Abstract
High sodium-to-potassium ratios are associated with elevated blood pressure levels and an increased risk of cardiovascular diseases. We aimed to determine whether urinary sodium-to-potassium ratios fluctuate diurnally during the day to understand measured values of casual urinary sodium-to-potassium ratios. A total of 13,277 casual urine specimens were collected under free-living conditions from 122 Japanese normotensive and hypertensive individuals. Participants collected all casual urine samples in aliquot tubes, reported urine volumes and the time at each voiding for 10–22 days. Then, specimens were classified into hourly data. Diurnal patterns of urinary sodium-to-potassium ratios and urinary concentrations of sodium and potassium were evaluated. Overall mean values of hourly urinary sodium-to-potassium ratios were highest (4.1–5.0) in the early morning, lower (3.3–3.8) in the daytime and higher (4.0–4.4) toward evening hours. The mean urinary sodium and potassium concentrations were the lowest (90–110 and 24–32 mmol l−1, respectively) during the early morning and higher (110–140 and 35–43 mmol l−1, respectively) after mid-morning. Diurnal variability of potassium concentrations was larger than for sodium concentrations. Diurnal variations in urinary sodium-to-potassium ratios were comparable between normotensive and hypertensive individuals, between hypertensive individuals with and without antihypertensive medications, and among age and gender-specific subgroups. Overall mean hourly urinary sodium-to-potassium ratios fluctuated diurnally under free-living conditions and were higher during the morning and evening and lower during the daytime compared with 24-h urinary sodium-to-potassium ratios. Diurnal variation in urinary sodium-to-potassium ratios should be considered to understand actual daily dietary levels and avoid over- and under-estimation in clinical practice.
Keywords: casual urine, circadian rhythm, diurnal variation, sodium potassium, sodium-to-potassium ratio
Introduction
Sodium-to-potassium (Na/K) ratios in 24-h urine are known to correlate well with blood pressure (BP) in epidemiological studies,1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 and it has been reported to be a superior metric to either sodium (Na) or potassium (K) alone in relation to BP outcomes and cardiovascular disease (CVD) risks.1, 2, 3, 4, 12 Errors due to under- or over-collection of 24-h urine specimens are lower for Na/K ratios compared with Na or K alone because the ratio is independent of urine volume. Methods for estimating 24-h urine Na and K excretions from casual urine specimens are dependent on urinary creatinine measurements, which may degrade if specimens are not well temperature-controlled;15, 16, 17, 18, 19, 20 however, the ratio is independent of creatinine measurements. The measurement of urinary Na/K ratios could provide both onsite prompt feedback by portable handy devices21 and good individual and population estimates of 24-h urinary Na/K ratios by using casual urine.22, 23, 24 The accuracy of these estimates depends on the timing of casual urine collection.23, 24 Casual urinary Na/K ratios usually fluctuate23, 24, 25 and are likely to reflect recent dietary habits, such as increases in the ratio after ingesting food with a high Na content and decreases in the ratio when such foods are avoided.26 Urinary Na/K ratios are higher in the first void after arising and in the last void before bedtime but lower in a second void after arising compared with 24-h urine values.23, 24 However, the overall trend remains unknown. Previous studies have examined fluctuations in Na and K excretion by means of experimental trials in isolated unrealistic circumstances,27, 28, 29 chronobiological assessments,30, 31 protocols requiring compulsory urine sampling at specific intervals,29, 31, 32, 33, 34, 35, 36 or experiments requiring urine withdrawal.37, 38, 39 However, the findings of such studies are not easy to translate into clinical values in terms of obtaining insight into daily dietary behavior. Therefore, the primary aim of this study was to determine the amplitude and timing of the fluctuations observed in the mean hourly values of urinary Na/K ratios to clarify whether diurnal variations in urinary Na/K ratios could be observed even under free-living conditions. The secondary aim was to clarify how the fluctuations of urinary Na/K ratios can be translated into daily clinical practice.
Methods
Study design
This study was a cross-sectional study.
Participants and measurements
A total of 122 participants (age 25–69 years; male, n=64; female, n=58; normotensive, n=45; hypertensive with and without anti-hypertensive medication, n=46 and n=31, respectively) were recruited from the general population by advertisements. Participants were instructed to collect all urine samples under free-living conditions for 10–22 days, unless urine collection was unsuccessful or contaminated by feces. If participants declared that they failed to complete urine collection, we asked them to retry urine collection. In detail, participants were asked to record the volume (ml) of each casual urine sample and the time of collection. Urine samples were first collected in a standardized measuring cup for urine volume measurement, and then some of the urine was transferred into an aliquot tube (10 ml) at each voiding for the measurement of sodium and potassium concentrations. Each void was collected in a separate aliquot tube. The number of casual urine samples obtained during the study was dependent on the voiding frequency of each individual. The voiding samples before and after the longest sleep were defined as ‘void before bedtime’ and ‘first void after arising’, respectively. All casual urine samples were sent to the central laboratory at room temperature for measurement. Urinary Na and K concentrations (mmol l−1) were measured using an ion-specific electrode method, and then the Na/K molar ratio (mmol/mmol) was calculated. The excretion of Na and K was calculated from reported urine volumes. Diurnal fluctuations in urinary Na/K ratios, urinary Na and K concentrations, and volumes and excretions were assessed in 13 277 specimens. Weight was measured twice using HBF-206IT (OMRON Healthcare Co., Ltd., Kyoto, Japan), and the height was measured using a stadiometer HP-M (Tsutsumi, Tokyo). The participants did not wear shoes during these measurements. Body mass index was calculated as weight divided by height squared (kg m−2). Written informed consent was obtained from all participants. The ethics committee of the Shiga University of Medical Science and Omron Healthcare Company approved the study protocol.
