ABSTRACT
Burkholderia mallei, a facultative intracellular bacterium and tier 1 biothreat, causes the fatal zoonotic disease glanders. The organism possesses multiple genes encoding autotransporter proteins, which represent important virulence factors and targets for developing countermeasures in pathogenic Gram-negative bacteria. In the present study, we investigated one of these autotransporters, BatA, and demonstrate that it displays lipolytic activity, aids in intracellular survival, is expressed in vivo, elicits production of antibodies during infection, and contributes to pathogenicity in a mouse aerosol challenge model. A mutation in the batA gene of wild-type strain ATCC 23344 was found to be particularly attenuating, as BALB/c mice infected with the equivalent of 80 median lethal doses cleared the organism. This finding prompted us to test the hypothesis that vaccination with the batA mutant strain elicits protective immunity against subsequent infection with wild-type bacteria. We discovered that not only does vaccination provide high levels of protection against lethal aerosol challenge with B. mallei ATCC 23344, it also protects against infection with multiple isolates of the closely related organism and causative agent of melioidosis, Burkholderia pseudomallei. Passive-transfer experiments also revealed that the protective immunity afforded by vaccination with the batA mutant strain is predominantly mediated by IgG antibodies binding to antigens expressed exclusively in vivo. Collectively, our data demonstrate that BatA is a target for developing medical countermeasures and that vaccination with a mutant lacking expression of the protein provides a platform to gain insights regarding mechanisms of protective immunity against B. mallei and B. pseudomallei, including antigen discovery.
KEYWORDS: aerosols, autotransporter proteins, biodefense, countermeasures, glanders, immunoprotective antibodies, melioidosis, virulence determinants
INTRODUCTION
Burkholderia mallei and Burkholderia pseudomallei are closely related bacteria causing often fatal infections in animals and humans. B. pseudomallei is a motile, Gram-negative bacillus commonly found in water and wet soils in countries bordering the equator. The organism can infect most mammals and causes the tropical and emerging global disease melioidosis (1–9). B. mallei is an immotile, host-adapted clone of B. pseudomallei that does not persist outside its equine reservoir for long periods. The bacterium causes the extremely contagious and incapacitating zoonosis glanders, which primarily affects horses, mules, and donkeys. The disease is endemic in parts of the Middle East, Asia, Africa, and South America and is closely monitored by the World Organization of Animal Health, as it is considered a reemerging biosafety and biosecurity threat (7, 10–23). The genetic relatedness between the organisms indicates that B. mallei evolved from B. pseudomallei through a process of genomic reduction (24–27). The genes retained by B. mallei have an average identity of 99% at the nucleotide level with their B. pseudomallei orthologs, and of those, 650 have been proposed to form a core virulome based on comparative genomic analysis and sequence similarity to known virulence factors (28). Consistent with these in silico predictions, many virulome genes have been shown to specify key determinants in the pathogenesis of glanders and melioidosis, including lipopolysaccharide (LPS) (29, 30), type 3 and type 6 secretion systems (31–37), and capsule (38–41).
In addition to their high level of genomic relatedness, the clinical and pathological manifestations of disease caused by B. mallei and B. pseudomallei are markedly similar. Infection typically occurs via the respiratory route or through punctured skin, and the most common presentations are life-threatening pneumonia and bacteremia (2, 3, 10, 11, 18). A key aspect of pathogenesis by both B. mallei and B. pseudomallei that complicates treatment is their ability to invade and to survive and replicate within host cells, including professional phagocytes (5, 7, 42, 43). The organisms use type 3 and type 6 secretion systems to inject effector proteins inside host cells and to subvert eukaryotic cellular functions. Once internalized, B. mallei and B. pseudomallei escape endocytic vacuoles and enter the cytoplasm, where they replicate. The organisms then spread to adjacent cells through a process involving the formation of actin tails that push the bacteria from one cell to another. This ability to thrive intracellularly promotes dissemination to target tissues (liver, lungs, spleen, and lymph nodes), where the organisms form hallmark chronic lesions and granulomas that are difficult to treat. Glanders and melioidosis are difficult to diagnose and require prolonged antibiotic therapy with low success rates (2, 3, 10, 11, 18, 44, 45). B. mallei and B. pseudomallei are also resistant to most antibiotics, which limits treatment options (46–50). There is no vaccine to protect against these highly pathogenic bacteria, and there is concern regarding their use as bioweapons because B. mallei has already been utilized in this manner on multiple occasions (10, 11, 51–56). For these reasons, the U.S. Federal Select Agent Program has classified B. mallei and B. pseudomallei as tier 1 agents (the highest biosecurity level), and there is a pressing need to develop efficacious medical countermeasures for the organisms.
There has been significant effort in the past decade to devise vaccines for glanders and melioidosis, but many challenges remain, including considerable gaps in understanding immune mechanisms and correlates of protection, the availability of efficient vaccine delivery platforms, and the identification and characterization of protective antigens (57–64). Given their important roles in pathogenicity, overall structure, and cellular location at the host-pathogen interface, autotransporter proteins (ATs) represent excellent targets for developing countermeasures (65–74). These molecules form one of the largest families of virulence factors in Gram-negative bacteria and contribute a wide range of phenotypes, such as serum resistance, lipolytic activity, biofilms, and host cell adhesion (75–79). Thus, targeting ATs may impede the ability of pathogenic organisms to establish themselves in a host, persist, and cause disease. Autotransporters also share 4 structural features: a signal sequence directing the protein to cell membranes for secretion, an N-terminal passenger domain that specifies the biological function, a C-terminal transporter domain anchoring the AT to the outer membrane, and a helical linker region of ∼40 amino acids that connects the passenger and transporter domains. Based on the structure of the transporter domain, ATs can be classified as oligomeric or conventional (65–74, 80). Oligomeric ATs have a short C terminus of ∼70 amino acids that forms 4 antiparallel β-strands and are produced as trimers. In contrast, conventional ATs have a large C terminus (∼300 amino acids) specifying 10 to 12 β-strands and are produced as monomers. The N-terminal passenger domain of both AT classes is displayed on the bacterial cell surface and is typically the main target for developing countermeasures because it is readily accessible to the immune system. Many studies have demonstrated that ATs are immunoprotective antigens (81–86), and the inclusion of the host cell adhesion AT proteins NadA and pertactin in licensed vaccines for Neisseria meningitidis (Bexsero) and Bordetella pertussis (Daptacel, Infanrix, Boostrix, and Adacel), respectively, underscores their value for devising medical intervention strategies.
