Abstract
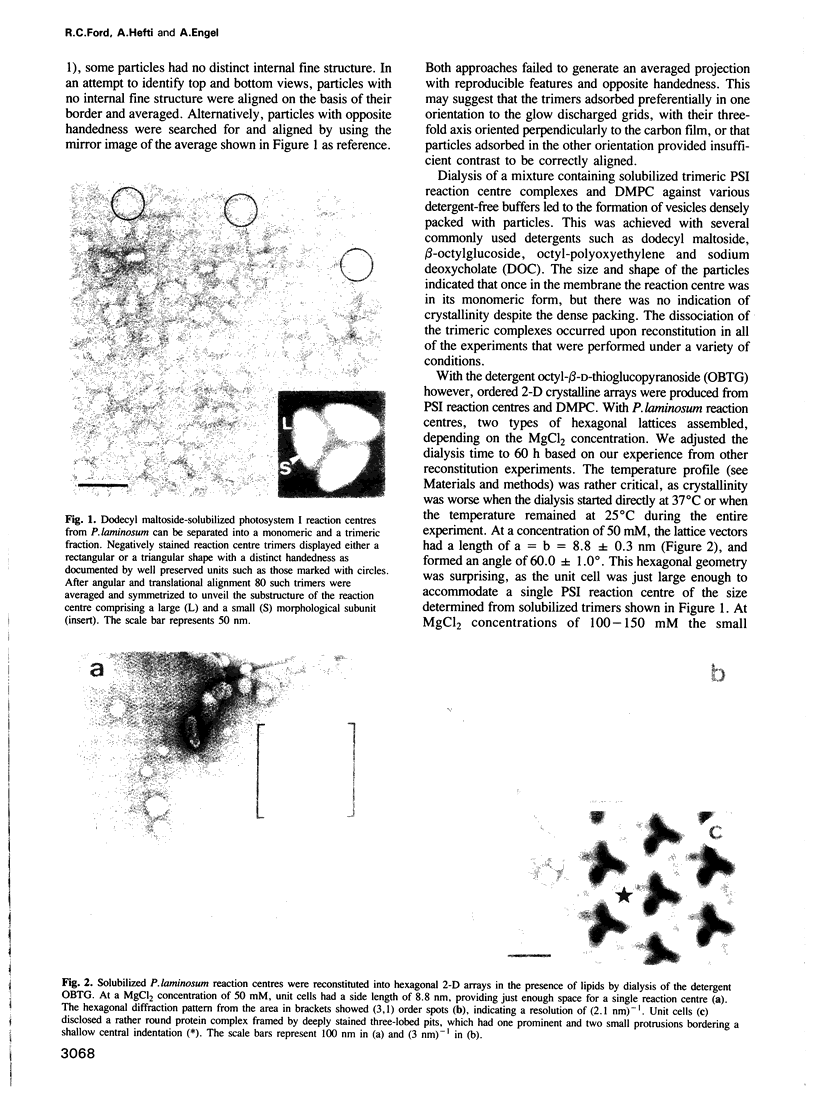
We present an electron microscopical analysis of the photosystem I reaction centre, the membrane complex involved in the second light-driven step of photosynthetic electron transfer in plants and cyanobacteria. To this end, ordered two-dimensional arrays were reconstituted from detergent solubilized photosystem I reaction centres and phospholipids, and studied by electron microscopy and digital image processing. Small (P1) and large (P3) hexagonal lattices obtained with reaction centres of the thermophilic cyanobacterium Phormidium laminosum had unit cell sizes of a = b = 8.8 nm and 15.8 nm, respectively. Reaction centres of a second thermophilic strain, Synechococcus sp. OD24, gave square lattices (a = b = 14.5 nm; P2(1)). Irrespective of the packing arrangement, projections of negatively stained photosystem I complexes showed elongated asymmetric shapes with a large domain at one end which was tilted with respect to a small domain forming the tip of the other end. Such features were also found in averaged projections of solubilized reaction centre trimers. Surface reliefs reconstructed from freeze-dried metal-shadowed P2(1) lattices revealed that reaction centres had a ridge of 2.5 nm height projecting from one side of the membrane while their other side was rather flat and exhibited a shallow, central indentation.
