MabTech) and developed using TMB substrate/stop solution. Imaging/counting was performed using a CTL Immunospot instrument (Cellular Technology, Shaker Heights, OH, USA).
Ebola virus infections
Laboratory mice were inoculated intraperitoneally with a target dose of 1000 pfu of mouse-adapted Ebola virus/H. sapiens-tc/COD/1976/Yambuku-Mayinga (ma-EBOV) on day 28 after the last vaccination or during secondary inoculations. All the live-virus studies were conducted under maximum (biosafety level 4) containment. Clinical observations were recorded throughout the study starting on the day of virus inoculation. Moribund mice were euthanized according to institution-approved clinical scoring.
Statistical analysis
All the statistical analyses were performed using GraphPad Prism (Windows V6). Data comparisons using unpaired parametric Student’s t-tests were performed for titer analysis and immune populations. A survival curve comparison was performed using a log-rank (Mantel–Cox) test.
RESULTS
VLP vaccination induces robust germinal center B-cell responses, which are augmented with the inclusion of poly-ICLC
Prior VLP vaccination studies have demonstrated the induction of EBOV GP1,2, antibody responses and that these responses can be heightened with the inclusion of adjuvants. Recently, we demonstrated that the dsRNA adjuvant poly-ICLC provided enhanced VLP-mediated anti-EBOV titers and influenced the antibody-isotype.19, 20 The production of high-affinity class-switched antibodies is dependent on germinal center (GC) formation;26, 33 however, limited characterization of this B-cell compartment has been reported for vaccine platforms that were developed against EBOV.28 Therefore, we examined if VLP vaccination resulted in the generation of GC reactions and if poly-ICLC would impact these responses. Mice were vaccinated with VLP (intramuscularly) in the presence or absence of poly-ICLC using a prime-boost schedule at three-week intervals (day 0, day 21). VLP-vaccinated mice were characterized by an approximate three-fold increase in draining lymph node (dLN, popliteal) cellularity at day 10 subsequent to prime vaccination, whereas the inclusion of poly-ICLC resulted in an approximate seven-fold increase (Figure 1A). However, the relative frequency of B220+ B-cells was unaltered (Figure 1A). On day 10, subsequent to prime vaccination, GC B-cells (B220+GL7+CD95+) were clearly identified within the dLN of both VLP-vaccinated groups. However, the relative frequencies and total numbers of GC B-cells were increased in the presence of poly-ICLC (Figure 1B).
Figure 1.
Poly-ICLC augments and sustains VLP-mediated germinal centers. Mice were vaccinated with VLP or VLP plus poly-ICLC (pICLC), and their draining lymph nodes (dLNs) were isolated. Single-cell suspensions were stained with B220, IgD, IgM, CD38, CD95, GL-7 and live/dead dye and collected by FACS. (A) Left, day 10 dLN cellularity; right, relative percentage of the B220+ population on day 10 dLN. (B) A representative FACS plot of the relative percentage and total number of day 10 dLN B220+CD95+GL7+ GC B-cells. (C) A representative day 28 (day 7 post-boosting) FACS plot and the relative frequency of B220+CD95+GL7+ GC B-cells (left panels and top right) and of B220+CD38loIgD−IgM− B-cells (right panels and top right). (D) The relative percentage of day 49 (day 28 post-boosting) dLN B220+CD95+GL7+ GC B-cells. (E) The relative frequency of day 25 (day 4 post-boosting) EBOV GP1,2-specific B-cells in the spleen as measured by ELISPOT. For clarity in the graphics, only significant comparisons have been labeled (means, s.e.m., n=3–5; *P<0.05, **P<0.005, and ***P<0.0005). All the other statistical comparisons are assumed to be non-significant.