The genomes of B. mallei and B. pseudomallei have in common 8 distinct autotransporter genes; 6 are predicted to encode oligomeric-type proteins, while the other 2 specify conventional ATs (87). Previous studies have examined the biological roles of all 8 ATs in B. pseudomallei (88–90). However, only the oligomeric ATs BimA (intracellular motility protein) (91), BoaA (92) and BpaC (93) (host cell adhesion proteins), and BpaB (biofilm and virulence factor) (77) have been functionally characterized in B. mallei. With this in mind, we sought to characterize the B. mallei ortholog of the conventional AT previously designated BatA in studies with the B. pseudomallei wild-type (WT) isolate K96243 (89) (the strains and plasmids used in the study are listed in Table 1). We constructed a B. mallei batA gene mutant strain, assessed its pathogenicity using both in vitro and in vivo infection models, and utilized the mutant as an experimental live attenuated vaccine to identify mechanisms of cross-protective immunity against glanders and melioidosis.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| B. mallei | ||
| ATCC 23344 | Wild-type strain; polymyxin B resistant, zeocin and kanamycin sensitive | 27 |
| batA KO | Isogenic batA mutant strain of ATCC 23344; resistant to polymyxin B and zeocin, sensitive to kanamycin | This study |
| ilv KO | Isogenic ilvB mutant strain of ATCC 23344; resistant to polymyxin B and kanamycin, sensitive to zeocin | This study |
| B. pseudomallei | ||
| 1026b | Wild-type strain | 148 |
| K96243 | Wild-type strain | 26 |
| B. thailandensis | ||
| DW503 | Laboratory strain; kanamycin sensitive | 143 |
| E. coli | ||
| EPI300 | Strain used for recombinant-DNA manipulations | Epicenter/Illumina |
| S17 | Strain used for conjugational transfer of plasmids to B. mallei; sensitive to polymyxin B, zeocin, and kanamycin | 149 |
| TUNER | Protein expression strain used to purify His- and GST-tagged BatA proteins | EMD Millipore |
| Plasmids | ||
| pBHR1 | Cloning vector; confers resistance to chloramphenicol and kanamycin | MoBiTec |
| pBHR1Δ Dra | pBHR1 containing a 339-nt deletion in the chloramphenicol resistance marker; confers resistance only to kanamycin | 77 |
| pBatA | pBHR1 in which the batA gene of B. pseudomallei K96243 was inserted; confers resistance only to kanamycin | This study |
| pCC1 | Cloning vector; confers resistance to chloramphenicol | Epicenter/Illumina |
| pCCbatA | pCC1 in which the batA gene of B. pseudomallei 1026b was inserted; confers resistance to chloramphenicol | This study |
| pEM7/ZEO | Source of the zeocin resistance cassette; confers resistance to zeocin | Thermo Fisher Scientific |
| pCCbatA.zeo | pCCbatA in which a 1.3-kb portion of the batA ORF was deleted and replaced with a 0.4-kb zeocin resistance cassette; confers resistance to chloramphenicol and zeocin | This study |
| pKAS46 | Gene replacement vector; confers resistance to kanamycin | 150 |
| pKASbatA.zeo | pKAS46 containing the insert from pCCbatA.zeo; confers resistance to kanamycin and zeocin | This study |
| pETcoco-1 | His-tagged protein expression vector; confers resistance to chloramphenicol | EMD Millipore |
| pHisBatA | pETcoco-1 in which a gene fragment encoding amino acids 30–307 of B. mallei ATCC 23344 BatA was inserted; confers resistance to chloramphenicol | This study |
| pGEX4T-2 | GST-tagged protein expression vector; confers resistance to ampicillin | GE Healthcare Life Sciences |
| pGSTBatA | pGEX4T-2 in which a gene fragment encoding amino acids 30–307 of B. mallei ATCC 23344 BatA was inserted; confers resistance to ampicillin | This study |
| pCCilvUP | pCC1 in which a 1-kb PCR product corresponding to the genomic sequence upstream of the B. mallei ATCC 23344 ilvB gene was inserted; confers resistance to chloramphenicol | This study |
| pCCilvDOWN | pCC1 in which a 1-kb PCR product corresponding to the genomic sequence downstream of the B. mallei ATCC 23344 ilvB gene was inserted; confers resistance to chloramphenicol | This study |
| pCCilvΔ | pCC1 containing the genomic sequences located upstream and downstream of the B. mallei ATCC 23344 ilvB gene joined by a unique NheI site; confers resistance to chloramphenicol | This study |
| pUC4K | Source of the kanamycin resistance cassette; confers resistance to kanamycin | GE Healthcare Life Sciences |
| pCCilv.kan | pCCilvΔ in which the kanamycin resistance cassette was inserted into the NheI site | This study |
| pKAS46-ZEO | pKAS46 in which the kanamycin resistance marker was replaced with a zeocin resistance cassette from pEM7/ZEO; confers resistance to zeocin | This study |
| pKASilv.kan | pKAS46-ZEO containing the insert from pCCilv.kan; confers resistance to zeocin and kanamycin | This study |
| pBR322 | Cloning vector; confers resistance to ampicillin | New England Biolabs Inc |
| pJTmcaP | pBR322 in which the gene encoding the M. catarrhalis mcaP gene was inserted; confers resistance to ampicillin | 76 |
RESULTS
In silico and in vitro characterization of the batA gene and its encoded product.
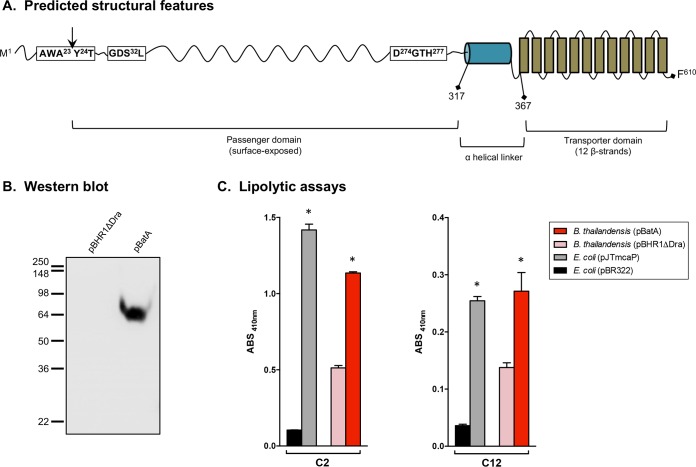
Comparative sequence analyses identified an ortholog of the B. pseudomallei K96243 batA gene (locus tag BPSL2237) on the complementary strand of chromosome I in the genome of B. mallei ATCC 23344 (locus tag BMA1647). The open reading frame (ORF) is 1,833 nucleotides (nt) in length and is predicted to encode an outer membrane protein of 610 amino acid residues with a molecular mass of 64 kDa. Consistent with this predicted location in the outer membrane, analysis with the SignalP 4.1 server detected a signal sequence cleavage site at the N terminus of BatA between amino acids 23 and 24 with a discrimination score of 0.592 (cutoff = 0.420). As shown in Fig. 1A, the batA gene product possesses key features of conventional ATs. Analysis with PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred) suggested that the last 243 amino acids form a C-terminal transporter domain consisting of 12 antiparallel β-strands and exhibit sequence similarity to the Simple Modular Architecture Research Tool (SMART) Autotransporter beta domain SM00869 (E value, 2.42e−22). Analysis with PSIPRED also indicated that amino acids 317 to 367 form a helical linker region that connects the transporter domain to an N-terminal passenger domain that is predicted to be exposed on the bacterial surface (residues 24 to 316). Searches using NCBI BLAST identified batA orthologs in the genomes of 30 B. mallei and 325 B. pseudomallei isolates. The encoded proteins were found to be highly conserved between all strains and both species (99 to 100% identity).
FIG 1.