Statistical analysis
Urinary Na/K ratios, Na and K concentrations and excretions, and urine volume were pooled and analyzed from 00:00–23:00 hours every hour on the hour. Midnight, before dawn, early morning, mid-morning, late morning, mid-afternoon, late afternoon and late evening were defined as 0:00, 1:00–4:00, 5:00–7:00, 8:00–10:00, 11:00, 14:00–15:00, 16:00–17:00 and 22:00–23:00 hours on the hour, respectively. Participants who changed their anti-hypertensive medication were excluded from the analysis. Adjusted overall mean values of hourly urinary variables were calculated by linear mixed models using the result of each urine sample as repeat measures for each participant to control intra-individual variation and inter-individual variation. Twenty-four time slots were classified into three time zones (overnight: 0:00–7:59 hours, daytime: 8:00–15:59 hours and evening: 16:00–23:59 hours). Statistical significance among time zones in the overall 122 participant and subgroup analyses, which evaluated the effects of time zones and subgroups, were conducted using a two-way analysis of variance with the GLM procedure in SAS statistical software version 9.4 (NC, USA).
Results
Table 1 shows the characteristics and urinary findings of the study participants. The mean age was 50.9 years, and normotensive individuals were younger than hypertensive individuals (38.9 vs. 58.3 years). The mean 24- h urinary Na/K molar ratio was 3.77, and this value was higher among normotensive than hypertensive individuals (4.21 vs. 3.51) and higher in men compared with women (3.88 vs. 3.66; Table 1 and Supplementary Table 1). The mean 24- h urinary volume was 1799.9 ml, and values were similar among normotensive and hypertensive individuals and between men and women (Table 1 and Supplementary Table 1). The mean voiding frequency was 7.11 per day, and values were similar between men and women (7.06 vs. 7.16 voids/day). However, voiding frequency was lower among normotensive than hypertensive individuals (6.37 vs. 7.54 voids/day; Table 1 and Supplementary Table 1). The mean times of the first void after arising was in the early morning (6:52 hours); second void was during mid-morning (9:57 hours); and the void before bedtime was during late evening (10:43 hours). These times were similar between men and women, whereas urine was voided earlier by hypertensive than normotensive individuals (Table 1, Supplementary Table 1 and Supplementary Figure 1). The anti-hypertensive medications administered to 14 (30%), 9 (20%), 15 (33%), 1 (1%), 3 (7%) and 4 (9%) participants comprised calcium channel blockers (CCBs), angiotensin 2 receptor blockers (ARBs), both CCBs and ARBs, both CCBs and angiotensin converting enzyme (ACE) inhibitors, both CCBs and ARBs with other drugs, and other drugs, respectively.
Table 1. Characteristics and urinary findings of normotensive and hypertensive study participants, 64 men and 58 women aged 25–69 years, free-living Japanese volunteers, in 2012 and 2014.
|
Normotensive individuals (N=45) |
Hypertensive individuals with anti-hypertensive medication (N=43) |
Hypertensive individuals without anti-hypertensive medication (N=34) |
Overall (N=122) |
|||||
|---|---|---|---|---|---|---|---|---|
| Variables | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| Age | 38.9 | 10.1 | 61.2 | 7.7 | 54.3 | 8.5 | 50.9 | 13.1 |
| Height (cm) | 165.0 | 10.3 | 161.2 | 7.2 | 163.7 | 7.5 | 163.5 | 8.7 |
| Weight (kg) | 62.4 | 13.0 | 63.5 | 11.3 | 64.8 | 13.1 | 63.7 | 12.6 |
| Body mass index (kg m−2) | 22.8 | 3.4 | 24.3 | 3.5 | 24.0 | 3.7 | 23.7 | 3.5 |
|
N (%) |
N (%) |
N (%) |
N (%) |
|||||
| Women (overall) | 23 (51.1) |
19 (44.2) |
16 (47.1) |
58 (47.5) |
||||
| Mean (s.d.) | Median | Mean (s.d.) | Median | Mean (s.d.) | Median | Mean (s.d.) | Median | |
| 24-Hour urine volume (ml) | 1797.6 (996.0) | 1540.0 | 1746.8 (582.4) | 1700.0 | 1870.0 (595.4) | 1780.0 | 1799.9 (765.1) | 1690.0 |
| 24-Hour Na excretion (mmol per 24 h) | 183.7 (90.1) | 157.7 | 187.7 (72.8) | 177.9 | 209.7 (75.7) | 202.9 | 192.4 (81.0) | 182.2 |
| 24-Hour K excretion (mmol per 24 h) | 45.4 (16.9) | 43.3 | 59.3 (18.7) | 59.1 | 58.6 (18.2) | 55.0 | 54.0 (19.1) | 52.8 |