We next determined the impact of vaccine boosting on VLP-mediated GC reactions. As with prime vaccination, GC B-cells were detected within the dLN on day 28 (day 7 post-boosting), and, consistent with the prime vaccination results, the inclusion of the adjuvant significantly augmented the relative frequency of GC B-cells (Figure 1C, left panel). Additional phenotypic characterization of the B-cell compartment supported the induction of activated class-switched (B220+GL7+CD95+CD38lowIgD−IgM−) B-cells following VLP vaccination with heightened frequencies observed when adding poly-ICLC (Figure 1C, right panel). Notably, the relative frequency of GC B-cells increased after boosting in the presence of poly-ICLC, whereas VLP-only-vaccinated mice presented comparable GC B-cell frequencies following both prime and boost vaccinations (Figures 1B and 1C). VLP-mediated GC reactions were still present within the dLN at four weeks post-boosting (day 49) with a continued increase in GC B-cell frequencies being observed in the presence of the adjuvant (Figure 1D). Consistent with GC B-cell dynamics, the VLP-induced antibody responses plateaued following vaccination in the absence of the adjuvant. However, the inclusion of poly-ICLC resulted in a continual rise of total anti-GP1,2 IgG titers and enhanced EBOV neutralization up to one month post-final vaccination (day 49), a time-point associated with acute protection from EBOV infection (Supplementary Figures S1A and S1C). Durable (>5 months post-vaccination) anti-EBOV GP1,2 IgG responses were additionally augmented in the presence of poly-ICLC (Supplementary Figure S1B, top). Interestingly, after one, two or three vaccinations, the EBOV GP1,2-specific IgM responses were marginal and were detected only in the presence of the adjuvant (Supplementary Figure S1B, bottom).
Because productive GC reactions result in the generation of antibody-secreting cells (ASCs), we measured the frequency of EBOV GP1,2-specific B-cells. Antigen-specific B-cells could not be detected after prime vaccination (data not shown), but we observed an approximate five-fold increase in ASC frequencies after the inclusion of poly-ICLC following boosting (Figure 1E). GP1,2-specific B-cells were still detectable up to day 49 in the presence of adjuvant, but they approached our lower limit of detection for VLP vaccination alone (data not shown). Altogether, we demonstrate that VLP-mediated B-cell responses are associated with GC formation and that the addition of poly-ICLC as an adjuvant resulted in augmented GC B-cell frequencies, increased generation of EBOV GP1,2-specific ASCs and elevated antibody titers.
VLP-mediated humoral immunity is established through follicular B-cell and T-cell-dependent mechanisms, but it is partially dispensable for protection
The quality and quantity of GC B-cell formation is dependent on TFH cells.34 Consistent with our recent findings and the relationship between TFH and the formation of GC reactions,19, 35 VLP-mediated TFH (CD3+CD4+PD-1+CXCR5hi) cells are generated during vaccination (Figures 2A and 2B). Moreover, the relative size of the TFH compartment is increased with the addition of adjuvant, suggesting that poly-ICLC augments humoral immunity by promoting TFH and GC B-cell reactions (Figures 2A and 2B). Consistent with the cellular phenotyping, high levels of inducible T-cell costimulator (ICOS, CD278) was detected on TFH cells following vaccination with a subtle increase of expression after adjuvant inclusion (Figure 2C). Interestingly, the relative frequency of dLN CD3+CD4+ T-cells declined subsequent to vaccination (Figure 2B).
Figure 2.
VLP-mediated humoral responses are dependent on follicular T-cell help. Mice were vaccinated with VLP or VLP plus poly-ICLC. Single-cell suspensions from the draining lymph nodes were stained with CD4, CD3, CXCR5, PD-1, ICOS, and live/dead dye and then analyzed by FACS. (A) The representative gating of day 7 prime TFH subsets. (B) Top, relative percentage of CD3+CD4+ and bottom, TFH frequency. (C) Top, ICOS mean fluorescent intensity of respective T-cell subsets subsequent to different vaccination regimes; Bottom, representative ICOS surface expression of CD3+CD4− (shaded) and CD3+CD4+PD-1+CXCR5hi (open) TFH cells. For graphic clarity, only the significant comparisons have been labeled (means, s.e.m.; n=5, *P<0.05, **P<0.005). All the other statistical comparisons are assumed to be non-significant.