Selected characteristics of the batA gene product and constitutive expression by recombinant B. thailandensis bacteria. (A) Different regions of the predicted BatA protein with positions of residues defining selected domains. The vertical arrow indicates the predicted signal sequence cleavage site. (B) Proteins were extracted from B. thailandensis DW503 bacteria carrying the plasmids pBHR1ΔDra (control) and pBatA (specifying the WT batA gene product) and analyzed by Western blotting with the monoclonal antibody BatA-MAb 1. Molecular mass markers are shown on the left in kilodaltons. (C) Freshly plate-grown bacteria were suspended in PBS, and equivalent numbers of cells were incubated with pNPs composed of 2 (C2) to 18 (C18) carbon chains. The absorbance (ABS) of triplicate samples was measured at a wavelength of 410 nm. The results are expressed as mean absorbances of samples and standard errors. Substrates were tested on at least 3 separate occasions. The asterisks indicate that recombinant bacteria producing BatA and McaP exhibited lipolytic activity levels significantly greater than those of their corresponding controls (P < 0.005; Mann-Whitney test). Data from representative experiments are shown. Only the substrates cleaved by BatA are shown (C2 and C12). The AT did not exhibit significant lipolytic activity toward pNP C4, C6, C8, C10, C14, C16, or C18.
To identify a potential function of batA, the gene sequence was analyzed using the NCBI Conserved Domain Database (CDD) service. The data suggest that the gene product belongs to the SGNH_hydrolase superfamily (E value, 9.21e−63), which comprises bacterial lipases and esterases specifying a highly conserved Gly-Asp-Ser-Leu (GDSL) motif in their N termini. While the substrates of these enzymes are diverse, the amino acids directly involved in their activity are conserved in molecules that have been experimentally characterized. These active-site residues form a prototypical Ser-His-Asp (SHD) catalytic triad and include the serine in the GDSL motif, which functions as the nucleophile. As illustrated in Fig. 1A, the BatA protein possesses a GDSL motif in its N terminus (amino acids 30 to 33), and residues S32, D274, and H277 are predicted to form a catalytic triad. Subsequent comparative sequence analyses and database searches identified two well-characterized conventional ATs belonging to the SGNH_hydrolase superfamily and exhibiting sequence similarities to BatA, Pseudomonas aeruginosa EstA (94–97), and Moraxella catarrhalis McaP (76, 98, 99). Both ATs have been shown to contain a GDSL motif in their N-terminal passenger domains and to function as esterases.
Based on similarities to EstA and McaP, we tested whether BatA might exhibit lipolytic activity. To accomplish this, the batA gene was cloned into the vector pBHR1, and the resulting plasmid, pBatA, was introduced into the Burkholderia thailandensis laboratory strain DW503. The latter was used as the host for recombinant work because of its low virulence and genetic relatedness to B. mallei and B. pseudomallei (100–104), which make the organism a useful surrogate for performing experiments under less restrictive biocontainment conditions. To determine if the BatA protein was produced by recombinant bacteria, Western blotting was performed. As shown in Fig. 1B, the BatA-specific monoclonal antibody no. 1 (BatA-MAb 1) bound to a protein with the expected molecular mass of 64-kDa in B. thailandensis harboring the plasmid pBatA, but not the vector control pBHR1ΔDra, demonstrating both antibody specificity and constitutive expression of the batA gene product. Next, we assessed the lipolytic activity of recombinant B. thailandensis cells using p-nitrophenyl esters (pNPs) with different carbon chain lengths. In these assays, the cleavage of pNPs releases nitrophenol, which is measured spectrophotometrically at a wavelength of 410 nm. Esterases typically cleave short-chain substrates (≤10 carbons), while lipases have a preference for longer chains (≥12 carbons). As shown in Fig. 1C, constitutive expression of the batA gene product by B. thailandensis cells carrying pBatA resulted in significantly enhanced cleavage (≥2-fold) of the pNPs C2 and C12 compared to the organism harboring the control plasmid pBHR1ΔDra. Benchmark levels of lipolytic activity were established using Escherichia coli carrying the vector control pBR322 and recombinant E. coli bacteria that harbor plasmid pJTmcaP and express the above-mentioned M. catarrhalis conventional AT McaP, which was previously shown by our group to cleave pNP substrates (76, 98, 99). Therefore, the results of pNP cleavage assays support the in silico analyses and indicate that BatA exhibits lipolytic activity.
Characterization of the B. mallei batA gene and its encoded product.
To investigate the biological function of the batA gene, we constructed an isogenic mutant strain of B. mallei ATCC 23344. Mutagenesis was accomplished via homologous recombination of a 1.4-kb DNA fragment corresponding to the batA gene in which an internal portion of the ORF was replaced with a zeocin resistance marker in the genome of the organism. To ascertain lack of BatA production by the mutant, protein lysates from both the parent and mutant strains were analyzed by Western blotting with the monoclonal antibody BatA-MAb 1. As expected, the antibody did not react with extracts from the isogenic batA knockout (KO) mutant strain. However, we also discovered that the monoclonal antibody does not react with protein preparations from WT bacteria. Extensive investigation using different culture media (broth, agar, rich, minimal, chemically defined, and low iron), growth conditions (a range of temperatures above and below 37°C, various incubation periods, and replication within macrophages), and detection methods (Western blotting, immunofluorescence labeling of bacteria, and immunoprecipitation) with the BatA-MAb 1 antibody and BatA-specific polyclonal antisera failed to show expression of the AT by B. mallei ATCC 23344. Based on these results, we concluded that BatA is not produced at detectable levels under any of the laboratory conditions examined.
Several B. mallei gene products have been reported to be selectively expressed in vivo (and/or under in vitro conditions that mimic the host environment), including the ATs BpaB (77), BpaC (93), and BimA (31, 105). With this in mind, we assessed whether BatA might be produced in vivo. To accomplish this, serum samples from mice that survived aerosol infection with B. mallei ATCC 23344 were tested by enzyme-linked immunosorbent assay (ELISA) using purified recombinant BatA protein. We found that the mice produced antibodies against BatA, with a reciprocal endpoint titer of 233 ± 88. As a positive control, we tested the serum samples for the presence of antibodies against capsular polysaccharides (CPS), which are known to be immunogenic during infection (106), and measured a titer of 933 ± 352. To confirm the validity of these data, we tested serum samples from horses experimentally infected with B. mallei ATCC 23344 by ELISA. We found that the horses produced antibodies against BatA and CPS with titers of 1,150 ± 695 and 5,600 ± 2,653, respectively. Altogether, these results indicate that B. mallei expresses the batA gene product in vivo at some stage of the infection, which in turn elicits the production of BatA-specific antibodies by the host.
The inability to detect the BatA protein in laboratory-grown bacteria suggests that it is not required for replication outside a host. Conversely, ELISA data indicate that the AT is produced during the course of infection and therefore may be important for agent replication in vivo. Since the ability to survive and replicate inside professional phagocytic cells is a key aspect of pathogenesis by B. mallei, we compared the fitness of the batA KO mutant to that of the WT strain in macrophage killing assays using J774 murine cells. These experiments revealed that the batA mutation caused a reduction in intracellular replication of nearly 40% over a period of 7 h (see Fig. S1A in the supplemental material). We also compared the growth of the WT and mutant strains in liquid broth cultures and found that they grew at equivalent rates (see Fig. S1B in the supplemental material). Therefore, the reduced intracellular fitness of the batA KO mutant does not appear to be the result of a generalized growth defect. Taken together, these data show a role for the batA gene in the replication and/or survival of B. mallei within host cells.