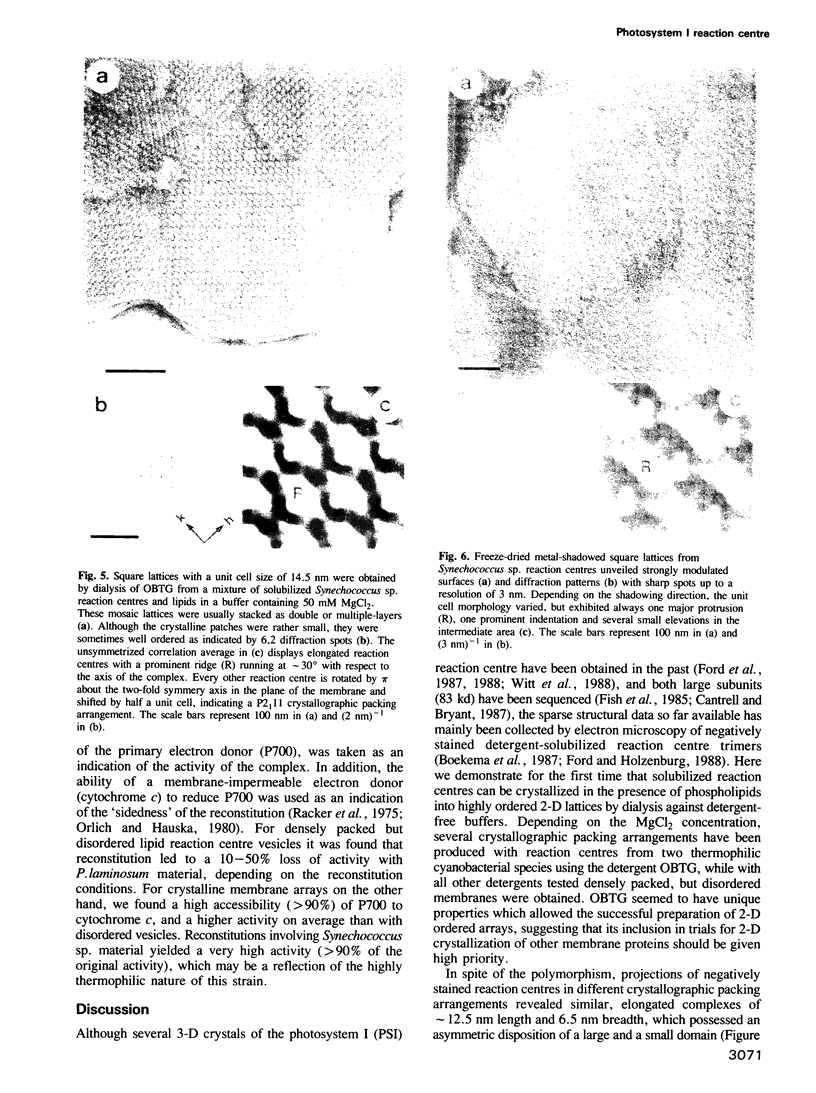
Full text
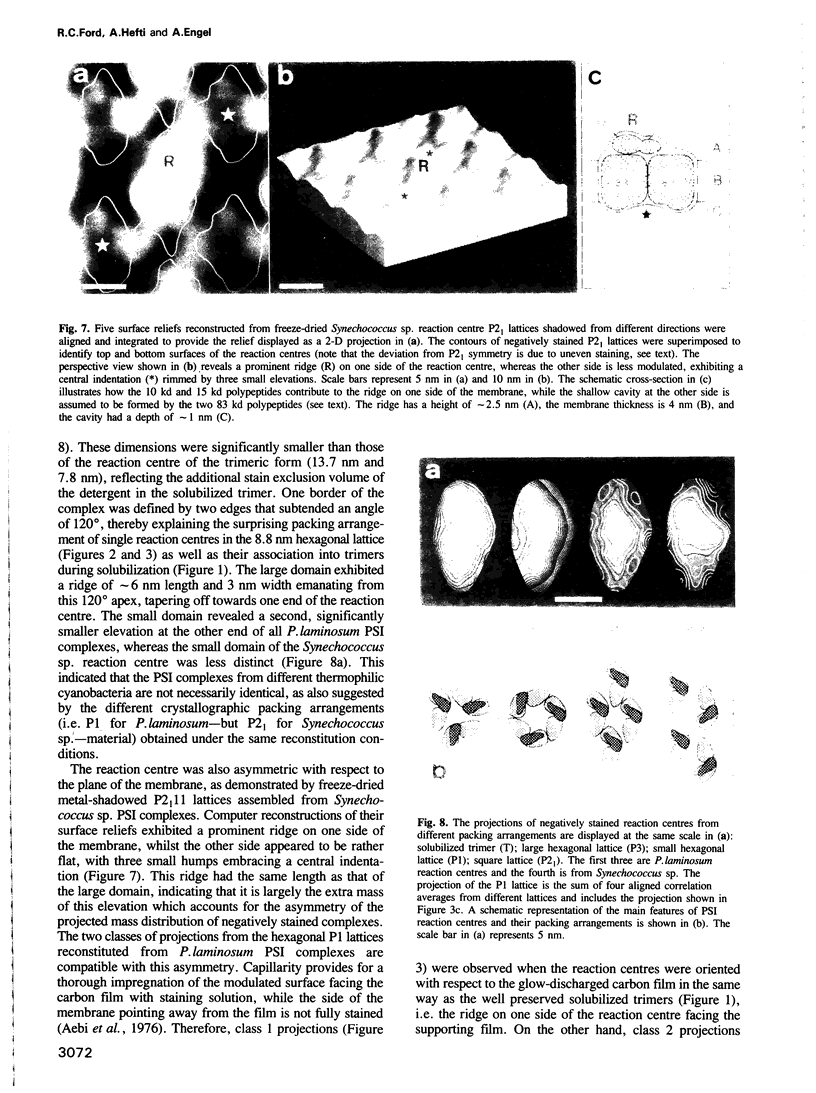
PDF
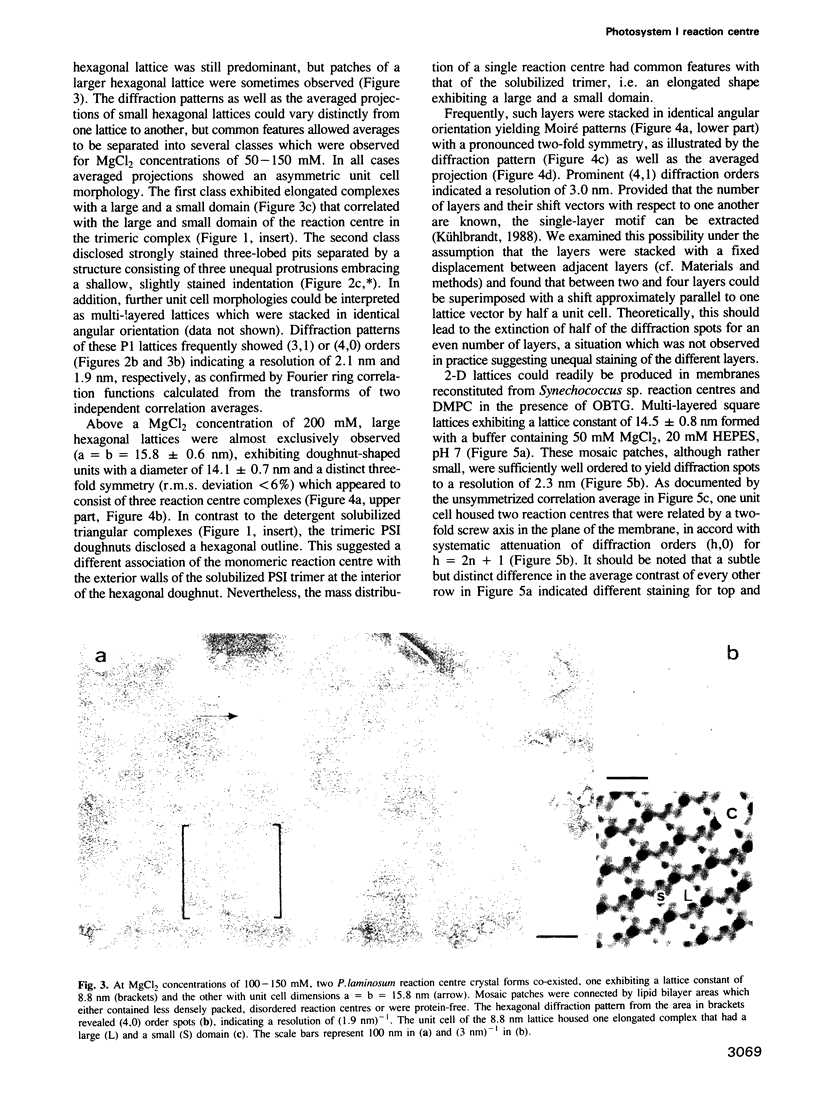
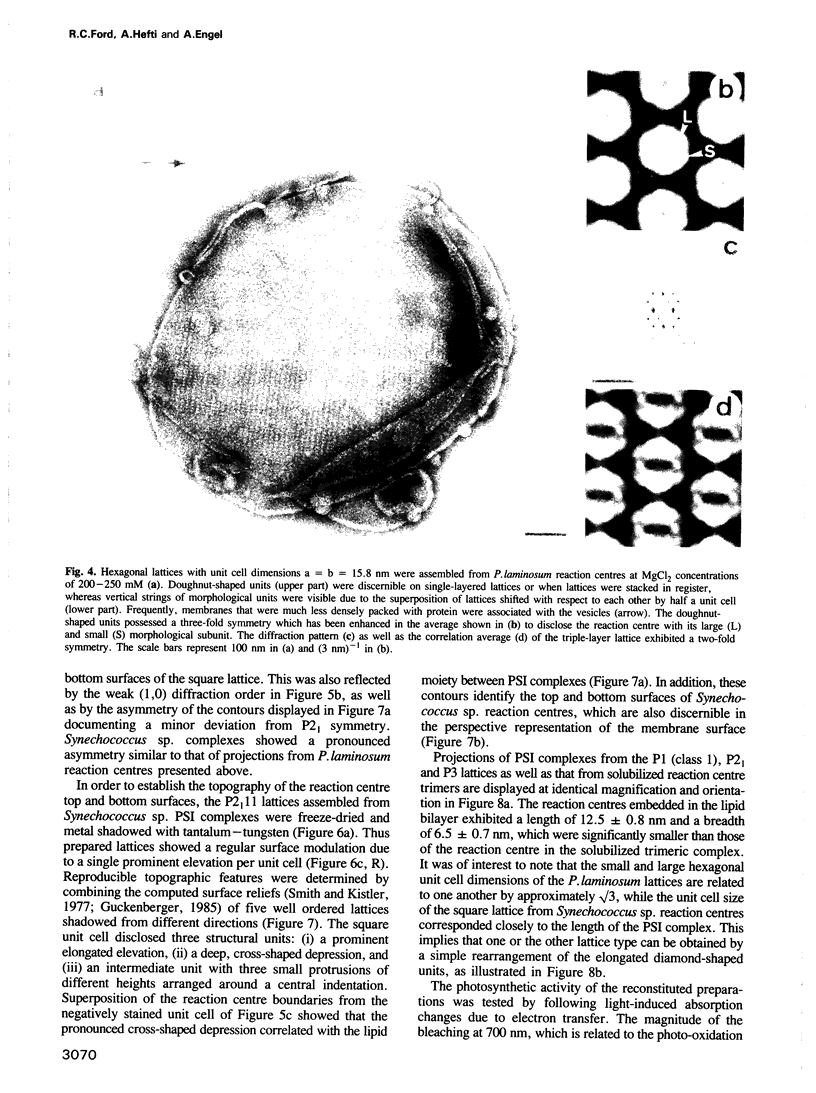



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Bijlenga R. K., ten Heggeler B., Kistler J., Steven A. C., Smith P. R. Comparison of the structural and chemical composition of giant T-even phage heads. J Supramol Struct. 1976;5(4):475–495. doi: 10.1002/jss.400050406. [DOI] [PubMed] [Google Scholar]
- Aebi U., Smith P. R., Dubochet J., Henry C., Kellenberger E. A study of the structure of the T-layer of Bacillus brevis. J Supramol Struct. 1973;1(6):498–522. doi: 10.1002/jss.400010606. [DOI] [PubMed] [Google Scholar]
- Bengis C., Nelson N. Purification and properties of the photosystem I reaction center from chloroplasts. J Biol Chem. 1975 Apr 25;250(8):2783–2788. [PubMed] [Google Scholar]
- Fish L. E., Kück U., Bogorad L. Two partially homologous adjacent light-inducible maize chloroplast genes encoding polypeptides of the P700 chlorophyll a-protein complex of photosystem I. J Biol Chem. 1985 Feb 10;260(3):1413–1421. [PubMed] [Google Scholar]