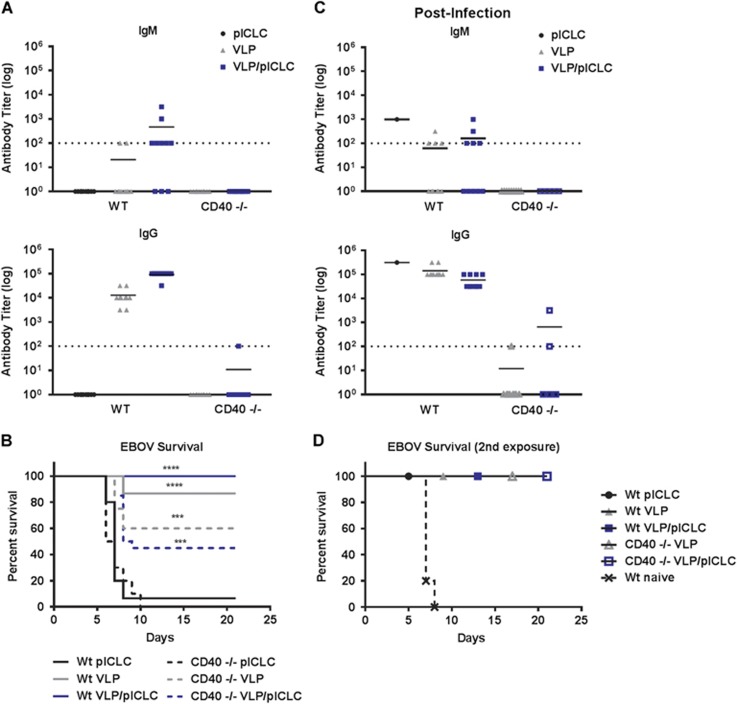
CD40-CD40L interactions between GC B-cells and TFH cells are required to induce productive T-cell-dependent (TD) antibody responses.36 We therefore determined the direct in vivo contribution of this pathway during VLP vaccination. The tumor necrosis factor superfamily, receptor 5 knockout (CD40−/−) mice have defective TD humoral immune responses while also being capable of establishing TI antibody responses.37 Wild-type and CD40−/− mice were prime-boost-vaccinated (day 0, day 21) with VLP and EBOV GP1,2-specific antibody titers were analyzed on day 35. Wild-type mice that were vaccinated with VLP displayed robust IgG antibody titers; however, the CD40−/− mice had no detectable anti-GP1,2-specific IgG or IgM responses (Figure 3A and Supplementary Figure S2A). The VLP candidate vaccine consists of both EBOV GP and VP-40; we therefore also determined antibody reactivity against VP-40. Consistent with the lack of GP1,2 antibody responses, the CD40−/− VLP-vaccinated mice also failed to establish anti-VP-40 humoral responses (Supplementary Figure S2B).
Figure 3.
EBOV GP antibody responses are established through T-cell-dependent mechanisms. (A) Wild-type or CD40−/− mice were vaccinated with VLP with or without poly-ICLC at day 0 and day 21. Their sera were collected at day 35 (day 14 post-boosting) and EBOV GP1,2-specific IgG and IgM responses were measured by ELISA. (B) On day 49 (four weeks post-vaccination), wild-type or CD40−/− VLP-vaccinated mice were inoculated with a typically lethal dose of EBOV. The combined survival from two independent vaccination studies (n=15/group for wild-type mice; n=20/group for CD40−/−-vaccinated mice; and n=10/group CD40−/− control). The control wild-type and CD40−/− mice displayed similar lethality in response to ma-EBOV with mean survivals of 7 and 6.5 days, respectively; P=0.62. ***P<0.0005, ****P<0.0001. (C) Sera were collected from the surviving mice on day 21 post-EBOV infection, and their GP1,2-specific IgG and IgM responses were measured by ELISA. (D) The naïve or vaccinated mice that survived the initial EBOV infection were once again inoculated with a typically lethal dose of EBOV on day 28 after initial infection and monitored for survival. All titers were calculated by reciprocal end-point dilutions with the background set at the control absorbance +0.2 O.D. Dashed lines represent the levels of detection.