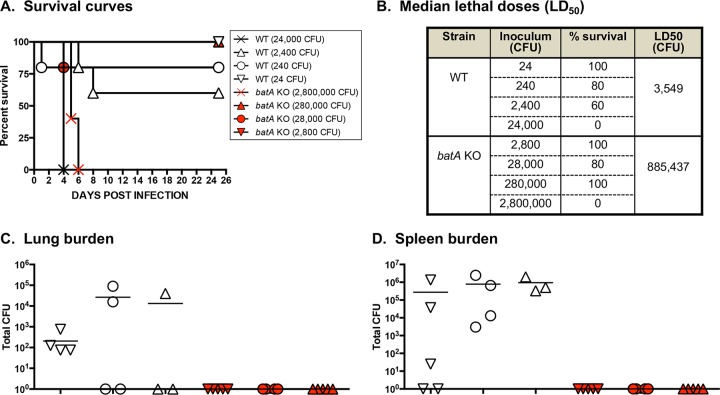
Based on the ELISA data suggesting in vivo expression during infection and the results of macrophage killing assays indicating a role in the ability to thrive intracellularly, we hypothesized that the batA gene contributes to the virulence of B. mallei. To test this, we determined the median lethal dose (LD50) of the batA KO mutant strain using a mouse model of aerosol infection. At study endpoints, tissues from the animals that survived infection were also collected, and the bacterial burden was determined as a measure of in vivo fitness. Compared to infection with WT organisms, the calculated LD50 values for the batA KO strain were considerably higher (Fig. 2A and B; see Fig. S2A and B in the supplemental material). These data are consistent with the results of macrophage assays showing reduced intracellular fitness of the mutant and suggest that the batA gene product is an important virulence factor of B. mallei. This belief is further supported by analyses of the bacterial burden in target tissues. While the batA KO strain was cultured from the lungs and spleens of survivors 15 days postchallenge (see Fig. S2C and D), no bacteria were detected in any of the mice infected with the mutant at day 25 (Fig. 2C and D). Moreover, the number of organisms in the tissues of mice infected with the batA KO strain at day 15 postchallenge was significantly less than in animals inoculated with a much lower dose of WT B. mallei (see Fig. S2C and D).
FIG 2.
Median lethal dose comparison for B. mallei WT and mutant strains. BALB/c mice were inoculated intratracheally using a Microsprayer device to aerosolize the indicated numbers of bacterial CFU into the lungs (n = 5 mice/dose). The animals were then monitored daily for clinical signs of illness and morbidity. (A) Kaplan-Meier survival curves. (B) Calculated LD50 values (by the method of Reed and Muench). (C and D) Tissues were collected from mice that survived challenge (day 25), homogenized, diluted, and spread on agar plates to determine bacterial loads. The symbols represent data for individual animals; the horizontal lines represent the mean total number of CFU for each group.
To confirm the contribution of the BatA protein to B. mallei virulence, we introduced the plasmid pBatA into the batA KO mutant and determined the LD50 of the complemented strain. Surprisingly, these experiments revealed that constitutive production of the AT does not restore virulence to WT levels but instead enhances attenuation. The batA KO strain harboring the control plasmid pBHR1ΔDra showed an approximately 1,000-fold reduction in the LD50, while the median lethal dose of the mutant carrying pBatA was >3,000-fold lower than that of WT B. mallei (Fig. 3B). To verify that the hyperattenuated phenotype of recombinant bacteria constitutively producing BatA was not due to a global growth defect, we measured the replication of WT B. mallei alongside the mutant carrying the plasmids pBHR1ΔDra and pBatA in liquid broth cultures. We found that all the strains grew at comparable rates (Fig. 4A) and that both plasmids were stably maintained in the absence of antibiotic selective pressure (Fig. 4B). Western blot analysis with the BatA-MAb 1 antibody also verified that the complemented batA KO strain produces the 64-kDa AT (Fig. 4C). In addition, macrophage killing assays demonstrated that the plasmid pBatA restores intracellular replication of the mutant to near WT levels (Fig. 4D). Therefore, the hyperattenuation of the batA KO strain constitutively producing BatA in vivo does not appear to be the result of an intracellular fitness deficiency. These data also confirm the results from macrophage killing assays showing reduced intracellular fitness of the batA KO mutant and demonstrate a role for BatA in the ability of B. mallei to thrive within host cells.
FIG 3.
Median lethal dose comparison for B. mallei WT and recombinant strains. BALB/c mice were inoculated intratracheally using a Microsprayer device to aerosolize the indicated numbers of bacterial CFU directly into the lungs (n = 5 mice/dose). The animals were then monitored daily for clinical signs of illness and morbidity. (A) Kaplan-Meier survival curves. (B) Calculated LD50 values (by the method of Reed and Muench). (C and D) Tissues were collected from mice that survived challenge (day 15), homogenized, diluted, and spread on agar plates to determine bacterial loads. The symbols represent data for individual animals; horizontal lines represent the mean total number of CFU for each group.
FIG 4.
In vitro replication rates and BatA production of B. mallei WT and recombinant strains. (A) Plate-grown bacteria were suspended in broth (without antibiotic supplementation) to an OD600 of 0.1. Following this, the bacteria were incubated at 37°C with shaking (200 rpm), and the optical densities of the cultures were measured from duplicate samples at the indicated time intervals. Strains were tested on 5 separate occasions. The error bars correspond to standard errors of the mean. The results of one representative experiment are shown. (B) At the endpoints of growth experiments, the liquid cultures were serially diluted and plated onto agar medium containing kanamycin (a selective marker encoded by plasmids pBHR1ΔDra and pBatA); duplicate aliquots were plated onto plain agar medium (no antibiotic added). The agar plates were incubated at 37°C for 48 h, and the CFU were counted to evaluate plasmid stability in recombinant strains. The error bars correspond to standard errors of the mean. The results of one representative experiment are shown. (C) Proteins were extracted from WT B. mallei ATCC 23344 bacteria and the batA KO mutant strain carrying the plasmids pBHR1ΔDra (control) and pBatA (specifying the WT batA gene) and analyzed by Western blotting with the monoclonal antibody BatA-MAb 1. Molecular mass markers are shown on the left in kilodaltons. (D) Plate-grown bacteria were suspended in PBS and used to infect 2 wells of duplicate tissue culture plates seeded with murine J774 macrophages (multiplicity of infection = 10:1). The infected cells were incubated for 1 h at 37°C to allow phagocytosis of the bacteria, washed, and treated with antibiotic for 2 h. Cells from one tissue culture plate were lysed, diluted, and plated onto agar medium to determine the number of bacteria phagocytized. The other tissue culture plate was incubated for an additional 7 h, after which the cells were washed, lysed, diluted, and spread onto agar plates to calculate the number of intracellular organisms. The results are expressed as the mean (plus standard error) intracellular replication index, which was calculated by dividing the number of intracellular bacteria at the endpoint of the assay (second tissue culture plate) by the number of bacteria phagocytized (first tissue culture plate). The assays were performed on 3 separate occasions. The asterisk indicates that the reduction in the intracellular replication index of the batA KO mutant carrying plasmid pBHR1ΔDra, compared to WT B. mallei, was statistically significant using a paired t test (P = 0.02). The hash mark indicates that the reduction in the intracellular replication index of the batA KO mutant carrying plasmid pBHR1ΔDra, compared to the batA KO mutant harboring the plasmid pBatA, was statistically significant using a paired t test (P = 0.04).
