The infiltration of leukocytes into uterine tissue is an essential step in parturition. This phenomenon, described as physiologic sterile inflammation, has been demonstrated over the past decade through the observation of multiple leukocyte subpopulations (monocytes, granulocytes and lymphocytes) migrating into various reproductive tissues such as the cervix, decidua, myometrium and fetal membranes both prior to and during human labor.1, 2, 3 This mechanism is initiated by an increase in the secretion of pro-inflammatory cytokines and chemokines (signaling molecules responsible for immune cell activation and migration) by the uterine tissues and fetal membranes.3, 4, 5 Cytokines activate both endothelial cells in the local uterine vasculature (by upregulating cell adhesion molecules, such as ICAM1 and VCAM1) and circulating peripheral maternal leukocytes (by upregulating specific extracellular matrix receptors, such as CD11b and CD44), which facilitate the transendothelial migration of immune cells into the target uterine tissues.6 Once the leukocytes infiltrate the uterus, the inflammatory signal is amplified by these activated immune cells through prostaglandin production, which induces myometrial contractility;7 matrix metalloproteinase synthesis, which initiates membrane degradation and cervix ripening;8, 9 and by additional cytokine production, which results in the onset of term labor. Unfortunately, in 10% of all pregnancies, the same events are triggered early, with cytokine expression and leukocyte infiltration occurring prematurely (<37 weeks of gestation), resulting in preterm birth (PTB) of the immature neonate.10 PTB is a critical public health problem with high morbidity, mortality and health care costs.11 There are multiple triggers of PTB, with infection and multiple gestations being the most commonly recognized risk factors.12 Numerous studies have been undertaken to elucidate the specific molecular events responsible for labor initiation at term to predict targets for drug development and novel therapies that would prevent PTB in high-risk pregnant women.
Previously, we explored the effects of mechanical stretching on the myometrium by modeling the physiologic distention of the uterine walls by the growing fetus(es) using an in vitro model, the immortalized human myometrial cell line hTERT-HM in combination with the Flexcell stretch system.13 The effect of stretching on the myometrium was measured by examining four consecutive events involved in human labor: (1) cytokine expression by the myometrium, (2) activation of uterine endothelial cells, (3) peripheral leukocyte activation and (4) transendothelial migration of activated immune cells. We found that the static mechanical stretching of hTERT-HM cells for 24 h significantly increased the expression of nine cytokines (IL-6, IL-12p70, IL-8, GROa, MIF, G-CSF, bFGF, VEGF and PDGF-bb) that are involved in leukocyte activation, migration and biosynthesis compared to non-stretched cells. Conditioned media collected from stretched myometrial cells, which contained all the secreted cytokines and chemokines, activated uterine endothelial cells by significantly upregulating gene and protein levels of cell adhesion molecules (E-selectin, ICAM1 and VCAM1) on their surface compared with the effect of media conditioned by non-stretched cells. Following endothelial cell activation, we explored peripheral leukocyte activation by stretch-induced myometrial cytokines. Conditioned media from stretched and non-stretched myometrial cells elicited a significant upregulation of the activation markers CD11b (integrin αM) and CD44 (hyaluronic acid receptor) on the surface of human monocytes (THP-1 cell line) and primary human granulocytes, suggesting that leukocyte activation is stretch-independent. Finally, stretch-conditioned media significantly increased granulocyte adhesion to uterine endothelial cells and the transendothelial migration of primary granulocytes and monocyte THP-1 cells compared with media conditioned by non-stretched myometrial cells.
Collectively, these data demonstrate that the static stretching of hTERT-HM myometrial cells results in upregulation of pro-inflammatory cytokines and chemokines, which leads to increased endothelial and leukocyte activation, leukocyte adhesion to endothelium, and transendothelial migration. The effects of stretch-conditioned media on monocyte and neutrophil transendothelial migration were attenuated by the addition of broad-spectrum chemokine inhibitor, a novel therapeutic drug that is capable of disrupting multiple cytokine signaling pathways, thus demonstrating its potential role in the prevention of PTB.
Results
Stemming from these findings, we decided to further confirm these results by using primary myometrial cells collected from pregnant women to obtain a more physiologically relevant insight into the inflammatory processes occurring in the pregnant human myometrium at term (37-40 gestational weeks). To obtain a broader understanding of the individual uterine tissues’ contributions during the labor process we collected primary cells from pregnant term decidua in addition to primary myometrial cells. Myometrium tissue biopsies (term not in labor) were used for the myometrial cell derivation, and decidua was collected from the fetal membranes (term not in labor) for the decidual cell isolation. Both tissues were enzymatically digested and isolated primary cells were grown until confluency, at which time both cultures were serum starved for 48 h followed by the collection of conditioned media. Forty-five cytokines secreted by these two adjoined uterine compartments were analyzed simultaneously by multiplex assay (data not shown). Media conditioned by term not in labor decidua (N=11) contained significantly higher (10- to 1000-fold) concentrations of 37 chemokines detected compared to media conditioned by term not in labor myometria (N=11). We hypothesized that the decidua plays a coordinating role at the maternal-fetal interface by contributing to myometrial inflammation.
