Patients with acute lymphoblastic leukaemia (ALL) are at high venous thromboembolism (VTE) risk (affecting between 1.5 and 37%1, 2) with up to 50% affecting the cerebral veins.3, 4, 5, 6
Thrombosis is a potentially avoidable source of morbidity and mortality in ALL patients, particularly during the early phases of chemotherapy.7, 8 Prevention and treatment of thrombosis with antithrombotic drugs may be complicated by co-existent bleeding risk due to factors such as thrombocytopenia and the need for procedures.
Conventional clotting tests (platelet count (PLT), prothrombin time (PT), partial thromboplastin time (PTT), Clauss fibrinogen) only measure isolated components of haemostasis with no measure of cellular/plasma interactions, platelet function, natural anticoagulants, Von Willebrand factor (VWF) and fibrinolysis. Therefore, when complex, multiple reciprocal changes occur, these tests are poor at predicting overall balance.2, 3 Consistent with this, in patients with ALL, conventional tests are often abnormal, implying a bleeding tendency when in fact, thrombosis is the greater risk. Global clotting tests are an alternative approach measuring the net effect of multiple pathways. Thromboelastography (for example, ROTEM) tests whole-blood clot formation and fibrinolysis, activated by intrinsic (INTEM) or extrinsic pathways (EXTEM). FIBTEM tests fibrinogen in isolation and thrombin generation reflects the net balance of pro- and anticoagulant proteins in plasma. In other similar complex coagulopathies (for example, liver failure, trauma and cardiac surgery), global tests are clinically useful and may be more reflective of haemostatic balance.9, 10, 11, 12
To comprehensively evaluate the coagulopathy in ALL patients before and during induction chemotherapy (the highest VTE risk period for current UK treatment protocols), we undertook a prospective, single centre longitudinal cohort study (GlobALL study REC approval ref 14/EM/1315) in which blood samples at multiple time points were analysed in parallel using global, conventional clotting tests, specific factor and anticoagulant levels.
Newly diagnosed adults and children with ALL commencing intensive chemotherapy at UHBristol NHS Foundation trust were eligible for study enrolment. Participants gave informed written consent. VTE and bleeding events were recorded for the first 90 days of treatment. ALL treatment was with UKALL2011 (patients <25 y, Peg-Asparaginase d4 and d18), UKALL14 (patients 25–65, Peg-Asparaginase D18 and d4 if <40 y) and UKALL60+ protocols (patients >65 no Peg-Asparaginase).13 Adult patients (>18 y) routinely received low molecular weight heparin (LMWH) thromboprophylaxis in hospital if no contraindications but not on discharge. There was no routine thromboprophylaxis in children <18 y. Samples from 20 healthy adult controls were also obtained.
Blood samples were collected into EDTA and trisodium citrate at d0 (pre-treatment), d4, d5 (only if d4 Peg-Asparaginase was administered), d10, d18, d19 (only if d18 Peg-Asparaginase was administered), d24, d29 and d36. Each blood sample underwent analysis using standard laboratory methods for PLT, PT, activated PTT, Clauss fibrinogen, D dimer level, activities of antithrombin, protein C, coagulation factor VIII, antigenic levels of VWF:Ag and protein S. Global coagulation analysis of whole blood was performed within 4 h of venepuncture using a ROTEM analyser and the EXTEM, INTEM and FIBTEM tests. Plasma samples were analysed by calibrated automated thrombography to measure thrombin generation using an Ascent fluorometer (Thermo-Lab systems, Helsinki, Finland) and Stago PPP LOW (1pM tissue factor) reagent.
Statistical analysis was performed using GraphPad Prism (La Jolla, CA, USA) and SAS (Buckinghamshire, UK). One-way analysis of variance (ANOVA) comparison between groups using Tukey’s multiple comparisons test was used. Longitudinal distribution of the data over the study period was measured with a repeated measures ANOVA using an autoregressive variance matrix for normally distributed data and Kruskal–Wallis test for non-parametric data. A P-value of less than 0.05 was considered significant.