| Urine voiding frequency | ||||||||
| (No. of voids per day) | 6.37 (2.37) | 6 | 7.87 (2.25) | 8 | 7.12 (1.70) | 7 | 7.11 (2.24) | 7 |
| Time frame of urine voiding | ||||||||
| First void after arising | 7:37 hours (1:26) | 7:30 hours | 6:42 hours (1:18) | 6:40 hours | 6:27 hours (1:20) | 6:25 hours | 6:52 hours (1:26) | 6:50 hours |
| Second void after arising | 11:13 hours (2:32) | 10:50 hours | 9:15 hours (1:56) | 9:05 hours | 9:24 hours (1:58) | 9:30 hours | 9:57 hours (2:21) | 9:43 hours |
| Void before bedtime | 23:13 hours (1:53) | 23:07 hours | 22:27 hours (1:06) | 22:31 hours | 22:35 hours (1:19) | 22:50 hours | 22:43 hours (1:28) | 22:50 hours |
| Na concentration (mmol l−1) | ||||||||
| 24-h urine | 112.2 (44.6) | 106.4 | 112.8 (41.0) | 112.0 | 117.5 (42.8) | 112.1 | 113.9 (42.9) | 110.4 |
| First void after arising | 100.8 (47.8) | 91.3 | 104.3 (46.7) | 100.0 | 113.7 (46.7) | 108.7 | 106.4 (47.2) | 100.0 |
| Second void after arising | 121.5 (61.3) | 117.4 | 116.0 (50.1) | 113.1 | 123.1 (47.7) | 126.1 | 119.7 (52.7) | 117.4 |
| Void before bedtime | 124.2 (62.3) | 117.4 | 117.4 (60.7) | 113.1 | 133.0 (59.7) | 134.8 | 124.1 (61.1) | 121.8 |
| K concentration (mmol l−1) | ||||||||
| 24-h urine | 28.9 (11.6) | 26.8 | 36.3 (13.1) | 33.8 | 33.6 (12.0) | 31.6 | 32.8 (12.7) | 31.1 |
| First void after arising | 26.0 (13.0) | 24.2 | 33.4 (18.7) | 30.7 | 27.8 (14.1) | 25.6 | 29.7 (16.2) | 25.6 |
| Second void after arising | 37.0 (20.7) | 33.2 | 47.0 (23.7) | 46.0 | 46.1 (24.4) | 43.5 | 44.0 (23.5) | 40.9 |
| Void before bedtime | 29.5 (16.2) | 28.1 | 40.3 (26.5) | 35.8 | 37.0 (19.9) | 35.8 | 36.8 (22.7) | 33.2 |
| Na/K ratio | ||||||||
| 24-h urine | 4.21 (1.71) | 3.93 | 3.35 (1.37) | 3.09 | 3.72 (1.31) | 3.56 | 3.77 (1.53) | 3.52 |
| First void after arising | 4.50 (2.55) | 3.86 | 3.92 (2.54) | 3.21 | 4.65 (2.16) | 4.20 | 4.30 (2.45) | 3.74 |
| Second void after arising | 3.88 (2.21) | 3.40 | 3.21 (2.54) | 2.49 | 3.23 (1.69) | 2.88 | 3.40 (2.23) | 2.83 |
| Void before bedtime | 4.99 (2.76) | 4.42 | 3.79 (2.37) | 3.27 | 4.23 (2.09) | 3.76 | 4.21 (2.42) | 3.68 |
Abbreviations: K, potassium; Na, sodium.
Urinary findings are the means of the 7 days.
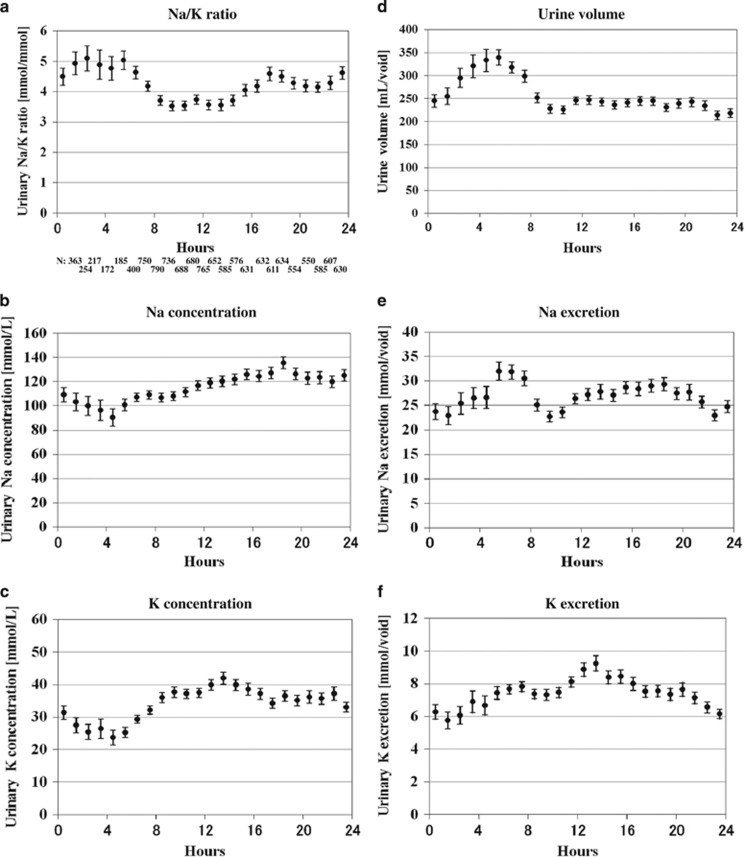
The overall mean value of the hourly urinary Na/K molar ratio was highest (4.1–5.0; biased ~0.6 higher than the 24-h urine) between midnight and early morning (overnight time zone), lower (3.3–3.8; biased ~0.4 lower than the 24-h urine) between mid-morning and mid-afternoon (daytime time zone), and higher (4.0–4.4; biased ~0.4 higher than the 24- h urine) between late afternoon and late evening hours (evening time zone; Figure 1a). Statistical significance was observed among time zones (overnight, daytime and evening) in participants overall (P<0.001). The amplitude of the fluctuations, defined as the difference between the maximum and minimum values of the overall mean hourly urinary Na/K molar ratios (mmol mmol−1), was ~1–1.5 (Figure 1a). The overall mean hourly urinary Na and K concentrations were the lowest (90–110 and 24–32 mmol l−1, respectively) between midnight and early morning (around the time of the first void after arising) and increased to 110–140 and 35–43 mmol l−1, respectively, after mid-morning and persisted until late evening (after the time of second void after arising; Table 1, Figures 1b and c). The diurnal variability of Na and K concentrations, defined as the s.d. divided by the mean value during a period of 24 h was 28.8% and 38.7%, respectively. The mean urine volume was the highest (320 ml per void) during the early morning (around the time of the first void after arising) and remained essentially constant at ~230 ml per void after the mid-morning (around the time of the second void; Figure 1d). The mean Na excretion was highest (32 mmol per void) during the early morning (around the time of the first void after arising), and it remained essentially constant at ~25 mmol per void after mid-morning (around the time of the second void after arising; Figure 1e). The mean K excretion was higher (8–9 mmol per void) between late morning and late afternoon, and it remained lower (6–7 mmol per void) from late evening until before dawn (Figure 1f).