Protection studies.
Our data indicate that at an inoculating dose of 104 CFU, the batA KO strain is detected in target tissues on day 15 postinfection (see Fig. S2C and D in the supplemental material) but is cleared by day 25 (Fig. 2C and D). Based on these findings, we hypothesized that transient colonization by the mutant elicits protective immunity against subsequent challenge with WT bacteria. To test this, we inoculated groups of mice via the aerosol route with 104 CFU of the batA KO strain and, 30 days later, “back-challenged” the animals with ∼10 LD50 of B. mallei ATCC 23344. As positive controls, mice were vaccinated via the aerosol route with 105 CFU of a B. mallei live attenuated strain harboring a mutation in the ilv locus. The latter is the equivalent of the B. pseudomallei vaccine strain 2D2, an established benchmark of protection in the field (107–109). As shown in Fig. 5A and B, all the naive mice succumbed to infection by day 5 postchallenge. In contrast, vaccination with the B. mallei ilv KO strain protected 40% of the mice against mortality through both acute and chronic stages of disease. Inoculation with the batA KO mutant provided the highest level of protection during acute and chronic infection (67% and 50% survival, respectively). In multiple independent experiments, protection against lethal aerosol exposure to WT B. mallei afforded by prior inoculation with the batA KO mutant was found to be highly reproducible. Cumulative survival numbers from 11 independent experiments showed 73% and 56% survival during acute and chronic infection, respectively (Table 2). As shown in Fig. S3 in the supplemental material, this high level of protection correlated with restricted growth of WT B. mallei in the lungs, as well as a significant delay in the dissemination of bacteria into the blood and liver. However, the immune response elicited by vaccination with the batA KO mutant did not interfere with dissemination and replication of the organism in the spleen (see Fig. S3B). Taken together, these data demonstrate that inoculation with the batA KO strain induces potent host immune responses, which provide excellent protection from death during the acute and chronic stages of disease caused by B. mallei and can delay the dissemination and replication of WT bacteria in distinct target organs.
FIG 5.
Vaccination with the batA KO mutant strain provides protective immunity against back-challenge with a lethal dose of WT B. mallei bacteria. BALB/c mice were vaccinated intratracheally with 104 CFU of the batA KO and 105 CFU of the ilv KO mutant strains using a Microsprayer device. Thirty days later, the animals were back-challenged with ∼10 LD50 of WT B. mallei ATCC 23344 and monitored daily for clinical signs of illness and morbidity. Age- and weight-matched naive BALB/c mice were used as controls. (A) Kaplan-Meier survival curves. (B) Survival data during the acute and chronic phases of infection. (C, D, and E) Tissues were collected from mice that survived back-challenge (day 45), homogenized, diluted, and spread on agar plates to determine bacterial loads. The symbols show data for individual animals; the horizontal lines represent the mean total CFU for each group.
TABLE 2.
Experiments demonstrating that vaccination with the batA KO mutant strain provides protective immunity against aerosol challenge with lethal doses of WT organisms
| No. of independent experiments | Vaccinea | WT agent used in challengeb | Total no. of mice challenged | % survivalc |
% sterile immunityd | |
|---|---|---|---|---|---|---|
| Acute infection | Chronic infection | |||||
| 11 | batA KO | B. mallei ATCC 23344 | 99 | 73 | 56 | 0 |
| Naive | 94 | 4 | 2 | 0 | ||
| 8 | batA KO | B. pseudomallei 1026b | 82 | 71 | 67 | 62 |
| Naive | 74 | 0 | 0 | 0 | ||
| 4 | batA KO | B. pseudomallei K96243 | 33 | 100 | 85 | 57 |
| Naive | 27 | 11 | 4 | 0 | ||
Mice were vaccinated intratracheally with 104 CFU of the batA KO mutant strain using a Microsprayer.
Thirty to 45 days postvaccination, mice were back-challenged intratracheally with lethal doses of WT bacteria (∼10 LD50 of WT B. mallei ATCC 23344 and ∼5 LD50 of B. pseudomallei 1026b and K96243) using a Microsprayer device. Age- and weight-matched naive mice were used as controls.
Acute infection, 1 to 10 days postchallenge; chronic infection, 11 to 55 days postchallenge.
The livers, spleens, and lungs of survivors were collected, homogenized, diluted, and plated onto agar medium to determine whether the tissues were colonized with WT bacteria.
To investigate which arm of the adaptive immune system contributes to protection, we examined the role of antibodies by passive transfer of immune serum. Groups of mice were vaccinated with the batA KO mutant and exsanguinated 30 to 45 days postvaccination, and sera from these animals were pooled (Fig. 6A). Naive mice were then injected intraperitoneally with 1 ml of the pooled immune sera and challenged 48 h later with ∼10 LD50 of WT B. mallei ATCC 23344 bacteria via the aerosol route. As shown in Fig. 6B and C, administration of a single dose of immune serum afforded protection against death that was equivalent to or greater than that provided by vaccination with the batA KO strain. Administration of immune serum provided a higher rate of survival during the acute stage of disease (80% versus 50% for mice vaccinated with the batA KO mutant), and the same number of animals (5/10) survived through the chronic phase in both experimental groups. In addition, the bacterial burden in target organs of mice that survived for the duration of the study were nearly identical whether the animals were vaccinated with the batA KO strain or administered immune serum (Fig. 6D, E, and F). These experiments also indicated that protection is dependent on the amount of immune serum administered, because the passive transfer of a 0.1-ml volume did not protect against lethal infection (Fig. 6B and C). As shown in Table 3, the results of passive-transfer experiments were highly reproducible. In 4 independent studies, immune serum provided cumulative survival rates of 91% and 66% during acute and chronic infection, respectively. Given the quality and levels of protection seen after transfer of immune serum, we determined the kinetics of bacterial accumulation in target tissues after challenge with WT B. mallei. These experiments revealed that administration of immune serum restricts bacterial growth in the lungs (see Fig. S4A in the supplemental material) and delays the dissemination and accumulation of WT B. mallei in the liver (see Fig. S4C) and blood (see Fig. S4D). However, the transfer of immune serum had no discernible effect on the bacterial burden in the spleen (see Fig. S4B). These results are consistent with the data shown in Fig. S3 and indicate that the effect of immune serum on the kinetics of bacterial accumulation in target organs during the early stage of infection is comparable to that afforded by vaccination with the batA KO mutant strain. Altogether, the data indicate that the humoral immune response elicited by vaccination with the batA KO mutant strain is sufficient to protect against lethal aerosol exposure to WT B. mallei and can aid in reducing the colonization of distinct target tissues.
FIG 6.
Passive transfer of immune serum provides protective immunity against challenge with a lethal dose of WT B. mallei bacteria. (A) Experimental timeline and details. BALB/c mice vaccinated intratracheally with 104 CFU of the batA KO mutant strain and back-challenged with WT B. mallei ATCC 23344 (30 days postvaccination) were used as an efficacy benchmark. (B) Kaplan-Meier survival curves. (C) Survival data during the acute and chronic phases of infection. (D, E, and F) Tissues were collected from mice that survived challenge (day 32), homogenized, diluted, and spread on agar plates to determine bacterial loads. The symbols show data for individual animals; the horizontal lines represent the mean total CFU for each group.