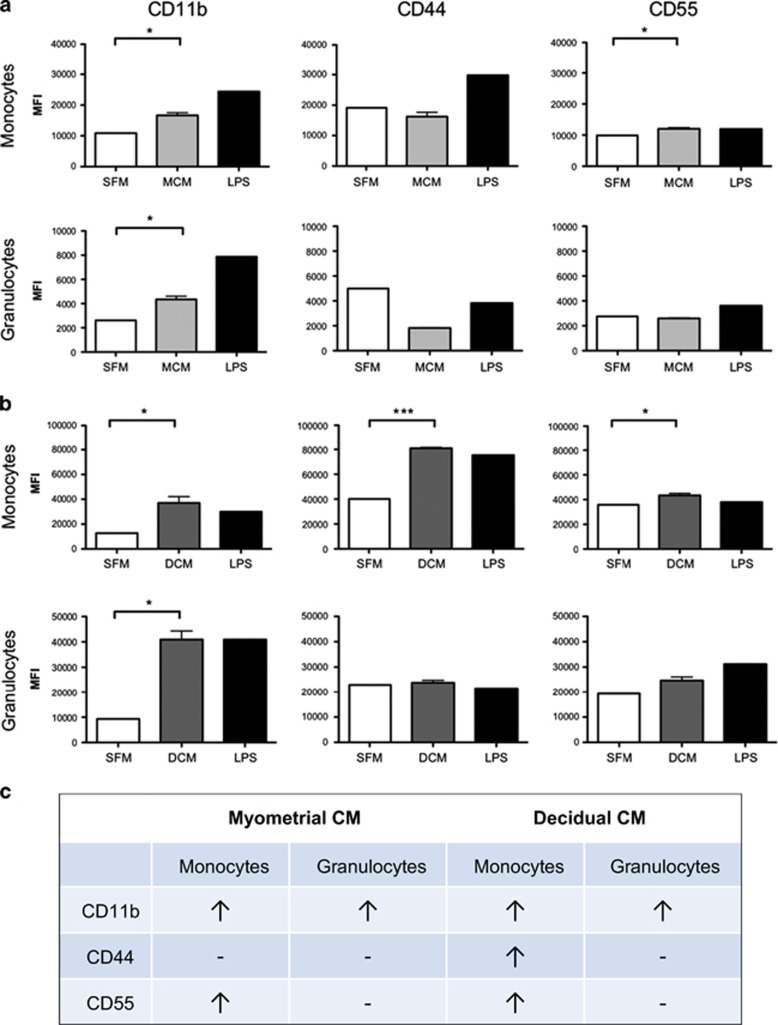
Here, we show the ability of numerous cytokines/chemokines secreted by the term myometria and decidua to activate primary maternal peripheral leukocytes in whole blood collected from early- to second-trimester pregnant women (Figure 1). The activation of peripheral leukocytes by the myometrial and decidual-secreted cytokines/chemokines was assessed on peripheral monocytes and granulocytes by flow cytometry using the expression of known activation markers (CD11b, CD44 and CD55). Briefly, total peripheral blood from pregnant women was incubated with myometrial conditioned media, decidual conditioned media, serum-free DMEM/RPMI (negative control) and lipopolysaccharide (LPS, 1 μg/ml, positive control) for 1 h (for CD11b activation) and 6 h (for CD44 and CD55 activation) at 37 °C. Next, the stimulated blood was incubated with fluorophore-conjugated antibodies for the detection of leukocyte sub-type markers (CD14 for monocytes, CD15 for granulocytes) and activation markers for 30 min at 4 °C in the dark. Then, the blood samples were mixed with FACS red blood cell lysing solution for 15 min at room temperature, fixed and analyzed with a Gallios flow cytometer the following day.
Figure 1.
Flow cytometry analysis of the protein expression of activation markers CD11b (PE-Cy7 conjugated antibody, BD), CD44 (PE conjugated antibody, BD) and CD55 (APC conjugated antibody, BD) on the surface of primary human peripheral granulocytes and monocytes following stimulation with (a) myometrial conditioned media (MCM, N=3, light gray) or (b) decidual conditioned media (DCM, N=3, dark gray). Protein expression is recorded as mean fluorescent intensity, MFI. Serum-free media (SFM) was used as a negative control and lipopolysaccharide (LPS, 1 μg/ml) was used as a positive control. (c) Summary table of significant results. CD11b expression was examined after 1 h of stimulation and CD44 and CD55 expressions were examined after 6 h of stimulation. Decidua and myometrium were collected from term not in labor women undergoing elective cesarean section, peripheral blood was collected from pregnant women (16-18 weeks of gestation). Significance was determined by paired t-tests. Data are presented as mean±s.e.m.
