The advent of novel therapies has significantly changed the therapeutic landscape in multiple myeloma (MM). However, MM remains largely incurable and patients undergo disease relapse frequently after a short remission period. This is partly because MM is characterized by the loss of critical mediators of immune surveillance and the development of an immunologic milieu that fosters both disease progression and resistance to therapy. In fact, novel anti-MM agents such as bortezomib and lenalidomide exert many of their effects via immunomodulation in addition to direct cytotoxic effect against myeloma cells.1 Despite this, there is a lack of clinically useful immune biomarkers that could reflect both the baseline host immune status and predict clinical outcomes.2 Using retrospective data from a large cohort, we show that the absolute lymphocyte count (ALC) to the absolute monocyte count (AMC) ratio in the peripheral blood (PB) serves as a powerful prognostic immune biomarker in newly diagnosed MM patients and may reflect the immunologic status of these patients. We also correlate this biomarker with known adverse cytogenetics in MM.
The bone marrow (BM) microenvironment plays a critical role in the development of MM from its precursor condition, monoclonal gammopathy of undetermined significance (MGUS), in part by allowing immune tumor evasion.2 In fact, the clones responsible for MGUS and later MM share cytogenetic abnormalities,3 underscoring the significance of immune escape in the development of MM. Among the BM cells implicated in this process are tumor-associated macrophages (TAMs).4 These are derived from circulating monocytes and create an immunosuppressive microenvironment that promotes the growth and survival of MM cells.4, 5 Prior studies have shown that the relative amount of BM-associated TAMs correlates with poor outcomes in MM.6 Studies in other lymphoid malignancies also show that the PB AMC correlates with TAM recruitment at the tumor site and has prognostic significance.7 TAMs also show a phenotypic and functional resemblance to the newly defined immunosuppressive monocytic myeloid-derived suppressor cells (MDSCs), highlighting their significance in tumor-induced immunosuppression that contributes to chemoresistance and poor outcomes.8
In addition to TAMs, the other immunologic biomarker associated with clinical outcome in a variety of cancers is the PB ALC. The ALC of healthy individuals stays in a narrow range in their lifespan, deviating significantly only during illness. Interestingly, a longitudinal study in elderly men demonstrated that a decrease in ALC is associated with a three-year mortality from any cause.9 Therefore, despite being a relatively crude measure, the ALC provides a useful assessment of immune function and general health. Retrospective studies in MM have shown that a higher ALC before bortezomib therapy is associated with better overall survival (OS).10 Furthermore, in MM patients undergoing autologous stem cell transplant (ASCT), both higher pre-ASCT ALC levels and early post-ASCT recovery of ALC were independent prognostic factors for OS.11 Taken together, the ratio of ALC to AMC may represent the relative strength of the host immune system (that is, ALC) to tumor-induced immune dysfunction (that is, AMC, reflective of TAMs). We thus hypothesized that the ALC/AMC ratio can serve as a better prognostic immune biomarker in newly diagnosed MM than ALC or AMC alone. Accordingly, we investigated ALC, AMC and ALC/AMC at diagnosis in predicting clinical outcome among newly diagnosed MM patients. We also correlated these immune subsets to known adverse cytogenetics to better understand how the latter correlate with immune dysfunction in MM.
Our study included 372 patients with newly diagnosed MM at the University Hospitals Cleveland Medical Center in Cleveland, OH and the University of Cincinnati in Cincinnati, OH from 2004–2014. The study was approved by the institutional review boards at both institutions. All patients fulfilled the criteria for symptomatic MM based on the ‘CRAB’ criteria. Patients with a history of HIV or immunosuppression therapy were excluded. The primary end-point was progression-free survival (PFS) from time of diagnosis. The correlation of ALC, AMC and the ALC/AMC ratio with various parameters was assessed with Pearson’s chi-square test (or Fisher’s exact test) for categorical parameters and with Mann–Whitney U-test for continuous parameters. The Cox proportional hazards model was used to evaluate ALC/AMC at diagnosis as a prognostic factor for PFS, as well as to assess and adjust with other known prognostic factors. Statistical analysis was performed using SAS software (version 9.4, SAS Institute, Cary, NC, USA). A P-value of <0.05 was considered statistically significant.
