Abstract
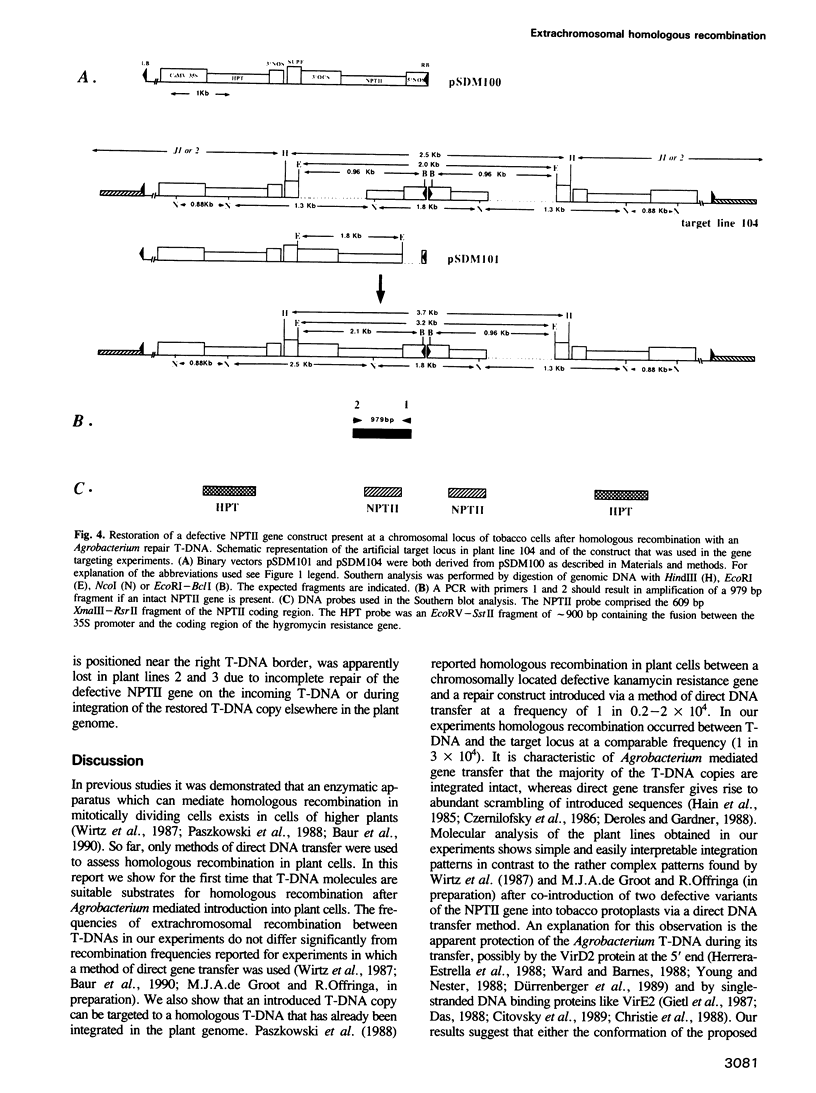
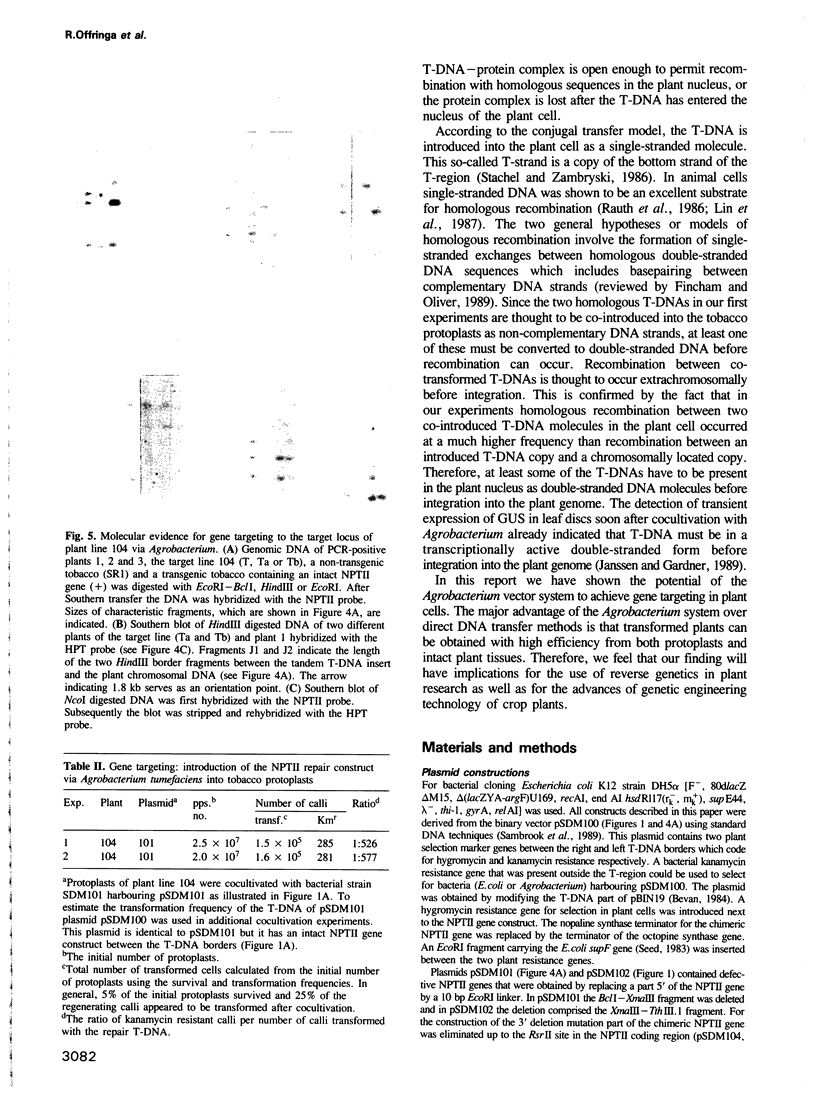
We determined whether T-DNA molecules introduced into plant cells using Agrobacterium are suitable substrates for homologous recombination. For the detection of such recombination events different mutant versions of a NPTII construct were used. In a first set of experiments protoplasts of Nicotiana tabacum SR1 were cocultivated with two Agrobacterium tumefaciens strains. Each strain contained a different T-DNA, one carrying a 5' deleted NPTII gene and the other a NPTII gene with a 3' deletion. A restored NPTII gene was found in 1-4% of the protoplasts that had been cotransformed with both T-DNAs. Restoration of the NPTII gene could only be the consequence of homologous recombination between the two different T-DNAs in the plant cell, since the possibility of recombination in Agrobacterium was excluded in control experiments. In subsequent experiments was investigated the potential use of Agrobacterium for gene targeting in plants. A transgenic tobacco line with a T-DNA insertion carrying a defective NPTII gene with a 3' deletion was transformed via Agrobacterium with a T-DNA containing a defective NPTII repair gene. Several kanamycin resistant plant lines were obtained with an intact NPTII gene integrated in their genome. In one of these lines the defective NPTII gene at the target locus had been properly restored. Our results show that in plants recombination can occur between a chromosomal locus and a homologous T-DNA introduced via A. tumefaciens. This opens the possibility of using the Agrobacterium transformation system for site directed mutagenesis of the plant genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Albright L. M., Yanofsky M. F., Leroux B., Ma D. Q., Nester E. W. Processing of the T-DNA of Agrobacterium tumefaciens generates border nicks and linear, single-stranded T-DNA. J Bacteriol. 1987 Mar;169(3):1046–1055. doi: 10.1128/jb.169.3.1046-1055.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. D., Pennell N., Bosnoyan L., Shulman M. J. Homologous recombination can restore normal immunoglobulin production in a mutant hybridoma cell line. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6432–6436. doi: 10.1073/pnas.85.17.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur M., Potrykus I., Paszkowski J. Intermolecular homologous recombination in plants. Mol Cell Biol. 1990 Feb;10(2):492–500. doi: 10.1128/mcb.10.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Christie P. J., Ward J. E., Winans S. C., Nester E. W. The Agrobacterium tumefaciens virE2 gene product is a single-stranded-DNA-binding protein that associates with T-DNA. J Bacteriol. 1988 Jun;170(6):2659–2667. doi: 10.1128/jb.170.6.2659-2667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., Wong M. L., Zambryski P. Cooperative interaction of Agrobacterium VirE2 protein with single-stranded DNA: implications for the T-DNA transfer process. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1193–1197. doi: 10.1073/pnas.86.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernilofsky A. P., Hain R., Herrera-Estrella L., Lörz H., Goyvaerts E., Baker B. J., Schell J. Fate of selectable marker DNA integrated into the genome of Nicotiana tabacum. DNA. 1986 Apr;5(2):101–113. doi: 10.1089/dna.1986.5.101. [DOI] [PubMed] [Google Scholar]