Figure 1.
Overall hourly mean values of casual urinary variables (urine specimens, N=13 277); Na/K ratios were highest from midnight to early morning, lower from mid-morning to mid-afternoon and higher toward evening, and statistical significance was observed between time zones (overnight, daytime, and evening) in overall participants (P<0.001) (a). Na and K concentrations were lowest from midnight to early morning, and higher from mid-morning to late evening (b, c). Urine volume was highest in the early morning and remained constant thereafter (d). Na excretion was highest in the early morning (around the time of first void after arising), and remained constant thereafter (e). K excretion increased between late morning and late afternoon, and decreased between late evening and before dawn (f). Bars indicate the 95% confidence interval of the mean.
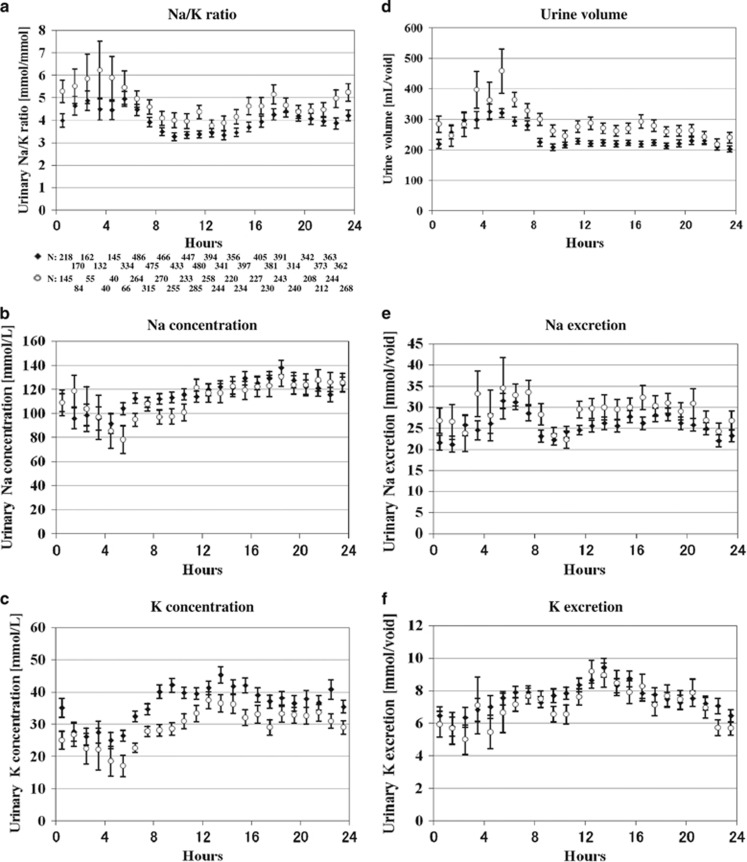
Subgroup analysis showed that diurnal fluctuations in the mean urinary Na/K ratios were comparable between normotensive and hypertensive individuals (Table 1, Figures 2a–f) and between hypertensive individuals taking and not taking antihypertensive medications (Supplementary Figures 2A–F), hypertensive individuals taking and not taking Renin–Angiotensin System (RAS) antagonists (Supplementary Figures 5A–F), men and women (Supplementary Table 1, Supplementary Figures 3A-F), and age-specific subgroups (Supplementary Table 2, Supplementary Figures 4A–R). Statistical significance was observed in urinary Na/K ratios between both time zones and subgroups, among subgroups of gender-specific subgroups (men and women), age-specific subgroups, hypertensive and normotensive individuals, hypertensive individuals with and without medication, and individuals with and without RAS inhibitors (all P<0.001).
Figure 2.
Overall hourly mean values of casual urinary variables in subgroups of normotensive and hypertensive individuals (urine specimens, N=13 277); Characteristics of the Na/K ratio, Na and K concentrations, urine volume, Na and K excretions were similar among subgroups of normotensive (♦ N=45) and hypertensive (○ N=77) (a–f). Bars indicate 95% confidence interval of the mean.