In our cohort, the median age was 67.3 years old (range: 30–92) and 196 patients (53%) were male. Overall, 256 patients (69%) had IgG, 81 (22%) had IgA and 33 (9%) had light chain disease. The median AMC, ALC and ALC/AMC ratio at diagnosis was 0.412 × 109 l−1, 1.461 × 109 l–1 and 3.9, respectively. To define a cutoff point, the choices of AMC⩾0.420 × 109 l–1, ALC⩾ 1.405 × 109 l–1 and ALC/AMC⩾3.6 yielded the greatest differential to segregate cohorts, based on the χ2-value (χ2=94.4, P<0.01) analyzed at different cutoff points between the 25 and 75% quartiles (2.9–4.3) from the log-rank test. Out of 372 patients, 175 patients had cytogenetics available at diagnosis—our panel included del(17p), t(4;14), t(11;14), and hyperdiploidy; 1q gain was not available. Our median follow up period was 37.5 months (range: 1.16–152.9). Patients who were lost to follow up were censored from the survival analysis. At the time of this analysis, 108 patients (29%) had died, with 92 deaths (24%) due to MM.
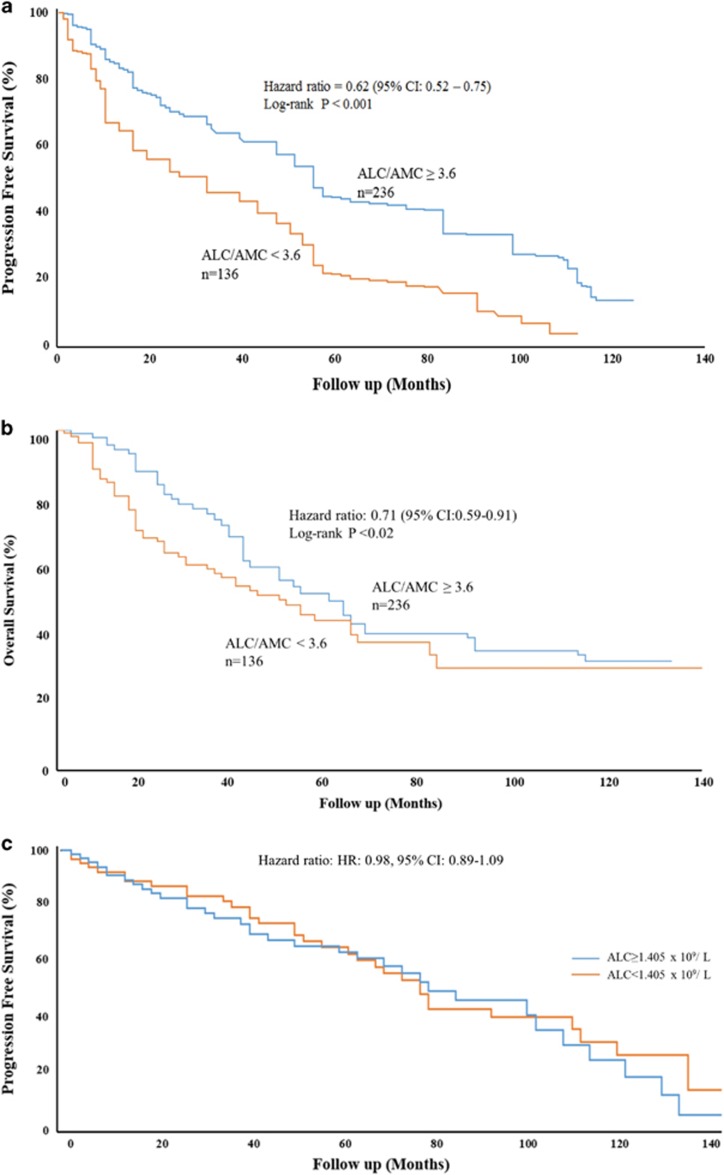
Patients with ALC/AMC<3.6 were older, had higher stage disease, increased bone marrow plasma cell percentage, and lower hemoglobin at presentation than those with ALC/AMC⩾3.6 (Table 1A). Baseline serum creatinine was not significantly different between these groups. Low ALC and high AMC individually showed significant correlation with these factors in univariate analysis, but failed to be significant in multivariate analysis (data not shown). The ALC/AMC ratio independently predicted del(17p) and t(4;14) (Hazard ratio (HR)=2.1, CI: 1.23–3.19, P-value=0.04 and HR=1.7, CI: 1.03–2.12, P-value=0.03, respectively). ALC/AMC as a continuous variable was identified as a predictor of PFS (HR=0.62, (95% CI: 0.52–0.75), P-value<0.001). Multivariate analysis including ALC, age, international staging system (ISS) stage and treatment type (no ASCT versus ASCT) showed that ALC/AMC (HR=0.49, CI: 0.41–0.58, P-value<0.001) as well as treatment type (HR=0.63, CI: 0.41–0.58, P-value<0.001) were independent