- Das A. Agrobacterium tumefaciens virE operon encodes a single-stranded DNA-binding protein. Proc Natl Acad Sci U S A. 1988 May;85(9):2909–2913. doi: 10.1073/pnas.85.9.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblaere R., Bytebier B., De Greve H., Deboeck F., Schell J., Van Montagu M., Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 1985 Jul 11;13(13):4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshayes A., Herrera-Estrella L., Caboche M. Liposome-mediated transformation of tobacco mesophyll protoplasts by an Escherichia coli plasmid. EMBO J. 1985 Nov;4(11):2731–2737. doi: 10.1002/j.1460-2075.1985.tb03996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrenberger F., Crameri A., Hohn B., Koukolíková-Nicola Z. Covalently bound VirD2 protein of Agrobacterium tumefaciens protects the T-DNA from exonucleolytic degradation. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9154–9158. doi: 10.1073/pnas.86.23.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham J. R., Oliver P. Meiosis: initiation of recombination. Nature. 1989 Mar 2;338(6210):14–15. doi: 10.1038/338014a0. [DOI] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser C. S., Fraley R. T. Genetically engineering plants for crop improvement. Science. 1989 Jun 16;244(4910):1293–1299. doi: 10.1126/science.244.4910.1293. [DOI] [PubMed] [Google Scholar]
- Gietl C., Koukolíková-Nicola Z., Hohn B. Mobilization of T-DNA from Agrobacterium to plant cells involves a protein that binds single-stranded DNA. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9006–9010. doi: 10.1073/pnas.84.24.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Estrella A., Chen Z. M., Van Montagu M., Wang K. VirD proteins of Agrobacterium tumefaciens are required for the formation of a covalent DNA--protein complex at the 5' terminus of T-strand molecules. EMBO J. 1988 Dec 20;7(13):4055–4062. doi: 10.1002/j.1460-2075.1988.tb03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooykaas P. J., den Dulk-Ras H., Ooms G., Schilperoort R. A. Interactions between octopine and nopaline plasmids in Agrobacterium tumefaciens. J Bacteriol. 1980 Sep;143(3):1295–1306. doi: 10.1128/jb.143.3.1295-1306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard E. A., Winsor B. A., De Vos G., Zambryski P. Activation of the T-DNA transfer process in Agrobacterium results in the generation of a T-strand-protein complex: Tight association of VirD2 with the 5' ends of T-strands. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4017–4021. doi: 10.1073/pnas.86.11.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen B. J., Gardner R. C. Localized transient expression of GUS in leaf discs following cocultivation with Agrobacterium. Plant Mol Biol. 1990 Jan;14(1):61–72. doi: 10.1007/BF00015655. [DOI] [PubMed] [Google Scholar]