Discussion
The main finding of the present study was that the mean hourly values of urinary Na/K ratios varied diurnally in individuals under free-living conditions. The amplitude of the fluctuation, defined as the difference between the maximum and minimum values of the overall mean of the hourly urinary Na/K molar ratio, was relatively large (~1–1.5). The mean hourly values of urinary Na/K ratios and 24-h urinary Na/K ratios differed systematically and depended on the time of day; that is, casual urine sampling only during one specific time frame will provide values that are biased toward being either lower (during the daytime) or higher (during the morning and evening) compared with the Na/K ratio of the 24-h urine. Thus, appropriate bias correction for time should improve the accuracy and precision of the 24-h urinary Na/K ratio derived from the casual urine Na/K ratio, for example, subtracting values of 0.4–0.6 from morning and evening casual urine Na/K ratios and adding the value 0.4 to the daytime casual urine Na/K ratio to estimate the true 24-h urine Na/K ratio. Furthermore, repeated casual urine sampling from diverse time frames is also known to improve the accuracy of identifying the 24-h urinary Na/K ratio,23, 24 and the present findings could explain this result by washing out values with lower and higher bias by averaging them to converge with the 24-h urinary Na/K ratio. Therefore, it can be inferred that estimation by repeated sampling of casual urinary Na/K ratios could improve its accuracy for identifying the 24-h urinary Na/K ratio with appropriate bias correction based on when they were measured.
The diurnal variations in casual urinary Na/K ratios could reflect postprandial surges, hormonal activities, or other factors. Previous studies have suggested that food intake is not an important contributor to the circadian rhythm.33, 40, 41 In the present study, the variation observed in the overall mean values of hourly urinary Na/K ratios were not likely explained by postprandial surges. Thus, it can be inferred that food intake was irregularly reflected in urinary Na and K excretions, and the fluctuation in overall mean hourly urinary Na/K ratios is mainly related to something other than food intake, for example, hormonal factors. One of the hormonal factors underlying this diurnal variation is renal Na and K excretion.42, 43, 44 Renal Na and K excretion is regulated in the distal tubule under the control of hormones such as aldosterone.42, 43, 44 High aldosterone secretion causes Na reabsorption, which induces an increase in renal K excretion and a decrease in Na excretion.42, 43, 44 Therefore, aldosterone functions in increasing Na absorption from the urine into serum, and in K release from serum to urine.42, 43, 44 Plasma aldosterone is subject to circadian rhythms, as it is inactive during sleeping hours and active after arising in humans and other animals.27, 45, 46 Thus, the diurnal variation of Na may be explained mainly by that of aldosterone. The bladder collects urine before casual urinary excretion and induces a time lag between diurnal variations in serum and urinary Na/K ratios. Therefore, the first urine void after arising would mainly reflect inactive Na reabsorption while asleep, causing relatively increased renal Na excretion that results in the highest urinary Na/K ratio during the early morning. Thereafter, the period of active Na reabsorption would be reflected in the urine void after mid-morning (from around the time of the second void after arising until daytime casual urine voids), causing relatively less renal Na excretion and lower Na/K ratios. Plasma aldosterone activity decreases toward nighttime and induces another increase in the urinary Na/K ratio. The reported diurnal fluctuation of urinary aldosterone is symmetrical and that of serum aldosterone is similar to fluctuations in the mean hourly urinary Na/K ratios;34, 45 however, further study is needed to clarify this issue.
Hypertensive individuals taking RAS inhibitors (N=28) also showed diurnal variation of Na/K ratios. This finding was reasonable because RAS inhibitors do not fully block the pathway of aldosterone production,47 and aldosterone is also regulated by other factors such as serum K and adrenocorticotropic hormones.47
The limitations of this study were that we did not evaluate the diurnal variation in hormonal factors, and the sample size was limited to N=122. It is not known whether these findings are also applicable to individuals taking blood pressure drugs that are not predominantly used in the Japanese general population because we could not include the complete variety of blood pressure drugs that patients may use. However, it is reasonable to infer that diuretics may not affect the diurnal variation because casual urine collected mainly during the daytime showed lower Na/K ratios compared with the 24-h urinary Na/K ratios in the INTERSALT study, which was carried out in the 1980 s when diuretics were mainly used for anti-hypertensive medication.22 In addition, blood flow into the kidneys might be variable under free-living conditions and may affect the secretion rates of urinary and hormonal factors during casual urine sample collection. It is unknown whether the findings are also applicable to hydroelectrolytic disorder patients, patients suffering acute illness, those who did not have recent medication, individuals with renal dysfunction, and children or the elderly. However, the strength of this study was that the overall mean hourly urinary variables were determined from more than 13 000 specimens collected from a general population, which might have washed out the noise and retained signals. In addition, some participants awoke on their own to urinate after sleep onset, which is a natural occurrence under free-living conditions. Thus, diverse and complex lifestyle factors such as dietary intake, hydration status, hemodynamic status, posture and acid–base balance might have been similar to the average value of the general population within each time frame. Epidemiological findings have shown consistencies in higher casual urinary Na/K ratios observed during early morning and night and lower urinary Na/K ratios during the daytime compared with 24-h urine values in worldwide diverse populations.23, 24, 48, 49, 50, 51, 52, 53 Reverse causality is known to affect the studies with diet as an exposure because sick individuals are likely to modify their diet toward healthier food either because of medical advice or an illness-related reduction in food consumption.54, 55 The difference observed in the level of urinary Na/K ratios between hypertensive individuals and normotensive individuals is likely explained by reverse causality; however, the diurnal fluctuations in overall mean hourly urinary Na/K ratios in subgroups of normotensive and hypertensive participants, hypertensive individuals with and without medication, and hypertensive individuals with and without RAS inhibitors were comparable in the present study. Similarly, findings observed among subgroups in gender- and age-specific individuals were comparable. Thus, the present findings might be applicable to diverse demographic backgrounds and would be useful in daily clinical practice and epidemiological studies.