Thirty-five patients were recruited between January 2015 and January 2016 (24 male, 31B, 4T lineage, median age 12 y, range 18 months −67 y). Twenty-nine patients received d4 and d18 Peg-Asparaginase, three received d18 only and three did not receive Asparaginase (as BCR-ABL positive). Thirty-four patients had central venous line for induction chemotherapy. Six patients received LMWH thromboprophylaxis during induction for a median duration of 15 days (range 4–42). Out of 232 blood samples collected, 221 were of sufficient quality to process.
The overall VTE incidence was 17% (6 out of 35) (median age 32 y, range 14–67 y), including 2 patients on preceding LMWH thromboprophylaxis. Two life-threatening cerebral VTE occurred, three line-associated clots and one deep vein thrombosis (latter patient did not receive Peg-Asparaginase). Thrombosis was diagnosed at a median 35 days after chemotherapy initiation (range day 10–50). All events occurred when PLTs >50 × 109/l. Two adults had clinically relevant non-major bleeding and overt consumptive coagulopathy prior to treatment.
Compared to 20 healthy controls (Table 1) and appropriate reference ranges for age and gender, before induction chemotherapy (d0), patients with ALL showed multiple abnormalities in the panel of haemostasis tests (Table 1). The tests which showed the highest proportion of values outside reference interval were reduced PLT, PTT, thrombin generation (ETP (endogenous thrombin potential) and peak) and EXTEM maximal clot firmness (MCF) (>25% of results below reference intervals), and increased Clauss fibrinogen, D dimer, VWF:Ag, FVIII, FIBTEM MCF (>25% of results above reference intervals; Table 1).
Table 1. Summary of haemostatic parameters measured in patients with ALL pre-treatment (d0) at d29 and over 36 days of induction chemotherapy.
| Parameter | Normal controls n=20 |
ALL study patients (n=35) d0 results compared to normal reference range |
ALL study patients d0 vs normal controls |
ALL study patients (n=26) d29 results compared to normal reference range |
ALL study patients (n=26) d29 vs d0 | VTE vs no VTE at d0 | VTE vs no VTE over 36 days | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean±s.e.m. | Mean±s.e.m. in ALL | Below range | Above range | P-value | Mean±s.e.m. in ALL | Below range | Above range | P-value | P-value | P-value | |
| PLT (× 109/l) | 258.7±14.5 | 106.0±25.88 | 80% (n=28) | 9% (n=3) | Lower in ALL P<0.0001 | 252.6±24.9 | 19% (n=5) | 12% (n=3) | Higher at d29 P<0.0001 | Higher in VTE P<0.05 | Higher in VTE P<0.001 |
| PT (s) | 10.2±0.1 | 10.97±0.24 | 3% (n=1) | 14% (n=5) | Longer in ALL P<0.05 | 12.22±0.30 | 0% | 42% (n=11) | Higher at d29 P=0.0027 | NS | NS |
| PTT (s) | 25.4±0.3 | 24.36±0.64 | 49% (n=17) | 3% (n=1) | NS | 37.5±5.20 | 8% (n=2) | 42% (n=11) | Higher at d29 P=0.0049 | NS | NS |
| Clauss fibrinogen (g/l) | 2.7±0.2 | 2.98±0.26 | 14% (n=5) | 29% (n=10) | NS | 1.02±0.09 | 85% (n=22) | 0% | Lower at d29 P<0.0001 | Higher in VTE P<0.001 | NS |
| D dimer (ng/ml) | <500 | 4072±572.4 | 0% | 97% (n=34) | Higher in ALL P<0.0001 | Not tested | Not tested | NS | Not done | ||