predictors of PFS and OS (Table 1B). Patients with baseline ALC/AMC⩾3.6 (N=236) versus ALC/AMC<3.6 (N=136) experienced both superior median PFS (43 months versus 24 months, respectively; HR=0.62, 95% CI: 0.52–0.75; P-value<0.001; Figure 1a) and superior median OS (62 months versus 48 months, respectively; HR=0.71, 95% CI: 0.59–0.91; P-value<0.02; Figure 1b). Cutoff points ALC⩾1.405 × 109 l–1 did not predict PFS (HR=0.98, 95% CI: 0.89–1.09; Figure 1c), nor did AMC⩾0.420 × 109 l–1 (HR=0.88, 95% CI: 0.76–1.21).
Table 1A. Baseline patient characteristics based on the absolute lymphocyte/monocyte ratio.
| Characteristics | ALC/AMC⩾3.6 (N=236) | ALC/AMC<3.6 (N=136) | P-value |
|---|---|---|---|
| Age, years, median (range) | 63 (30–89) | 68 (34–92) | <0.001 |
| Age, years | <0.001 | ||
| ⩾65 | 109 (46%) | 91 (68%) | |
| <65 | 127 (54%) | 45 (32%) | |
| Ethnicity | 0.29 | ||
| Black | 63 (27%) | 36 (26%) | |
| Caucasian | 168 (71%) | 96 (70%) | |
| Others | 5 (2%) | 4 (4%) | |
| Monoclonal protein class | |||
| IgG | 163 (69%) | 94 (69%) | 0.19 |
| IgA | 54 (23%) | 29 (21%) | 0.09 |
| Light chain | 19 (8%) | 11 (10%) | 0.04 |
| β2-microglobulin (mg l–1), median (range) | 3.1 (1.1–54) | 4.1 (1.1–72) | <0.01 |
| ⩾3.5 | 114 (48%) | 78 (57%) | |
| <3.5 | 118 (50%) | 55 (40%) | |
| Hemoglobin (g dl–1), median (range) | 12.8 (6.8–19.1) | 10.1 (6.2–18.3) | 0.02 |
| Serum creatinine (mg dl–1), median (range) | 1.67 (0.44–9.1) | 2.16 (0.47–15) | 0.22 |
| Presence of extra-medullary disease | 1 | 7 | <0.001 |
| Plasma cell percentage, median (%)a | 23 | 41 | 0.030 |
| ISS | 0.02 | ||
| Stage I | 66 (27%) | 31 (22%) | |
| Stage II | 117 (49%) | 53 (40%) | |
| Stage III | 44 (18%) | 34 (25%) | |
| Cytogenetics | (N=100) | (N=75) | |
| del(17p) | 5 (4%) | 12 (17%) | 0.04 |
| t(4;14) | 11 (20%) | 18 (22%) | 0.03 |
| t(11;14) | 25 (22%) | 15 (17%) | 0.20 |
| Hyperdiploidy | 59 (45%) | 30 (30%) | 0.12 |
Abbreviations: ALC, absolute lymphocyte count; AMC, absolute monocyte count; ISS, international staging system.
On bone marrow biopsy.