In conclusion, we identified diurnal variations in the overall mean hourly urinary Na/K ratios under free-living conditions. The ratios were higher in the morning and evening but lower in the daytime compared with 24-h urine values. Diurnal variations in urinary Na/K ratios should be considered when measuring casual urine Na/K ratios under free-living conditions to avoid over- and under-estimating actual daily dietary values in clinical practice.
Acknowledgments
This study was conducted by OMRON Healthcare Co. Ltd. in cooperation with the Department of Public Health, Shiga University of Medical Science. The running cost of this observational study was funded by OMRON Healthcare Co. Ltd.
Author contributions
All authors contributed to the design of the study. TI, ST and YS participated in data collection. TI participated in data analysis. TI participated in writing the report. HU, ST, KK, HA and KM participated in critical revision of the manuscript. All authors approved the final version of the report for submission.
Footnotes
Supplementary Information accompanies the paper on Hypertension Research website (http://www.nature.com/hr)
TI is an employee of OMRON Healthcare Co., Ltd. HU served as a consultant for this project. KM received a research grant from OMRON Healthcare Co., Ltd.
Supplementary Material
References
- INTERSALT Co-operative Research Group. INTERSALT: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Br Med J 1988; 297: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J, Rose G, Stamler R, Elliott P, Dyer A, Marmot M. INTERSALT study findings. Public health and medical care implications. Hypertension 1989; 14: 570–577. [DOI] [PubMed] [Google Scholar]
- Tzoulaki I, Patel CJ, Okamura T, Chan Q, Brown IJ, Miura K, Ueshima H, Zhao L, Van Horn L, Daviglus ML, Stamler J, Butte AJ, Ioannidis JP, Elliott P. A nutrient-wide association study on blood pressure. Circulation 2012; 126: 2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Trials of Hypertension Prevention Collaborative Research Group. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med 2009; 169: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Chang MH, Gwinn M, Dowling N, Khoury MJ, Hu FB. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2011; 171: 1183–1191. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Barrett-Connor E. The association between blood pressure, age, and dietary sodium and potassium: a population study. Circulation 1988; 77: 53–61. [DOI] [PubMed] [Google Scholar]
- Dyer AR, Elliott P, Shipley M. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study. II. Estimates of electrolyte-blood pressure associations corrected for regression dilution bias. The INTERSALT Cooperative Research Group. Am J Epidemiol 1994; 139: 940–951. [DOI] [PubMed] [Google Scholar]
- Cook NR, Kumanyika SK, Cutler JA. Effect of change in sodium excretion on change in blood pressure corrected for measurement error. The Trials of Hypertension Prevention, Phase I. Am J Epidemiol 1998; 148: 431–444. [DOI] [PubMed] [Google Scholar]
- Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, Marmot M. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group [published erratum appears in BMJ 1997;315:458]. BMJ 1996; 312: 1249–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins CE, O’Reilly S, Brinkman M, Hodge A, Giles GG, English DR, Nowson CA. Relationship of urinary sodium and sodium-to-potassium ratio to blood pressure in older adults in Australia. Med J Aust 2011; 195: 128–132. [DOI] [PubMed] [Google Scholar]
- Hedayati SS, Minhajuddin AT, Ijaz A, Moe OW, Elsayed EF, Reilly RF, Huang CL. Association of urinary sodium/potassium ratio with blood pressure: sex and racial differences. Clin J Am Soc Nephrol 2012; 7: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez V, Chang ET. Sodium-to-potassium ratio and blood pressure, hypertension, and related factors. Adv Nutr 2014; 5: 712–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara Y, Takahashi Y, Kumagai K, Setoh K, Kawaguchi T, Takahashi M, Muraoka Y, Tsujikawa A, Gotoh N, Terao C, Yamada R, Kosugi S, Sekine A, Yoshimura N, Nakayama T, Matsuda F. Nagahama Study Group. Descriptive epidemiology of spot urine sodium-to-potassium ratio clarified close relationship with blood pressure level: the Nagahama study. J Hypertens 2015; 33: 2407–2413. [DOI] [PubMed] [Google Scholar]
- Okayama A, Okuda N, Miura K, Okamura T, Hayakawa T, Akasaka H, Ohnishi H, Saitoh S, Arai Y, Kiyohara Y, Takashima N, Yoshita K, Fujiyoshi A, Zaid M, Ohkubo T, Ueshima H. NIPPON DATA80 Research Group. Dietary sodium-to-potassium ratio as a risk factor for stroke, cardiovascular disease and all-cause mortality in Japan: the NIPPON DATA80 cohort study. BMJ Open 2016; 6: e011632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol 1993; 20: 7–14. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, Hashimoto T. A simple method to estimate populational 24- h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens 2002; 16: 97–103. [DOI] [PubMed] [Google Scholar]
- Brown IJ, Dyer AR, Chan Q, Cogswell ME, Ueshima H, Stamler J, Elliott P. INTERSALT Co-Operative Research Group. Estimating 24- hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. Am J Epidemiol 2013; 177: 1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierto FW, Hannon WH, Gunter EW, Smith SJ. Stability of urine creatinine. Clin Chim Acta 1997; 264: 227–232. [DOI] [PubMed] [Google Scholar]
- Fuller NJ, Elia M. Factors influencing the production of creatinine: implications for the determination and interpretation of urinary creatinine and creatine in man. Clin Chim Acta 1988; 175: 199–210. [DOI] [PubMed] [Google Scholar]
- Okuda M, Asakura K, Sasaki S, Shinozaki K. Twenty-four-hour urinary sodium and potassium excretion and associated factors in Japanese secondary school students. Hypertens Res 2016; 39: 524–529. [DOI] [PubMed] [Google Scholar]
- Urinary Sodium/potassium monitor. Available at http://www.healthcare.omron.co.jp/medical/products/HEU-001F/index.html (accessed on 15 January 2017).