- Kucherlapati R. S., Eves E. M., Song K. Y., Morse B. S., Smithies O. Homologous recombination between plasmids in mammalian cells can be enhanced by treatment of input DNA. Proc Natl Acad Sci U S A. 1984 May;81(10):3153–3157. doi: 10.1073/pnas.81.10.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeurier G., Hirth L., Hohn B., Hohn T. In vivo recombination of cauliflower mosaic virus DNA. Proc Natl Acad Sci U S A. 1982 May;79(9):2932–2936. doi: 10.1073/pnas.79.9.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sperle K. M., Sternberg N. L. Extrachromosomal recombination in mammalian cells as studied with single- and double-stranded DNA substrates. Mol Cell Biol. 1987 Jan;7(1):129–140. doi: 10.1128/mcb.7.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A. K., Roginski R. S., Gregg R. G., Smithies O., Skoultchi A. I. Regulated expression of genes inserted at the human chromosomal beta-globin locus by homologous recombination. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3845–3849. doi: 10.1073/pnas.85.11.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkowski J., Baur M., Bogucki A., Potrykus I. Gene targeting in plants. EMBO J. 1988 Dec 20;7(13):4021–4026. doi: 10.1002/j.1460-2075.1988.tb03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauth S., Song K. Y., Ayares D., Wallace L., Moore P. D., Kucherlapati R. Transfection and homologous recombination involving single-stranded DNA substrates in mammalian cells and nuclear extracts. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5587–5591. doi: 10.1073/pnas.83.15.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. Purification of genomic sequences from bacteriophage libraries by recombination and selection in vivo. Nucleic Acids Res. 1983 Apr 25;11(8):2427–2445. doi: 10.1093/nar/11.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. Agrobacterium tumefaciens and the susceptible plant cell: a novel adaptation of extracellular recognition and DNA conjugation. Cell. 1986 Oct 24;47(2):155–157. doi: 10.1016/0092-8674(86)90437-x. [DOI] [PubMed] [Google Scholar]
- Valvekens D., Van Montagu M., Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Vernaleone F., Wilson J. H. Topological requirements for homologous recombination among DNA molecules transfected into mammalian cells. Mol Cell Biol. 1985 Aug;5(8):2080–2089. doi: 10.1128/mcb.5.8.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz U., Schell J., Czernilofsky A. P. Recombination of selectable marker DNA in Nicotiana tabacum. DNA. 1987 Jun;6(3):245–253. doi: 10.1089/dna.1987.6.245. [DOI] [PubMed] [Google Scholar]
- Young C., Nester E. W. Association of the virD2 protein with the 5' end of T strands in Agrobacterium tumefaciens. J Bacteriol. 1988 Aug;170(8):3367–3374. doi: 10.1128/jb.170.8.3367-3374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Li E., Sajjadi F., Subramani S., Jaenisch R. Germ-line transmission of a disrupted beta 2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature. 1989 Nov 23;342(6248):435–438. doi: 10.1038/342435a0. [DOI] [PubMed] [Google Scholar]
- Zimmer A., Gruss P. Production of chimaeric mice containing embryonic stem (ES) cells carrying a homoeobox Hox 1.1 allele mutated by homologous recombination. Nature. 1989 Mar 9;338(6211):150–153. doi: 10.1038/338150a0. [DOI] [PubMed] [Google Scholar]