Previous in vivo characterizations of EBOV candidate vaccines have suggested an obligate requirement for anti-EBOV antibodies for efficacy.16, 17, 21, 22 We therefore tested the protective contribution of humoral immunity using CD40−/− mice, which displayed a lack of VLP-specific antibody responses. The prime-boost vaccination of wild-type mice with VLP or VLP/poly-ICLC resulted in acute protection against a typically lethal dose of EBOV (Figure 3B). Surprisingly, we also observed partial protection in VLP-vaccinated CD40−/− mice (Figure 3B). In two separate studies, the control mice succumbed to EBOV infection by day 10, whereas a combined 60% (n=24/40) of the VLP-vaccinated mice were protected from disease in the absence of detectable EBOV GP1,2-specific antibody responses. Importantly, we observed similar morbidity within unvaccinated wild-type (mean survival= 7 days) and CD40−/− mice (mean survival=6.5 days; P-value= 0.62), suggesting comparable EBOV lethality across murine strains. Poly-ICLC did not significantly alter the protection in VLP-vaccinated CD40−/− mice (Figure 3B).
We speculated that despite the lack of TI responses upon VLP vaccination, EBOV infection may result in the generation of protective low-affinity antibody responses. To test this hypothesis, we analyzed the antibody responses following EBOV infection in the mice that had survived EBOV infection. All the surviving wild-type mice had robust IgG titers against EBOV GP1,2, whereas the CD40−/− mice had a near complete absence of antibody responses, even subsequent to EBOV infection (Figure 3C). Of the 14 CD40−/− survivors analyzed after EBOV infection, only 3 had marginal anti-EBOV GP1,2 IgG titers and none mounted detectable IgM responses (Figure 3C). On day 28 subsequent to primary EBOV infection, the mice were once again inoculated with a typically lethal dose of EBOV. Consistent with the initial survival results, all the mice were protected from secondary EBOV infection, including those mice without detectable anti-GP1,2 antibody responses (Figure 3D). Altogether, these results support the idea that EBOV-specific antibody responses following either VLP vaccination or EBOV infection are generated through follicular B-cell and TD mechanisms. However, protection from EBOV lethality could be achieved in the absence of these TD humoral immune responses.
Protection from EBOV infection in the absence of antibody affinity maturation or class-switched humoral responses
Our finding in which mice survived a typical lethal EBOV inoculation in the absence of anti-EBOV GP1,2 antibodies is contrary to previous reports. To confirm our results, we used activation-induced cytidine deaminase-deficient (AICDA/AID−/−) mice that fail to generate high-affinity IgG isotype antibodies.38 Wild-type or AID−/− mice were prime-boost-vaccinated with VLP as described above. VLP vaccination of wild-type mice generated robust IgG responses; however, AID−/− mice were characterized by a complete absence of class-switched EBOV GP1,2-specific IgG titers (Figure 4A). To determine the protective role of these IgG responses, wild-type and AID−/− mice were inoculated with a typically lethal dose of EBOV. In accordance with our CD40−/− studies, we observed ~60% protection against EBOV infection in mice lacking IgG antibody responses (Figure 4B). Moreover, wild-type and AID−/− control mice succumbed to EBOV infection at similar rates (wild-type mean survival=7 days, AID−/− mean survival=7.5 days; P=0.44). VLP-vaccinated AID−/− mice mounted marginal IgM responses that were not seen in wild-type mice; these responses do not appear to be required for protection (Figure 4A).
Figure 4.
Protection from EBOV lethality without high-affinity or class-switched GP-specific antibodies. (A) Wild-type or AID−/− mice were vaccinated with VLP on day 0 and day 21. Their sera were collected on day 14 and day 35 (day 14 post-boosting), and their EBOV GP1,2-specific IgG and IgM responses were measured by ELISA. The titers were calculated by reciprocal end-point dilutions with the background set at the control absorbance +0.2 O.D. Dashed lines represent the levels of detection. (B) The survival of mice that were inoculated with a typically lethal dose of EBOV on day 49 (n=5 wild-type mice/group; n=8 AID−/− VLP-vaccinated mice, and n=4 AID−/− control). The control wild-type and AID−/− mice displayed similar lethality in response to ma-EBOV with mean survivals of 7 and 7.5 days, respectively; P=0.44. *P<0.05, **P<0.005.