TABLE 3.
Passive-transfer experiments demonstrating that immune sera from mice vaccinated with the batA KO mutant strain provide protective immunity against challenge with lethal doses of WT B. mallei
| No. of independent experimentsa | Treatmentb | Total no. of mice challenged | % survivalc |
% sterile immunityd | |
|---|---|---|---|---|---|
| Acute infection | Chronic infection | ||||
| 4 | Immune serum | 35 | 91 | 66 | 0 |
| Naive serum | 34 | 12 | 3 | 0 | |
| batA KO vaccination | 38 | 63 | 56 | 0 | |
Immune serum was generated for each independent experiment.
Mice were administered 1 ml of serum intraperitoneally. Forty-eight hours later, the animals were challenged intratracheally with ∼10 LD50 of WT B. mallei ATCC 23344 using a Microsprayer device. Mice vaccinated with 104 CFU of the batA KO strain 30 to 45 days prior to challenge with WT bacteria were used as efficacy benchmarks.
Acute infection, 1 to 10 days postchallenge; chronic infection, 11 to 55 days postchallenge.
The livers, spleens, and lungs of survivors were collected, homogenized, diluted, and plated onto agar medium to determine whether the tissues were colonized with WT bacteria.
To examine the functionality of antibodies in the immune serum, we measured B. mallei-specific immunoglobulin titers and isotypes by ELISA using whole bacteria fixed with paraformaldehyde. As shown in Fig. 7A, single-dose vaccination with the batA KO strain resulted in robust IgG titers in the immune serum (a mean reciprocal endpoint titer of 24,533 ± 6,274) with a Th1 bias IgG2a/IgG1 ratio of 8.5, and the levels of IgG3 and IgG2b immunoglobulins were consistently the highest. Flow cytometry analysis of whole WT B. mallei bacteria with the immune serum revealed that the antibodies bind to the surface of the organism (Fig. 7B), and opsonophagocytic killing assays demonstrated that the antibodies increase phagocytosis of B. mallei by macrophages (Fig. 7C) and promote effective intracellular killing (Fig. 7D).
FIG 7.
ELISA, flow cytometry, and opsonophagocytic killing assays using WT B. mallei and immune serum (from mice vaccinated with the batA KO strain). (A) Immune serum samples were serially diluted and placed in duplicate wells of plates coated with whole paraformaldehyde-fixed B. mallei ATCC 23344 bacteria. Alkaline-phosphatase-conjugated goat anti-mouse isotype-specific antibodies were used for detection. The results are expressed as mean (plus standard error) reciprocal endpoint titers of 5 independently generated batches of immune serum. Naive serum was used to establish background reactivity. (B) Whole paraformaldehyde-fixed B. mallei ATCC 23344 bacteria were incubated with immune serum, labeled with a goat anti-mouse antibody conjugated to Alexa Fluor 488, and analyzed using a BD LSRII flow cytometer. The number of cells analyzed and the percentage binding antibodies on their surfaces are shown. Bacteria incubated with naive serum were used as controls. (C and D) Freshly grown B. mallei ATCC 23344 bacteria were incubated with immune serum for 30 min at 37°C, and the opsonized organisms were used to infect 2 wells of duplicate tissue culture plates seeded with murine J774 macrophages. (C) The infected cells were incubated for 1 h at 37°C to allow phagocytosis of the bacteria, washed, and treated with antibiotic for 2 h. Cells from one tissue culture plate were lysed, diluted, and plated onto agar medium to determine the number of bacteria phagocytized. The results are expressed as the mean numbers of CFU plus standard errors. (D) The other tissue culture plate was incubated for an additional 7 h, after which the cells were washed, lysed, diluted, and spread onto agar plates to calculate the number of intracellular organisms. The results are expressed as the mean (plus standard error) intracellular replication index, which was calculated by dividing the number of intracellular bacteria at the endpoint of the assay (second tissue culture plate) by the number of bacteria phagocytized (first tissue culture plate); an index below 1 (blue dashed line) indicates intracellular killing of bacteria. The assays were performed on 15 separate occasions. Bacteria incubated with PBS and naive serum (prior to infecting macrophages) were used as controls. The P values indicate that the differences observed between bacteria incubated with naive and immune sera were statistically significant using a paired t test.
It is possible that whole immune sera from mice vaccinated with the batA KO mutant contain soluble factors (e.g., cytokines, chemokines, and antimicrobial peptides) responsible for the protection observed in passive-transfer experiments. To address this, we purified IgG antibodies from immune serum (Fig. 8A), injected naive mice with a volume of immunoglobulins reflective of protective titers (see Fig. S5 in the supplemental material), and challenged the animals with ∼10 LD50 of WT B. mallei via the aerosol route. For the negative-control groups, the volume of purified naive IgG preparations was adjusted to reflect the quantity of purified immune IgG passively transferred to the mice. As shown in Fig. 8B and C, purified IgG antibodies provided protection against death during acute and chronic infection, albeit at levels slightly lower than whole immune serum. Similar to immune serum, the purified IgG antibodies increased phagocytosis of B. mallei by macrophages (see Fig. S6A in the supplemental material), as well as intracellular killing of the organism (see Fig. S6B). Taken together, these data conclusively demonstrate that vaccination with the batA KO mutant elicits the production of IgG antibodies that are sufficient to protect against lethal aerosol infection with B. mallei and to promote bacterial clearance by professional phagocytic cells.
FIG 8.
Passive transfer of immune serum fractions provides protective immunity against challenge with a lethal dose of WT B. mallei. (A) Experimental timeline and details. Naive serum was used as a control. (B) Kaplan-Meier survival curves. (C) Survival data during the acute and chronic phases of infection. (D, E, and F) Tissues were collected from mice that survived challenge, homogenized, diluted, and spread on agar plates to determine bacterial loads. The symbols show data for individual animals; the horizontal lines represent the mean total CFU for each group. The experiments were performed on 2 separate occasions (each time with freshly generated immune serum), and the graphs and table show cumulative results.
To gain insight into the antigens recognized by protective antibodies, we also performed passive-transfer experiments using immune serum that had been adsorbed to plate-grown B. mallei cells. The ELISA data in Fig. S5 in the supplemental material demonstrate that this treatment effectively removed antibodies against antigens constitutively expressed in vitro, including capsular polysaccharides and LPS, which are the most abundant molecules on the surface of the organism and lead targets for vaccine development in the field (110–114). Remarkably, the adsorbed serum, which was completely depleted of antibodies binding to laboratory-grown bacteria, retained the ability to protect against lethal aerosol exposure to WT B. mallei ATCC 23344 at a level that was comparable to that of IgG antibodies purified from whole immune serum (Fig. 8B and C). We found that 56% of mice injected with adsorbed serum survived the acute phase of infection (compared to 58% of mice given purified IgG) and that 31% of the infected animals survived for the entire duration of the studies (versus 33% of mice administered IgG). In addition, the transfer of adsorbed serum promoted more effective clearance of bacteria from the lungs (Fig. 8D) and liver (Fig. 8E) than purified IgG antibodies and whole immune serum. No bacteria could be detected in these organs from 4 out of 5 mice (80%) that were given adsorbed serum. In contrast, 12 of 13 mice (92%) that received immune serum and IgG antibodies were colonized in these tissues. Based on these results, we conclude that the adsorbed immune serum retained protective antibodies binding to antigens that are specifically expressed in vivo by the batA KO strain during vaccination (while it transiently colonizes the animals).