| VWF:Ag (μ/ml) | 1.01±0.09 | 1.84±0.11 | 0% | 37% (n=13) | Higher in ALL P<0.0001 | 2.22±0.21 | 0% | 42% (n=11) | NS | Higher in VTE P<0.01 | Higher in VTE P<0.05 |
| Factor VIII (μ/ml) | 1.07±0.06 | 2.18±0.14 | 0% | 77% (n=27) | Higher in ALL P<0.0001 | 2.00±0.13 | 0% | 73% (n=19) | NS | NS | NS |
| Antithrombin (μ/ml) | 1.01±0.02 | 1.08±0.03 | 9% (n=3) | 11% (n=4) | NS | 0.70±0.04 | 69% (n=18) | 0% | Lower at d29 <0.0001 | NS | NS |
| Protein C (μ/ml) | 1.08±0.04 | 1.15±0.07 | 9% (n=3) | 23% (n=8) | NS | 0.96±0.07 | 19% (n=5) | 0% | NS | NS | NS |
| Free protein S (μ/ml) | 1.03±0.05 | 0.85±0.04 | 6% (n=2) | 0% (n=0) | Lower in ALL P<0.01 | 0.66±0.05 | 19% (n=5) | 0% | Lower at d29 P=0.0197 | NS | NS |
| ETP (nm/min) | 1585±56.2 | 1148±73.31 | 66% (n=23) | 11% (n=4) | Lower in ALL P<0.001 | 1306±132.8 | 42% (n=11) | 15% (n=4) | NS | NS | Higher in VTE P<0.001 |
| Peak (nm) | 273.7±19.6 | 193.3±16.48 | 57% (n=20) | 9% (n=3) | Lower in ALL P<0.01 | 159±25.23 | 69% (n=18) | 8% (n=2) | NS | NS | Higher in VTE P<0.001 |
| EXTEM MCF (mm) | 63.9±1.1 | 53.71±3.04 | 40% (n=14) | 14% (n=5) | Lower in ALL P<0.05 | 50.82±2.95 | 35% (n=9) | 0% | NS | NS | Higher in VTE P<0.05 |
| EXTEM CT (s) | 74.0±2.4 | 96.69±15.41 | 3% (n=1) | 23% (n=8) | NS | 87.09±7.78 | 4% (n=1) | 35% (n=9) | NS | NS | Shorter in VTE P<0.001 |
| FIBTEM MCF (mm) | 14.8±0.8 | 22.38±1.78 | 3% (n=1) | 34% (n=12) | Higher in ALL P<0.01 | 13.95±1.75 | 27% (n=7) | 12% (n=3) | Lower at d29 P=0.0036 | Higher in VTE P<0.05 | NS |
| INTEM CT (s) | 185.3±4.2 | 176.5±11.36 | 0% | 9% (n=3) | NS | 192.4±14.94 | 4% (n=1) | 12% (n=3) | NS | NS | Shorter in VTE P<0.05 |
Abbreviations: ALL, acute lymphoblastic leukaemia; ETP, endogenous thrombin potential (area under curve); EXTEM CT, clotting time initiated by extrinsic pathway using ROTEM; EXTEM MCF, maximal clot firmness initiated by extrinsic pathway using ROTEM; INTEM CT, clotting time initiated by intrinsic pathway using ROTEM; INTEM MCF, maximal clot firmness intrinsic pathway using ROTEM; NS, non-significant (P>0.05); Peak, peak thrombin generation; PT, prothrombin time; PTT, partial thromboplastin time; VTE, venous thromboembolism; VWF:Ag, Von Willibrand Factor antigen. Test results were classified as ‘below range’ or above range’ according to whether the results were outside 95% institutional reference intervals that were age and gender specific as appropriate. Pre-treatment data are expressed as mean±s.e.m. Pre-treatment comparison of results in patients who later went on to develop VTE (n=6) with those who didn’t (n=29). D29 results and comparison of d29 vs d0 results only included those patients who received Peg-Asparaginase and had a sample available for both time points (n=26). P-values generated using one-way ANOVA with Dunnett’s post hoc test. Comparison of results between ALL patients with and without VTE over 36 days: P-values generated using repeated measures ANOVA for normally distributed data and Kruskal–Wallis test for non-parametric data (to assess for difference between VTE and non-VTE groups).