Adjuvant-enhanced protection against EBOV infection in the complete absence of B-cells
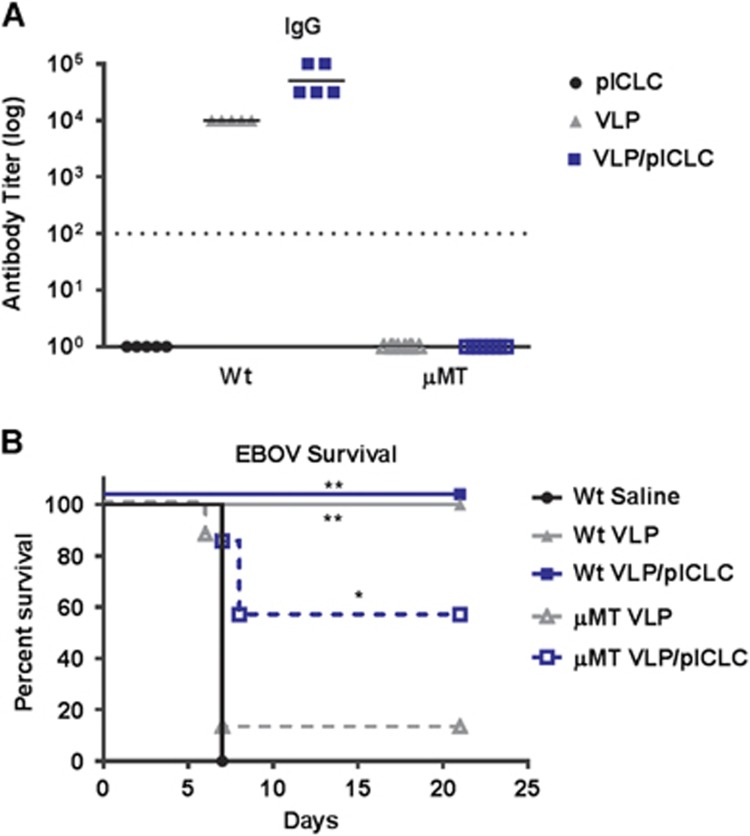
Despite the agreement of our CD40−/− and AID−/− mouse study results, previous experiments utilizing B-cell-deficient mice (for example, μMT, Jh−/−) have suggested that vaccine-induced antibody responses are obligatory for protection against EBOV.12, 17, 21 However, μMT and Jh−/− mice have a developmental block in B-cell lymphopoiesis and therefore display a complete loss of the B-cell compartment.39 Indeed, we observed that VLP-vaccinated μMT mice failed to establish EBOV GP1,2-specific antibody responses and were not protected from EBOV infection (Figures 5A and 5B). Consistent with previous reports, in the absence of B-cells, VLP vaccination alone failed to protect mice from a typically lethal dose of EBOV. However, surprisingly, the inclusion of poly-ICLC during μMT VLP vaccination rescued the protection of the mice from EBOV lethality despite the complete absence of B-cells (Figure 5B). This finding suggests that adjuvant signaling is inducing a B-cell-independent mechanism to protect against EBOV infection.
Figure 5.
Poly-ICLC rescues VLP efficacy in B-cell-deficient animals. (A) Wild-type or μMT mice were vaccinated on day 0 and day 21 with or without poly-ICLC. Their sera were collected on day 35 (day 14 post-boosting), and EBOV GP1,2-specific IgG responses were measured by ELISA. The titers were calculated by reciprocal end-point dilutions with the background set at the control absorbance +0.2 O.D. Dashed lines represent the levels of detection. (B) The survival of mice that were inoculated with a typically lethal dose of EBOV on day 49 (n=5 wild-type mice; n=8 μMT VLP, and n=7 μMT poly/VLP). *P<0.05, **P<0.005.
DISCUSSION
The mechanism of protection established by EBOV vaccine platforms has been much-debated, with no definitive correlate identified to date. As EBOV candidate vaccines progress towards Food and Drug Administration (FDA) licensure in the United States, increased focus on identifying correlates of immune protection will be required. Moreover, the potential lack of human efficacy data from EBOV vaccine trials may require licensure in the United States via the FDA ‘Animal Rule’.10, 40 Therefore, our ability to interpret EBOV vaccine responses accurately depends upon well-defined animal models.