- Iwahori T, Miura K, Ueshima H, Chan Q, Dyer AR, Elliott P, Stamler J. for the INTERSALT Research Group. Estimating 24- hour urinary sodium/potassium ratio from casual (“spot”) urinary sodium/potassium ratio: The INTERSALT Study. Int J Epidemiol (e-pub ahead of print 09 September 2016; doi:10.1093/ije/dyw287).
- Iwahori T, Ueshima H, Miyagawa N, Ohgami N, Yamashita H, Ohkubo T, Murakami Y, Shiga T, Miura K. Six random specimens of random daytime casual urine on different days sufficient to estimate daily sodium/potassium ratio in comparison to 7- day 24- h collections. Hypertension Res 2014; 37: 765–771. [DOI] [PubMed] [Google Scholar]
- Iwahori T, Ueshima H, Torii S, Saito Y, Fujiyoshi A, Ohkubo T, Miura K. Four to seven random casual urine are sufficient to estimate 24-hr urinary sodium/potassium ratio in individuals with high blood pressure. J Hum Hypertens 2016; 30: 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Koyama H, Moji K, Aoyagi K, Takemoto T, Suzuki S, Satoh H. Daily response of blood pressure to day-to-day variation of urinary sodium to potassium ratio. Clin Exp Hypertens 1999; 21: 1189–1202. [DOI] [PubMed] [Google Scholar]
- Yatabe J, Yatabe MS, Takano K, Watanabe A, Kurosawa S, Yonemoto M, Nochi M, Ikeda Y, Iwahori T, Shiga T, Watanabe T, Kimura J, Oral IX. Concurrent B: Salt and Hypertension Abstract 072: newly developed personal device can detect changes and variations of urinary Na/K ratio with standardized low- and high-salt meals in healthy volunteers. Hypertension 2014; 64: A072. [Google Scholar]
- Rakova N, Jüttner K, Dahlmann A, Schröder A, Linz P, Kopp C, Rauh M, Goller U, Beck L, Agureev A, Vassilieva G, Lenkova L, Johannes B, Wabel P, Moissl U, Vienken J, Gerzer R, Eckardt KU, Müller DN, Kirsch K, Morukov B, Luft FC, Titze J. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab 2013; 17: 125–131. [DOI] [PubMed] [Google Scholar]
- Millet C, Custaud MA, Allevard AM, Zaouali-Ajina M, Monk TH, Arnaud SB, Claustrat B, Gharib C, Gauquelin-Koch G. Influence of head-down bed rest on the circadian rhythms of hormones and electrolytes involved in hydroelectrolytic regulation. Eur J Appl Physiol 2001; 85: 74–81. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kawasaki T, Kawazoe N, Abe I, Uezono K, Ueno M, Fukiyama K, Omae T. Circadian variations of urinary dopamine, norepinephrine, epinephrine and sodium in normotensive and hypertensive subjects. Nephron 1990; 55: 277–282. [DOI] [PubMed] [Google Scholar]
- Cugini P, Murano G, Lucia P, Letizia C, Lisanu M, Scavo D, Gillum RF, Lee JY, Halberg F, Koga Y. Effects of a mild and prolonged restriction in sodium or food intake on the circadian rhythm of aldosterone and related variables. Chronobiol Int 1987; 4: 245–250. [DOI] [PubMed] [Google Scholar]
- Reinberg A, Dupont W, Touitou Y, Lagoguey M, Bourgeois P, Touitou C, Muriaux G, Przyrowsky D, Guillemant S, Guillemant J, Brière L, Zeau B. Clinical chronopharmacology of ACTH 1-17. II. Effects on plasma testosterone, plasma aldosterone, plasma and urinary electrolytes (K, Na, Ca and Mg). Chronobiologia 1981; 8: 11–31. [PubMed] [Google Scholar]
- Uezono K, Kawasaki T, Itoh K, Cugini P. Effects of a 1 day fast on biohumoral variables associated with human circadian rhythmicity. Clin Exp Pharmacol Physiol 2002; 29: 582–588. [DOI] [PubMed] [Google Scholar]
- Morise T, Okamoto S, Ikeda M, Takeda R. The possible role of endogenous digitalis-like substance in the regulation of circadian changes in urinary electrolyte excretion in man. Endocrinol Jpn 1989; 36: 845–850. [DOI] [PubMed] [Google Scholar]
- Bultasová H, Veselková A, Brodan V, Pinsker P. Circadian rhythms of urinary sodium, potassium and some agents influencing their excretion in young borderline hypertensives. Endocrinol Exp 1986; 20: 359–369. [PubMed] [Google Scholar]
- Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, Bonny O, Firsov D. The circadian clock modulates renal sodium handling. J Am Soc Nephrol 2012; 23: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochel JP, Fink M, Bon C, Peyrou M, Bieth B, Desevaux C, Deurinck M, Giraudel JM, Danhof M. Influence of feeding schedules on the chronobiology of renin activity, urinary electrolytes and blood pressure in dogs. Chronobiol Int 2014; 31: 715–730. [DOI] [PubMed] [Google Scholar]