- Ford R. C., Holzenburg A. Investigation of the structure of trimeric and monomeric photosystem I reaction centre complexes. EMBO J. 1988 Aug;7(8):2287–2293. doi: 10.1002/j.1460-2075.1988.tb03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford R. C., Picot D., Garavito R. M. Crystallization of the photosystem I reaction centre. EMBO J. 1987 Jun;6(6):1581–1586. doi: 10.1002/j.1460-2075.1987.tb02403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Goldfarb W., Eisenberg D., Baker T. S. Reconstruction of glutamine synthetase using computer averaging. Ultramicroscopy. 1978;3(3):283–290. doi: 10.1016/s0304-3991(78)80038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. B., Stroud R. M. Projected structure of purple membrane determined to 3.7 A resolution by low temperature electron microscopy. J Mol Biol. 1981 Sep 25;151(3):491–517. doi: 10.1016/0022-2836(81)90007-3. [DOI] [PubMed] [Google Scholar]
- Høj P. B., Svendsen I., Scheller H. V., Møller B. L. Identification of a chloroplast-encoded 9-kDa polypeptide as a 2[4Fe-4S] protein carrying centers A and B of photosystem I. J Biol Chem. 1987 Sep 15;262(26):12676–12684. [PubMed] [Google Scholar]
- Kühlbrandt W. Structure of light-harvesting chlorophyll a/b protein complex from plant photosynthetic membranes at 7 A resolution in projection. J Mol Biol. 1988 Aug 20;202(4):849–864. doi: 10.1016/0022-2836(88)90563-3. [DOI] [PubMed] [Google Scholar]
- Miller M., Pedersen J. Z., Cox R. P. Effect of growth temperature on membrane dynamics in a thermophilic cyanobacterium: a spin label study. Biochim Biophys Acta. 1988 Sep 1;943(3):501–510. doi: 10.1016/0005-2736(88)90383-5. [DOI] [PubMed] [Google Scholar]
- Orlich G., Hauska G. Reconstitution of photosynthetic energy conservation. I. Proton movements in liposomes containing reaction center of photosystem I from spinach chloroplasts. Eur J Biochem. 1980 Oct;111(2):525–533. doi: 10.1111/j.1432-1033.1980.tb04968.x. [DOI] [PubMed] [Google Scholar]
- Racker E., Knowles A. F., Eytan E. Resolution and reconstitution of ion-transport systems. Ann N Y Acad Sci. 1975 Dec 30;264:17–33. doi: 10.1111/j.1749-6632.1975.tb31473.x. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Smith P. R., Kistler J. Surface reliefs computed from micrographs of heavy metal-shadowed specimens. J Ultrastruct Res. 1977 Oct;61(1):124–133. doi: 10.1016/s0022-5320(77)90011-9. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Koike H., Katoh S. Multiple forms of chlorophyll-protein complexes from a thermophilic cyanobacterium Synechococcus sp. Arch Biochem Biophys. 1982 Nov;219(1):209–218. doi: 10.1016/0003-9861(82)90151-5. [DOI] [PubMed] [Google Scholar]
- Tamm L. K. Incorporation of a synthetic mitochondrial signal peptide into charged and uncharged phospholipid monolayers. Biochemistry. 1986 Nov 18;25(23):7470–7476. doi: 10.1021/bi00371a032. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]