The ma-EBOV model has been critical for the characterization of protective Ebola vaccines and therapeutics.41, 42 In addition, the ability to conduct studies in the context of wild-type and knockout (for example, Jh4 −/−, β2−/−, CD4−/−, CD8−/−, CD11−/−) mouse models has provided insight into the mechanisms of protection against EBOV infection.18, 21, 43, 44 Previous studies have demonstrated the critical importance of anti-EBOV antibody vaccine responses.16, 17, 22 The preclinical success of EBOV antibody therapeutics additionally supports an essential role for humoral immunity in the protection against EVD.23, 24, 45 However, little is known about the early EBOV B-cell initiating events that are required for the establishment of these responses.28
Within this study, we show that the EBOV GP1,2-specific humoral responses that were induced by both VLP vaccination and EBOV infection are predominately established through T-cell-dependent mechanisms. We find that VLP vaccinations generate robust and sustained GC B-cell reactions that are detectable at least four weeks post-vaccination, and these responses were augmented through dsRNA signaling as provided by poly-ICLC. Of interest, GC B-cell responses were not detectable following vaccination with equal amounts of soluble recombinant EBOV GP1,2, suggesting differences in GC formation associated with the display of EBOV GP1,2 (data not shown). Finally, our studies indicated that antibody responses promote protection against typically lethal EBOV infection, but they are neither obligate nor predictive of survival.
Our finding of protection from Ebola lethality in the absence of EBOV-specific antibodies builds upon the results of previous reports.16, 17, 22 Prior murine studies that defined an obligate role for antibody-mediated protection against EVD were obtained from knockout models with a complete loss of the B-cell compartment.16, 21 These studies demonstrated that B-cell-deficient mice were not protected following EBOV vaccination, which is consistent with our findings. Immune depletion studies within NHP models have additionally been used to delineate the requirement for B-cells in vaccine-induced EBOV immunity, and previous EBOV rVSV vaccine platform studies within NHPs demonstrated a critical role for CD4+ T-cells in the establishment of anti-GP1,2 antibody responses.17 Our data support a similar mechanism within mice and extend this observation by linking the requirement for CD40/CD40L interaction.
However, contrary to previous reports, our studies demonstrate that an absence of antibodies does not result in a complete loss of protection from EVD. The animal models (CD40−/− and AID−/−) used within this study have no known defects in T or B-cell development and are fully capable of producing T-cell-independent antibodies. We observed ~60% protection against EBOV infection in both murine models following VLP vaccination, despite a failure to produce anti-EBOV GP1,2-specific antibodies. Moreover, in support of antibody-independent protection, we found that the inclusion of poly-ICLC in VLP preparations could partially rescue protection against EBOV lethality, even in animals which have a complete loss of B-cells. Understanding the mechanism by which this is achieved will be the focus of future studies.
Our results support the idea that anti-EBOV GP1,2-specific antibody responses are generated via follicular B-cell and TD mechanisms. Although we failed to detect TI antibody responses following either VLP vaccination or EBOV infection, we cannot preclude contributions from low-affinity antibodies that were unmeasurable within our studies. However, our finding of poly-ICLC-mediated protection in VLP-vaccinated B-cell-deficient mice would suggest a minor, if any, contribution by such mechanisms within our model. Previously, we demonstrated that EBOV-specific T-cell responses following VLP vaccination are essential for protection.19, 20, 21 Prior studies using the VLP as a pre- or post-exposure therapeutic agent have reported a role for innate immune components in protecting against EVD.43, 46 Therefore, these T-cell or innate immune responses may provide unknown compensatory mechanisms in protecting against EBOV lethality in the absence of anti-EBOV antibodies. Questions about the mechanisms of VLP efficacy in the absence of anti-EBOV antibodies are currently being investigated.