Table 1B. Univariate and multivariate analysis for progression-free survival and overall survival.
| Characteristics |
Progression-free survival |
Overall survival |
||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Median PFS (months) | Hazard ratio | 95% CI | P-value | Median OS (months) | |
| Univariate analysis | ||||||||
| Agea | 1.036 | 1.029–1.049 | <0.0003 | — | 1.125 | 1.062–1.219 | <0.01 | — |
| ALC/AMC<3.6 | 0.54 | 0.49–0.61 | <0.001 | 24 | 0.62 | 0.42–0.82 | <0.003 | 48 |
| ALC<1.4 × 109 l–1 | 0.98 | 0.89–1.09 | <0.00001 | 37 | 0.91 | 0.82–1.29 | <0.09 | 69 |
| Hemoglobin <12 g dl–1 | 0.72 | 0.62–0.82 | <0.0002 | 35 | 0.89 | 0.72–1.02 | <0.02 | 65 |
| Non-IgG isotype | 0.82 | 0.72–0.92 | <0.0001 | 33 | 0.89 | 0.73–1.09 | <0.01 | 68 |
| β2-microglobulin <3.5 | 1.20 | 1.16–1.31 | <0.0001 | 25 | 1.16 | 1.02–1.29 | <0.06 | 78 |
| No ASCT versus ASCT | 0.61 | 0.55–0.69 | <0.0001 | 30 | 0.69 | 0.51–0.92 | <0.002 | 60 |
| Multivariate analysis | ||||||||
| ALC/AMC <3.6 | 0.49 | 0.41–0.58 | <0.001 | 24 | 0.59 | 0.44–0.92 | <0.001 | 48 |
| No ASCT versus ASCT | 0.63 | 0.56–0.68 | <0.001 | 30 | 0.69 | 0.54–0.95 | <0.009 | 60 |
Abbreviations: ALC, absolute lymphocyte count; AMC, absolute monocyte count; ASCT, autologous stem cell transplant.
Age is assessed as a continuous variable.
Figure 1.
Progression-free survival (PFS) and overall survival (OS) of myeloma patients based on baseline immune parameters. Patients with baseline ALC/AMC⩾3.6 (N=236) versus ALC/AMC<3.6 (N=136) experienced superior median PFS (43 months versus 24 months, respectively; (a)) and superior median OS (62 months versus 48 months, respectively; (b)). ALC alone did not predict PFS (c).
To summarize, myeloma patients in our cohort with a higher ALC/AMC at diagnosis enjoyed a longer PFS and OS (either as a continuous or dichotomized variable). Also, multivariate analysis demonstrated that patients harboring del(17p) or t(4;14) had a lower ALC/AMC, suggesting possible immune dysfunction in these groups. In our study, ALC alone at diagnosis was not associated with improved PFS or OS. Interestingly, although previous studies had suggested ALC as a prognostic marker, recent investigations with the novel agents failed to demonstrate statistical significance.12, 13 Our study is novel in that we show that the ALC/AMC is a more robust prognostic biomarker than ALC alone, as it assesses the relative strength of the host immune system to myeloma-induced immune dysfunction. To our knowledge, these findings have only once been suggested in a smaller Korean cohort14—our study in a larger cohort confirms and expands these findings in an immune context. We also present novel findings of the correlation of low ALC/AMC with del(17p) and t(4;14). Although both adverse cytogenetics and immunoparesis are known adverse prognostic factors in MM, it is unknown whether there exists a pathophysiological mechanism. Our study supports research into mechanisms linking these cytogenetics and immune dysfunction in the MM microenvironment.
The search for immune biomarkers that help stratify patients based on their immune status is ever important in an era of upcoming immunotherapies. Although several immune-based modalities carry promise in changing the anti-myeloma therapy landscape, they suffer from major drawbacks that include variable response rates, induction of de novo autoimmune disease, and other inflammatory and autoimmune toxicities.15 Thus, there is an unmet need to identify which subsets of patients are most likely to benefit from immunotherapies and to have minimal adverse effects by assessing the degree of overall immunosuppression. Our findings suggest that the ALC/AMC is an easily measurable biomarker that could help stratify patients based on their baseline immune status. Ongoing and future studies could incorporate this readily available biomarker in identifying treatment-naive MM patients that are best suited for immunotherapies.