Over the first 36 days of induction chemotherapy, (Table 1), there were dynamic changes in haemostasis tests. By d29, the tests which showed the highest proportion of values outside reference interval were reduced Clauss Fibrinogen, thrombin generation (ETP and peak), antithrombin, FIBTEM MCF and EXTEM MCF (>25% of results below reference intervals) and increased PT, PTT, VWF:Ag, FVIII and EXTEM clotting time (CT) (>25% of results above reference intervals; Table 1). The most clinically and statistically significant changes (d29 compared to d0) were increased PLT, PT, PTT and a simultaneous reduction of Clauss fibrinogen, FIBTEM MCF, antithrombin and protein S (P<0.05).
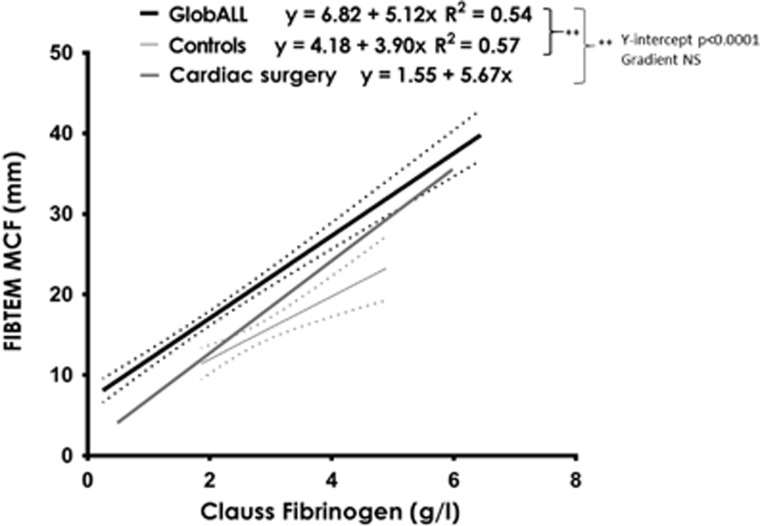
Correlation has previously been reported between Clauss fibrinogen and FIBTEM MCF, and between PLT and EXTEM MCF.14, 15 In this ALL cohort, Clauss fibrinogen correlated with FIBTEM MCF (r2=0.5417, n=221; P<0.0001). However, in comparison to healthy controls (n=20) and a cohort of cardiac surgery patients (n=2146) the y intercept of the correlation curves was higher in ALL patients (P<0.0001) indicating that for any given Clauss fibrinogen results, the corresponding FIBTEM MCF was higher (Figure 1). PLT and EXTEM MCF also correlated (r2=0.4080; n=221; P<0.0001). There was no correlation between any other ROTEM and conventional clotting test result in ALL patients or controls including PT vs EXTEM CT (measuring time to clot, extrinsic pathway), PTT vs INTEM CT (measuring time to clot, intrinsic pathway) confirming that these assays are not interchangeable.
Figure 1.
Correlation of FIBTEM MCF and Clauss fibrinogen results in ALL patients on GlobALL study (221 samples from 35 patients) and normal controls (n=20). Solid line demonstrates line of best fit with 95% confidence intervals (CIs) in dotted lines. Line is also drawn for data from patients undergoing cardiac surgery immediately before chest closure (n=2146): Fibtem MCF=1.55+5.67 × Clauss fibrinogen (95% CI on the slope is 5.49–5.84 and y intercept is 1.138–1.95).