In opposition to an obligate effort by humoral components in protecting against EBOV lethality, we illustrate a division of labor for VLP-mediated anti-EBOV immunity. The finding of a loss of VLP-mediated efficacy in B-cell-deficient mice, yet protection without detectable anti-EBOV antibodies, potentially suggests an unappreciated role for antibody-independent B-cell mechanisms in promoting protection.47, 48 The additional finding that the requirement for B-cells could be bypassed with the inclusion of dsRNA signaling suggests further unknown redundancies or compensatory mechanisms in the protection against EBOV lethality. Together, the data in this work provide a comprehensive description of the establishment and requirement of VLP-mediated EBOV humoral immunity. Moreover, they highlight intriguing gaps in our knowledge of less conventional roles for B-cell immune functions.
Acknowledgments
We thank Drs Sheli Radoshitzky (Molecular and Translational Sciences, USAMRIID) and Jens Kuhn (NIH/NIAID Integrated Research Facility at Fort Detrick (IRF-Frederick), MD; USA) for their careful manuscript review. We also thank Dr Andres Salazar (Scientific Director) of Oncovir (Washington, DC, USA) for providing the clinical-grade dsRNA adjuvant poly-ICLC (Hiltonol). This research was conducted under an Institutional Animal Care and Use Committee-approved protocol in compliance with the US Animal Welfare Act, Public Health Service Policy, and other federal statutes and regulations relating to animals and experiments involving animals. The facility in which this research was conducted (USAMRIID) is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and adheres to the principles stated in the Guide for the Care and Use of Laboratory Animals, by the National Research Council, 2011. This research was funded by grants provided by the US Department of Defense’s Defense Threat Reduction Agency (DTRA).
Footnotes
Supplementary Information for this article can be found on the Emerging Microbes & Infections website (http://www.nature.com/emi)
Supplementary Material
References
- Wong G, Kobinger GP. Backs against the wall: novel and existing strategies used during the 2014-2015 Ebola virus outbreak. Clin Microbiol Rev 2015; 28: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi A, Feldmann H. Ebola virus vaccines: an overview of current approaches. Expert Rev Vaccines. 2014; 13: 521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CL, Bavari S. A race for an Ebola vaccine: promises and obstacles. Trends Microbiol 2015; 23: 65–66. [DOI] [PubMed] [Google Scholar]
- De Santis O, Audran R, Pothin E et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis 2016; 16: 311–320. [DOI] [PubMed] [Google Scholar]
- Huttner A, Dayer JA, Yerly S et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 2015; 15: 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regules JA, Beigel JH, Paolino KM et al. A recombinant vesicular stomatitis virus ebola vaccine—preliminary report. N Engl J Med 2017; 376: 330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins KA, Jahrling PB, Bavari S et al. Ebola virus disease candidate vaccines under evaluation in clinical trials. Expert Rev Vaccines 2016; 15: 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzarano D, Geisbert TW, Feldmann H. Progress in filovirus vaccine development: evaluating the potential for clinical use. Expert Rev Vaccines 2011; 10: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PR, Bryant PR, Clark T et al. Immunology of protection from Ebola virus infection. Sci Transl Med 2015; 7: 286ps11. [DOI] [PubMed] [Google Scholar]
- Sullivan NJ, Martin JE, Graham BS et al. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol 2009; 7: 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfute SB, Bavari S. Correlates of immunity to filovirus infection. Viruses 2011; 3: 982–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G, Kobinger GP, Qiu X. Characterization of host immune responses in Ebola virus infections. Expert Rev Clin Immunol 2014; 10: 781–790. [DOI] [PubMed] [Google Scholar]
- Sullivan NJ, Hensley L, Asiedu C et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med 2011; 17: 1128–1131. [DOI] [PubMed] [Google Scholar]
- Olinger GG, Bailey MA, Dye JM et al. Protective cytotoxic T-cell responses induced by venezuelan equine encephalitis virus replicons expressing Ebola virus proteins. J Virol. 2005; 79: 14189–14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Olinger GG. Protective role of cytotoxic T lymphocytes in filovirus hemorrhagic fever. J Biomed Biotechnol 2011; 2011: 984241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G, Richardson JS, Pillet S et al. Immune parameters correlate with protection against ebola virus infection in rodents and nonhuman primates. Sci Transl Med 2012; 4: 158ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi A, Engelmann F, Feldmann F et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci USA 2013; 110: 1893–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Stroher U, Fernando L et al. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis 2007; 196: S404–S412. [DOI] [PubMed] [Google Scholar]