Cross-protection studies.
To evaluate the breadth of protective immunity, we tested whether vaccination with the batA KO strain could protect against B. pseudomallei. The data in Table 2 demonstrate that vaccination with the batA KO mutant reproducibly elicited protective immunity against lethal aerosol exposure to the WT B. pseudomallei strains 1026b and K96243, and the overall survival rate was comparable to that measured in back-challenge experiments with B. mallei ATCC 23344 (Table 2). However, a key difference from the B. mallei studies is that up to 62% of the mice that survived B. pseudomallei infection developed sterile immunity (compare the values in the last column of Table 2), and gross pathology analysis of the lungs, spleens, and livers from these animals was unremarkable compared to those from uninfected naive mice (data not shown). Passive transfer of whole immune serum to naive mice also resulted in high levels of protection against lethal challenge with B. pseudomallei K96243 (Fig. 9A and B). The serum provided 100% and 83% survival during acute and chronic infection, respectively, and 17% of the mice that survived had no detectable bacterial burden in target tissues by day 25 postchallenge. The data in Fig. S7 in the supplemental material demonstrate that protection is not dependent on the mouse background. Vaccination of C57BL/6 mice with the batA KO mutant provided complete protection against death during acute and chronic infection with both Burkholderia species. All the mice that survived B. pseudomallei infection developed sterile immunity, and 60% of the animals infected with B. mallei completely cleared the bacteria (see Fig. S7A and B). Likewise, passive transfer of whole immune serum to naive C57BL/6 mice resulted in high levels of protection against lethal challenge with both organisms. Taken together, these data indicate that vaccination with the B. mallei batA KO mutant strain elicits cross-protective immunity against WT B. mallei and B. pseudomallei isolates and that antibodies alone are sufficient for protection against both species of bacteria.
FIG 9.
Passive transfer of immune serum provides protective immunity against challenge with a lethal aerosol dose of WT B. pseudomallei K96243. Naive BALB/c mice were administered 1 ml of immune or naive serum intraperitoneally and challenged 48 h later with 7 LD50 of WT B. pseudomallei strain K96243. BALB/c mice vaccinated with 104 CFU of the B. mallei batA KO mutant and back-challenged with B. pseudomallei K96243 (30 days postvaccination) were used as an efficacy benchmark. Vaccination with the batA KO mutant and challenge with B. pseudomallei K96243 were both performed intratracheally using a Microsprayer device. (A) Kaplan-Meier survival curves. (B) Survival data during the acute and chronic phases of infection. (D, E, and F) Tissues were collected from mice that survived challenge (day 25), homogenized, diluted, and spread on agar plates to determine bacterial loads. The symbols show data for individual animals; the horizontal lines represent the mean total CFU for each group.
DISCUSSION
This study demonstrated that the conventional AT BatA exhibits lipolytic activity and contributes to the virulence of B. mallei in a mouse model of aerosol infection. We also report that BatA is highly conserved among sequenced B. mallei and B. pseudomallei isolates, is produced in vivo, and elicits the production of antibodies during infection. Hence, BatA possesses properties of an excellent target for developing medical countermeasures.
We discovered that the WT B. mallei strain ATCC 23344 does not produce detectable amounts of BatA protein when cultured in vitro. Published proteomic and transcriptome data for the organism support our findings and suggest stringent regulation of batA. The gene is not significantly expressed by B. mallei isolates under routine growth conditions (115) or in culture medium depleted of iron (116). In addition, the BatA protein was not detected in the outer membrane of B. mallei ATCC 23344 grown under conditions that mimic environments encountered by the bacterium (117). Precise regulation of batA does not appear to be a strain- or species-specific phenomenon, as Western blot analysis of lysates from B. mallei ATCC 10399 (China 5), B. pseudomallei K96243, and B. pseudomallei 1026b showed lack of reactivity with anti-BatA monoclonal and polyclonal antibodies even though the genomes of these isolates contain a WT copy of the gene (data not shown). Consistent with this, Ooi et al. (118) established the transcriptional landscape of B. pseudomallei K96243 exposed to 80 physical, chemical, and biological conditions, and batA was expressed only during high osmotic stress (LB broth supplemented with 2 M sorbitol). However, we could not immunodetect BatA in B. mallei ATCC 23344 or B. pseudomallei K96243 cells grown in this particular medium with our panel of antibodies, suggesting protein levels below the limits of detection (data not shown).
Although we cannot demonstrate BatA production by B. mallei cultured in the laboratory, our data indicate that the AT is produced in vivo. Serum samples from mice that survived aerosol infection with strain ATCC 23344 contained BatA-specific antibodies, as did sera from horses experimentally infected with the organism. These results are particularly relevant given that the horse is the natural (and highly susceptible) reservoir host for B. mallei. These findings, together with the reduced virulence of the batA KO mutant (Fig. 2; see Fig. S2 in the supplemental material), reinforce the value of BatA as a target for developing medical countermeasures. The protein is produced during the course of infection and contributes to pathogenesis. Therefore, targeting BatA may interfere with the ability of B. mallei to establish itself and persist in the host. Macrophage killing assays support this hypothesis and show a role for the AT in replication and/or survival within host cells (see Fig. S1A), presumably via degradation of lipids (Fig. 1C). Cleavage of short-chain esters by BatA may generate carbon and energy sources necessary for growth, as reported for P. aeruginosa EstA (119, 120). It is possible that BatA also facilitates phagosomal escape and entry of B. mallei in the cytoplasm of host cells, as previously shown for lipolytic enzymes expressed by the intracellular pathogens Listeria monocytogenes (121–123) and Rickettsia prowazekii (124–126). Ongoing investigation of the molecular mechanisms and factors driving expression of batA and a detailed structure-function analysis of the AT will clarify its role in B. mallei intracellular fitness and virulence.
The conclusion that BatA aids in the ability of B. mallei to thrive intracellularly is supported by in vitro complementation experiments. The construct pBatA, which specifies constitutive production of the AT (Fig. 4C), restores replication of the batA KO strain within macrophages to near WT levels (Fig. 4D). The plasmid, however, did not rescue the reduced virulence of the mutant in vivo and instead further attenuated the organism (Fig. 3). In light of our data showing production of BatA-specific antibodies during infection, it is tempting to speculate that the ostensibly hyperattenuated phenotype of the complemented mutant is in fact the result of increased immune clearance via targeting the constitutively expressed AT. Alternatively, anachronistic overexpression of the batA gene product may attenuate B. mallei. This phenomenon has been reported in the literature as a platform to develop live attenuated vaccines, termed attenuated gene expression (127). The molecular basis of attenuation is not fully understood and varies between model systems. For instance, constitutive production of the Cryptococcus neoformans adhesin Cfl1 appears to reduce virulence through untimely and excessive adhesion to host cells (128). In contrast, overproduction of the Salmonella enterica flagellar apparatus has been shown to disrupt the structural integrity of bacterial membranes, which promotes clearance of the organism by innate immune mechanisms (129). Interestingly, constitutive production of the B. mallei oligomeric AT BpaB was also found to attenuate the virulence of the organism (77). Thus, it is possible that disproportionate amounts of ATs (especially their C-terminal transporter domains forming porin-like structures) may destabilize bacterial membranes and cause attenuation through increased osmotic permeability. The mechanism by which constitutive production of BatA impacts the pathogenicity of B. mallei is currently being investigated.