Before induction chemotherapy (d=0), patients with ALL who later developed VTE (n=6) had higher PLT, VWF:Ag, Clauss fibrinogen and FIBTEM MCF than those who did not (P<0.05, Table 1). During induction chemotherapy (over 36 days), patients who developed VTE had higher thrombin generation (ETP and peak), PLT, VWF:Ag, EXTEM MCF and shorter INTEM CT and EXTEM CT compared to patients without VTE (P<0.05, Table 1). There were no other differences between the VTE and no VTE groups either at d0 or during induction chemotherapy, including lowest values of antithrombin (trough), PT or PTT. None of the study patients had positive IgM or IgG anticardiolipin antibodies or prothrombin gene mutation, and only one had factor V Leiden (homozygous). Interestingly, this latter patient did not develop VTE.
The GlobALL study confirms VTE is an important ALL complication and may occur in spite of LMWH thromboprophylaxis. Before and during the first 36 days of treatment, patients with ALL have deranged complex, dynamic abnormalities in haemostasis. The results of this study are consistent with the existing literature describing haemostatic abnormalities in patients with ALL.1 However, to our knowledge this is the most comprehensive evaluation at multiple time points and includes assessment of novel approaches such as ROTEM.
Many of the significant prothrombotic changes, such as high VWF, VIII and low antithrombin, are not tested by conventional tests explaining why these are poorly representative and may overestimate bleeding and underestimate VTE risk. The raised VIII, VWF and fibrinogen before treatment suggest a potential role for endothelial activation in VTE pathogenesis, which could represent a new target for prevention strategies.
This is the first study to have systematically evaluated ROTEM in ALL. Although PT and PTT (conventional tests) measure similar haemostasis pathways to EXTEM CT and INTEM CT (global haemostasis tests), respectively, results did not correlate. In addition, patients with abnormal PT and PTT results frequently had EXTEM CT and INTEM CT within the normal range. For example, 49% of patients had an abnormally short PTT pre-treatment and at d29, 42% had an abnormally long APTT. In comparison, only 9% of patients had an abnormally long INTEM CT pre-treatment, and at d29 only 12% of patients had an abnormally long INTEM CT. This combined with the fact that INTEM CT and EXTEM CT were significantly shorter over the 36 days in patients who went on to develop VTE and suggests these may be more clinically useful (Table 1). However, larger prospective studies would be needed to clarify if ROTEM parameters are better at predicting haemostatic balance and clinical outcome.
Importantly, this is also the first study to demonstrate that for any Clauss fibrinogen, ALL patients had significantly higher FIBTEM MCF compared to normal controls and cardiac surgery patients. Consistent with this at d29, 85% of Clauss fibrinogen results were below the reference range, whereas only 27% of FIBTEM MCF result were below the reference range. It has long been known that although Clauss fibrinogen levels fall following Asparaginase administration, VTE occurs more frequently than bleeding including some thrombotic events when fibrinogen levels <1 g/l. Therefore, many clinical trials recommend avoidance of monitoring in the absence of bleeding. The results of this study provide some reassurance that the fibrinogen component of clot strength may in fact be underestimated by using the Clauss assay. It is possible that FIBTEM results may be more physiologically reflective, perhaps because the FIBTEM MCF measures a broader or different aspect of fibrinogen function or because a plasma component interferes with the Clauss assay.
This study also provides preliminary evidence that it may be possible to define a group of ALL patients at high risk for thrombosis before they commence treatment with ongoing prothrombotic changes during treatment. It is possible that basic laboratory markers (PLT, VWF:Ag, Clauss fibrinogen) could be combined with known clinical risk factors (such as age) to enable prevention strategies to be targeted to patients at the highest VTE risk. Reassuringly, the same laboratory parameters would be associated with a low bleeding risk for thromboprophylaxis administration with all VTE events occurring when platelets were >50 × 109/l.