- Martins KA, Cooper CL, Stronsky SM et al. Adjuvant-enhanced CD4 T cell responses are critical to durable vaccine immunity. EBioMedicine 2016; 3: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins KA, Steffens JT, van Tongeren SA et al. Toll-like receptor agonist augments virus-like particle-mediated protection from Ebola virus with transient immune activation. PLoS ONE 2014; 9: e89735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Olinger G, Deal EM et al. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J Immunol 2005; 175: 1184–1191. [DOI] [PubMed] [Google Scholar]
- Blaney JE, Marzi A, Willet M et al. Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLoS Pathog 2013; 9: e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Audet J, Wong G et al. Sustained protection against Ebola virus infection following treatment of infected nonhuman primates with ZMAb. Sci Rep 2013; 3: 3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Wong G, Audet J et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt J, Zeitlin L, Kim DH et al. Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci Transl Med 2013; 5: 199ra13. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M, Okitsu S, Wang N et al. Molecular programming of B cell memory. Nat Rev Immunol 2012; 12: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesin L, Ersching J, Victora GD. Germinal center B cell dynamics. Immunity 2016; 45: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson KL, Song H, Stertman L et al. Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine 2016; 34: 1927–1935. [DOI] [PubMed] [Google Scholar]
- Warfield KL, Dye JM, Wells JB et al. Homologous and heterologous protection of nonhuman primates by Ebola and Sudan virus-like particles. PLoS ONE 2015; 10: e0118881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Bosio CM, Welcher BC et al. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci USA 2003; 100: 15889–15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Swenson DL, Olinger GG et al. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis 2007; 196: S430–S437. [DOI] [PubMed] [Google Scholar]
- Cazares LH, Ward MD, Brueggemann EE et al. Development of a liquid chromatography high resolution mass spectrometry method for the quantitation of viral envelope glycoprotein in Ebola virus-like particle vaccine preparations. Clin Proteomics 2016; 13: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol 2015; 15: 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Deenick EK, Batten M et al. The origins, function, and regulation of T follicular helper cells. J Exp Med 2012; 209: 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumjohann D, Preite S, Reboldi A et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 2013; 38: 596–605. [DOI] [PubMed] [Google Scholar]
- Elgueta R, Benson MJ, de Vries VC et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009; 229: 152–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T, Naka T, Yoshida K et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1994; 1: 167–178. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000; 102: 553–563. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kuhn R et al. B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 1991; 350: 423–426. [DOI] [PubMed] [Google Scholar]
- Allio T. Product development under FDA's animal rule: understanding FDA's expectations and potential implications for traditional development programs. Therap Innov Regul Sci 2016; 50: 660–670. [DOI] [PubMed] [Google Scholar]
- Bray M, Davis K, Geisbert T et al. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J Infect Dis 1998; 178: 651–661. [DOI] [PubMed] [Google Scholar]
- Nakayama E, Saijo M. Animal models for Ebola and Marburg virus infections. Front Microbiol 2013; 4: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfute SB, Anthony SM, Stuthman KS et al. Mechanisms of immunity in post-exposure vaccination against Ebola virus infection. PLoS ONE 2015; 10: e0118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfute SB, Warfield KL, Bray M. Mouse models for filovirus infections. Viruses 2012; 4: 1477–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama W, Marzi A, Nanbo A et al. Discovery of an antibody for pan-ebolavirus therapy. Sci Rep 2016; 6: 20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Perkins JG, Swenson DL et al. Role of natural killer cells in innate protection against lethal ebola virus infection. J Exp Med 2004; 200: 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanton MR, Way SS, Shlomchik MJ et al. Cutting edge: B cells are essential for protective immunity against Salmonella independent of antibody secretion. J Immunol 2012; 189: 5503–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi I, Whitmire JK. B cell depletion curtails CD4+ T cell memory and reduces protection against disseminating virus infection. J Immunol 2014; 192: 1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.