In response to myometrial conditioned media treatment (N=3), there was a significant increase in the expression of activation marker CD11b in monocytes and granulocytes and CD55 in monocytes. No significant changes were seen in activation marker CD44. In response to decidual conditioned media treatment (N=3), there was a significant increase in the expression of CD11b in monocytes and granulocytes, and CD44 and CD55 expression in monocytes. CD44 and CD55 expression in granulocytes was not affected. These results demonstrate that the factors (cytokines/chemokines) that are secreted by primary myometrial and decidual cells are capable of significantly upregulating the expression of various activation markers in primary peripheral immune cells. Decidual conditioned media treatment resulted in the upregulation of all three activation markers (CD11b, CD44 and CD55); whereas myometrial conditioned media treatment activated only CD11b and CD44. Furthermore, greater activation was seen in monocytes compared with granulocytes.
Comments
It has been well documented that an increase in pro-inflammatory cytokine secretion coupled with an increase in leukocyte invasion into the uterine compartments occurs prior to and during term labor as well as during spontaneous or infection-induced preterm labor. The data described above suggests that (1) multiple cytokines released from primary human decidual cells and primary myometrial cells activate maternal peripheral leukocytes. (2) The decidua may play a larger role than the myometrium (perhaps acting in a ‘cascade of activation’ manner) in the activation of peripheral leukocytes by upregulating a greater number of activation markers, which play a role in adhesion and migration. (3) Greater activation is seen in response to both myometrial and decidual conditioned media in monocytes compared with granulocytes, which is in agreement with previous findings suggesting a greater role for monocytes and macrophages in preparation of labor-onset both in humans and rodents.2
While studying human primary cells provides a closer representation of physiological processes occurring in the human body compared to cell lines, in vivo experiments are one step closer to accurate representation. The major limitation in this study is its inability to capture the immense complexity which occurs within this system, such as communication between different tissues and secreted factors, both spatially and temporally. We attempted to address this limitation by using conditioned media, which would allow us to test the effects of multiple secreted factors rather than focus on one cytokine of interest, and by examining multiple tissues.
A better understanding of the mechanisms responsible for the onset of physiologic inflammation will enhance our ability to combat pregnancy-related pathologies such as preterm labor and find novel targets for therapeutic treatments. This is especially critical in light of the fact that global rates of PTB have not declined over the past several decades, and remain a major challenge in perinatal health care, as it accounts for 75% of perinatal mortality and more than 50% of the long-term morbidity.11 This new finding may result in a greater focus on the decidua with respect to cytokine secretion and monocytes as a leukocyte sub-type of interest in the initiation of labor. Future studies will examine whether there is a difference between the effect of labor and non-labor myometrial- and decidual-secreted factors on endothelial and leukocyte activation, leukocyte adhesion and transendothelial migration.
Acknowledgments
This work was supported by a grant from Burroughs Wellcome Fund (grant #1013759). We are grateful to Mrs Anna Dorogin for her assistance in processing uterine tissues, Dr Caroline Dunk for her assistance with decidual cultures and Dr Jianhong Zhang for his assistance with flow cytometry. We thank obstetricians and nurses at Mount Sinai Hospital, Toronto, for helping with tissue collection and the LTRI Biobank for helping with blood collection.
Footnotes
The authors declare no conflict of interest.
References
- Thomson AJ, Telfer JF, Young A et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod 1999; 14: 229–236. [PubMed] [Google Scholar]
- Hamilton S, Oomomian Y, Stephen G et al. Macrophages infiltrate the human and rat decidua during term and preterm labour: evidence that decidual inflammation precedes labor. Biol Reprod 2011; 86: 39. [DOI] [PubMed] [Google Scholar]
- Osman I, Young A, Ledingham MA et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 2003; 9: 41–45. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Nedd-Roderique T, Li Y et al. Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. J Cell Mol Med 2013; 17: 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shynlova O, Nedd-Roderique T, Li Y et al. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J Cell Mol Med 2013; 17: 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol 2007; 120: 3–10. [DOI] [PubMed] [Google Scholar]
- Molnar M, Romero R, Hertelendy F. Interleukin-1 and tumor necrosis factor stimulate arachidonic acid release and phospholipid metabolism in human myometrial cells. Am J Obstet Gynecol 1993; 169: 825–829. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Mackler AM, Kirby MA. The role of leukocyte trafficking and activation in parturition. J Soc Gynecol Investig 2003; 10: 323–338. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, St Louis D, Lehr MA et al. Immune cells in term and preterm labor. Cell Mol Immunol 2014; 11: 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Preterm Birth Fact Sheet WHO: Geneva, Switzerland. 2015. [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD et al. Epidemiology and causes of preterm birth. Lancet 2008; 371: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracie S, Pennell C, Ekman-Ordeberg G et al. An integrated systems biology approach to the study of preterm birth using “-omics” technology – a guideline for research. BMC Pregnancy Childbirth 2011; 11: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Shynlova O, Lye SJ. Stretch-induced human myometrial cytokines enhance immune cell recruitment via endothelial activation. Cell Mol Immunol 2015; 12: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]