Acknowledgments
We would like to thank Above and Beyond Charity and David Telling Charitable Trust for funding this research in addition to the patients, parents and nursing staff for their invaluable contribution. We would also like to thank Alan Hedges for his help with the statistical analysis of data. AM and KB are supported by the Bristol NIHR Cardiovascular Biomedical Research Unit.
Footnotes
The authors declare no conflict of interest.
References
- Payne JH, Vora AJ. Thrombosis and acute lymphoblastic leukaemia. Br J Haematol 2007; 138: 430–445. [DOI] [PubMed] [Google Scholar]
- Truelove E, Fielding AK, Hunt BJ. The coagulopathy and thrombotic risk associated with L-asparaginase treatment in adults with acute lymphoblastic leukaemia. Leukemia 2013; 27: 553–559. [DOI] [PubMed] [Google Scholar]
- Grace RF, Dahlberg SE, Neuberg D, Sallan SE, Connors JM, Neufeld EJ et al. The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute consortium protocols. Br J Haematol 2011; 152: 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A, Mitchell C, Richards S, Vora A, Goulden N. Asparaginase-related venous thrombosis in UKALL 2003- re-exposure to asparaginase is feasible and safe. Br J Haematol 2010; 149: 410–413. [DOI] [PubMed] [Google Scholar]
- Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Mariani G, de Gaetano G et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood 2006; 108: 2216–2222. [DOI] [PubMed] [Google Scholar]
- Hough R, Rowntree C, Goulden N, Mitchell C, Moorman A, Wade R et al. Efficacy and toxicity of a paediatric protocol in teenagers and young adults with Philadelphia chromosome negative acute lymphoblastic leukaemia: results from UKALL 2003. Br J Haematol 2016; 172: 439–451. [DOI] [PubMed] [Google Scholar]
- Lauw MN, Van der Holt B, Middeldorp S, Meijers JC, Cornelissen JJ, Biemond BJ. Venous thromboembolism in adults treated for acute lymphoblastic leukaemia: effect of fresh frozen plasma supplementation. Thromb Haemost 2013; 109: 633–642. [DOI] [PubMed] [Google Scholar]
- Hunault-Berger M, Chevallier P, Delain M, Bulabois CE, Bologna S, Bernard M et al. Changes in antithrombin and fibrinogen levels during induction chemotherapy with L-asparaginase in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma. Use of supportive coagulation therapy and clinical outcome: the CAPELAL study. Haematologica 2008; 93: 1488–1494. [DOI] [PubMed] [Google Scholar]
- Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology 2005; 41: 553–558. [DOI] [PubMed] [Google Scholar]
- Hagemo JS, Christiaans SC, Stanworth SJ, Brohi K, Johansson PI, Goslings JC et al. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Crit Care 2015; 19: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stravitz RT, Lisman T, Luketic VA, Sterling RK, Puri P, Fuchs M et al. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol 2012; 56: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman T, Bakhtiari K, Adelmeijer J, Meijers JC, Porte RJ, Stravitz RT. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J Thromb Haemost 2012; 10: 1312–1319. [DOI] [PubMed] [Google Scholar]
- UKALL 2011 Eudract Number: 2010-020924-22, UKALL14 EudraCT Number: 2009-012717-22. Available from: http://www.hra.nhs.uk/news/research-summaries/ukall-2011/; http://www.hra.nhs.uk/news/research-summaries/ukall14/.
- Fluger I, Maderova K, Simek M, Hajek R, Zapletalova J, Lonsky V. Comparison of functional fibrinogen assessment using thromboelastography with the standard von Clauss method. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2012; 156: 260–261. [DOI] [PubMed] [Google Scholar]
- Giaccherini C, Verzeroli C, Marchetti M, Gamba S, Piras F, Russo L et al. PO-26 - Whole blood rotational thromboelastometry (ROTEM) to detect hypercoagulability in patients with myeloproliferative neoplasms (MPN). Thromb Res 2016; 140(Suppl 1): S185–S186. [DOI] [PubMed] [Google Scholar]