Footnotes
The authors declare no conflict of interest.
References
- Hoyos V, Borrello I. The immunotherapy era of myeloma: monoclonal antibodies, vaccines, and adoptive T-cell therapies. Blood 2016; 128: 1679–1687. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Otero P, Paiva B, Engelhardt M, Prosper F, San Miguel JF. Is immunotherapy here to stay in multiple myeloma? Haematologica 2017; 102: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber M, Gutierrez ML, Perez-Andres M, Paiva B, Rasillo A, Tabernero MD et al. Cytogenetic profiles in multiple myeloma and monoclonal gammopathy of undetermined significance: a study in highly purified aberrant plasma cells. Haematologica 2013; 98: 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi S, Ria R, Reale A, De Luisi A, Catacchio I, Moschetta M et al. Multiple myeloma macrophages: pivotal players in the tumor microenvironment. J Oncol 2013; 2013: 183602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beider K, Bitner H, Leiba M, Gutwein O, Koren-Michowitz M, Ostrovsky O et al. Multiple myeloma cells recruit tumor-supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget 2014; 5: 11283–11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyanı E, Sucak GT, Akyürek N, Şahin S, Baysal NA, Yağcı M et al. Tumor-associated macrophages as a prognostic parameter in multiple myeloma. Ann Hematol 2013; 92: 669–677. [DOI] [PubMed] [Google Scholar]
- Koh YW, Kang HJ, Park C, Yoon DH, Kim S, Suh C et al. The ratio of the absolute lymphocyte count to the absolute monocyte count is associated with prognosis in Hodgkin's lymphoma: correlation with tumor-associated macrophages. Oncologist 2012; 17: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek E, de Lima M, Letterio JJ, Kim B-G, Finke JH, Driscoll JJ et al. Myeloid-derived suppressor cells: the green light for myeloma immune escape. Blood Rev 2016; 30: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender BS, Nagel JE, Adler WH, Andres R. Absolute peripheral blood lymphocyte count and subsequent mortality of elderly men. J Am Geriatr Soc 1986; 34: 649–654. [DOI] [PubMed] [Google Scholar]
- Song MK, Chung JS, Joo YD, Lee SM, Lee GW, Lee HS et al. Clinical value of absolute lymphocyte counts before bortezomib-dexamethasone therapy in relapsed multiple myeloma patients. Acta Haematol 2010; 124: 34–39. [DOI] [PubMed] [Google Scholar]
- Jimenez-Zepeda VH, Reece DE, Trudel S, Chen C, Franke N, Winter A et al. Absolute lymphocyte count as predictor of overall survival for patients with multiple myeloma treated with single autologous stem cell transplant. Leuk Lymph 2015; 56: 2668–2673. [DOI] [PubMed] [Google Scholar]
- Napolitano M, Saccullo G, Bono R, Branca A, Cangialosi C, Mancuso S et al. Absolute lymphocyte count is unrelated to overall survival in newly diagnosed elderly patients with multiple myeloma treated with immunomodulatory drugs. Leuk Lymph 2015; 56: 1507–1509. [DOI] [PubMed] [Google Scholar]
- Suriu C, Akria L, Azoulay D, Shaoul E, Barhoum M, Braester A. Absolute lymphocyte count as a prognostic marker in newly diagnosed multiple myeloma patients. Int J Lab Hematol 2016; 38: e56–e59. [DOI] [PubMed] [Google Scholar]
- Shin SJ, Roh J, Kim M, Jung MJ, Koh YW, Park CS et al. Prognostic significance of absolute lymphocyte count/absolute monocyte count ratio at diagnosis in patients with multiple myeloma. Korean J Pathol 2013; 47: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci GV, Cesano A, Hawtin R, Janetzki S, Zhang J, Kirsch I et al. Validation of biomarkers to predict response to immunotherapy in cancer: volume I—pre-analytical and analytical validation. J Immunother Cancer 2016; 4: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]