- Moore-Ede MC. Physiology of the circadian timing system: predictive versus reactive homeostasis. Am J Physiol 1986; 250 (5 Pt 2): R737–R752. [DOI] [PubMed] [Google Scholar]
- Kohn CW, Strasser SL. 24- hour renal clearance and excretion of endogenous substances in the mare. Am J Vet Res 1986; 47: 1332–1337. [PubMed] [Google Scholar]
- Mellor DJ, Slater JS. The composition of maternal plasma and foetal urine after feeding and drinking in chronically catheterized ewes during the last two months of pregnancy. J Physiol 1973; 234: 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Stamler J. Assessment of sodium intake in epidemiological studies on blood pressure. Ann Clin Res 1984; 16 (Suppl 43): 49–54. [PubMed] [Google Scholar]
- Muratani H, Kawasaki T, Ueno M, Kawazoe N, Fujishima M. Circadian rhythms of urinary excretions of water and electrolytes in patients receiving total parenteral nutrition (TPN). Life Sci 1985; 37: 645–649. [DOI] [PubMed] [Google Scholar]
- Bulpitt CJ. Blood pressure and potassium consumption. In Bulpitt CJ (ed), Handbook of Hypertension, Vol 6: Epidemiology of Hypertension. Elsevier Science Publishers BV: New York, NY, USA, 1985, pp. 191–206.
- Soundararajan R, Pearce D, Hughey RP, Kleyman TR. Role of epithelial sodium channels and their regulators in hypertension. J Biol Chem 2010; 285: 30363–30369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen BM, Perrier R, Wang Q, Zuber AM, Maillard M, Mordasini D, Malsure S, Ronzaud C, Stehle JC, Rossier BC, Hummler E. Sodium and potassium balance depends on αENaC expression in connecting tubule. J Am Soc Nephrol 2010; 21: 1942–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugini P, Scavo D, Cornelissen G, Lee JY, Meucci T, Halberg F. Circadian rhythms of plasma renin, aldosterone and cortisol on habitual and low dietary sodium intake. Hormone Res 1981; 15: 7–27. [DOI] [PubMed] [Google Scholar]
- Chittavong M, Jansson A, Lindberg JE. Effects of high dietary sodium chloride content on performance and sodium and potassium balance in growing pigs. Trop Anim Health Prod 2013; 45: 1477–1483. [DOI] [PubMed] [Google Scholar]
- Massie BM. Heart failure and cardiomyopathy: congestive heart failure: Management of the patient with chronic heart failure. In Crawford MH, DiMarco JP, Paulus WJ (eds), Cardiology, Ch. 72, 3rd edn, Elsevier Ltd.: Philadelphia, PA, USA, 2009, pp. 979–997.
- Liu LS, Zheng DY, Jin L, Liao YL, Liu K, Stamler J. Variability of urinary sodium and potassium excretion in north Chinese men. J Hypertens 1987; 5: 331–335. [DOI] [PubMed] [Google Scholar]
- Pan WH, Chen JY, Chen YC, Tsai WY. Diurnal electrolyte excretion pattern affects estimates of electrolyte status based on 24- hour, half-day, and overnight urine. Chin J Physiol 1994; 37: 49–53. [PubMed] [Google Scholar]
- Miura T, Fukuda M, Naito T, Togawa H, Sato R, Sasagawa Y, Tomonari T, Kato Y, Mizuno M, Ono M, Ichikawa T, Shirasawa Y, Ito A, Yoshida A, Kimura G. Circadian rhythm of urinary potassium excretion in patients with CKD. Clin Nephrol 2012; 78: 169–173. [DOI] [PubMed] [Google Scholar]
- Dyer AR, Stamler R, Grimm R, Stamler J, Berman R, Gosch FC, Emidy LA, Elmer P, Fishman J, Van Heel N, Civinelli G. Do hypertensive patients have a different diurnal pattern of electrolyte excretion? Hypertens 1987; 10: 417–424. [DOI] [PubMed] [Google Scholar]
- Dyer AR, Martin GJ, Burton WN, Levin M, Stamler J. Blood pressure and diurnal variation in sodium, potassium, and water excretion. J Hum Hypertens 1998; 12: 363–371. [DOI] [PubMed] [Google Scholar]
- Wang CY, Cogswell ME, Loria CM, Chen TC, Pfeiffer CM, Swanson CA, Caldwell KL, Perrine CG, Carriquiry AL, Liu K, Sempos CT, Gillespie CD, Burt VL. Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24- hour calibration study. J Nutr 2013; 143: 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton PK, Appel LJ, Sacco RL, Anderson CA, Antman EM, CampbellN, Dunbar SB, Frohlich ED, Hall JE, Jessup M, Labarthe DR, Macgregor GA, Sacks FM, Stamler J, Vafiadis DK, Van Horn LV. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation 2012; 126: 2880–2889. [DOI] [PubMed] [Google Scholar]
- Cobb LK, Anderson CA, Elliott P, Hu FB, Liu K, Neaton JD, Whelton PK, Woodward M, Appel LJ. American Heart Association Council on Lifestyle and Metabolic Health. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation 2014; 129: 1173–1186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.