The attenuated-virulence phenotype of the batA KO mutant provides a compelling platform to gain novel insights into correlates of protective immunity and the role of adaptive immune responses in controlling aerosol infection by B. mallei and B. pseudomallei. Passive-transfer experiments demonstrated that serum antibodies (elicited by vaccination with the batA KO mutant) are sufficient to protect against lethal challenge (Table 3 and Fig. 9; see Fig. S7 in the supplemental material). These antibodies bind to the bacterial surface, increase phagocytosis of the organisms by host immune cells, and promote effective intracellular killing (Fig. 7). This, in turn, delays the seeding and replication of bacteria in target tissues (see Fig. S4) and provides high levels of protection against death during both the acute and chronic stages of disease (Table 3 and Fig. 9; see Fig. S7 in the supplemental material). Our results suggest that a reciprocal endpoint ELISA titer against whole organisms of 24,500 and an IgG2a/IgG1 ratio of 8.5 are good serological correlates of protective immunity against aerosol glanders and melioidosis in mice (Fig. 7A). These findings are consistent with previous reports indicating that high Th1 bias antibody titers afford superior protection (109, 130–133). Our data also underscore a protective role for antibodies of the IgG subclass (Fig. 8; see Fig. S6). Bearing in mind their high titers in immune serum (Fig. 7A) and their demonstrated effector functions in opsonophagocytic killing and complement-mediated cytotoxicity (134), IgG3 and IgG2b antibodies elicited by vaccination with the batA KO mutant are particularly relevant. Robust IgG3 titers induced by vaccination with B. pseudomallei outer membrane vesicles have previously been shown to correlate with passive and active protection against septicemic infection of BALB/c mice with B. pseudomallei strain K96243 and to promote complement-mediated killing of the organism (135). Capsule- and LPS-based vaccines against B. pseudomallei have also been reported to elicit strong IgG3 and IgG2b responses that provide protection in mice (136).
While the constitutively expressed capsule and LPS are lead vaccine targets for B. mallei and B. pseudomallei (110–114), the results of our passive-transfer experiments with adsorbed immune serum demonstrate the protective value of antigens selectively expressed in vivo during vaccination with the batA KO mutant. The data show that a single dose of immune serum depleted of antibodies binding to whole B. mallei bacteria cultured in vitro provides significant protection against lethal challenge during acute and chronic infection (Fig. 8). The reduced survival rates in mice receiving adsorbed serum (56% acute; 31% chronic) compared to animals given whole immune serum (94% acute; 56% chronic) are likely due to the removal of antibodies against capsule and LPS (see Fig. S5 in the supplemental material). Importantly, these experiments also revealed that passive transfer of adsorbed serum promotes superior bacterial clearance from the lungs and liver, as well as development of sterile immunity in these tissues (Fig. 8). Hence, antigens selectively expressed in vivo and recognized by antibodies in the adsorbed serum represent key vaccine candidates for preventing chronic colonization of target tissues and are worth identifying, as the current pool of Burkholderia antigens used in vaccine generation is limited and there is a need to expand the library of high-value immunoprotective targets (57–64, 137). The pathogenesis of B. mallei and B. pseudomallei is complex, as it involves extracellular and intracellular replication of the organisms, as well as dissemination and seeding of deep tissues. With this in mind, a multivalent platform is the most relevant path to develop a vaccine, and our data demonstrate that both the BatA protein and in vivo-expressed antigens recognized by antibodies in adsorbed serum are promising candidates for inclusion alongside established targets, such as capsule and LPS.
Previous studies have investigated the role of antibodies in protection against glanders and melioidosis. For example, prime-boost subcutaneous vaccination with the live attenuated B. pseudomallei purM strain Bp82 was shown to provide high levels of protection against intranasal challenge with lethal doses of WT B. pseudomallei 1026b in BALB/c and C57BL/6 mice (133). This protection was associated with reduced bacterial burden in the lungs, spleen, and liver at 72 h postchallenge, which is strikingly similar to what we observed in our experiments (see Fig. S3 and S4 in the supplemental material). Passive transfer of immune serum (elicited by vaccination with Bp82) to BALB/c mice resulted in ∼40% survival rates during the acute and chronic stages of infection, and vaccination of mice lacking B cells with the attenuated strain did not protect against subsequent lethal intranasal challenge with WT organisms. Passive transfer of immune serum elicited by vaccination with B. pseudomallei 1026b outer membrane vesicles was shown to provide 80% survival against intraperitoneal challenge of BALB/c mice with a lethal dose of B. pseudomallei K96243 (135), and monoclonal antibodies targeting LPS passively protected BALB/c mice against aerosol infection with 20 LD50 of B. mallei ATCC 23344 (up to 100% survival) by reducing bacterial numbers below the lethal threshold (138). Hyperimmune sera from horses vaccinated with mallein extract have also been successfully used to treat human patients with glanders (139–141). Our findings complement prior studies and expand upon them by demonstrating that antibodies elicited by vaccination with the batA KO mutant are not only sufficient to protect against the homologous WT B. mallei strain ATCC 23344, but also provide excellent cross-protection (and significant levels of sterile immunity) against multiple strains of B. pseudomallei in BALB/c, as well as C57BL/6, mice (Table 3 and Fig. 9; see Fig. S7 in the supplemental material). Taken together, these data support the feasibility of developing a single vaccine to protect against both organisms.
Given their ability to thrive intracellularly, it is widely accepted that an ideal vaccine for B. mallei and B. pseudomallei should also generate robust cellular immune responses in order to eliminate infected host cells and reduce the risk of developing chronic disease (57–64, 137). Our results underscore this paradigm, as vaccination with the batA KO mutant consistently provided higher levels of sterile immunity than passive transfer of antibodies alone in mice that survived lethal aerosol challenge (Fig. 9B; see Fig. S7B in the supplemental material), emphasizing the importance of cellular immunity. Back-challenge studies also produced two additional key findings. We found that while vaccination with the batA KO strain provided excellent protection against death during the acute and chronic stages of infection, BALB/c mice challenged with WT B. mallei were all colonized at the study endpoints (Table 2). Conversely, up to 62% of vaccinated BALB/c mice that survived challenge with WT B. pseudomallei strains 1026b and K96243 cleared the infection (Table 2). These data indicate that sterile immunity against the organisms can be achieved under the appropriate conditions and that vaccination with the batA KO mutant provides a powerful platform to investigate the mechanisms determining this outcome. Future work comparing and contrasting the kinetics, quality, levels, and functionality of immune responses in mice vaccinated with the batA KO mutant during infection with WT B. mallei (low levels of sterile immunity) and B. pseudomallei (high levels of sterile immunity) will identify key components of the immune system associated with complete clearance of the organisms.
MATERIALS AND METHODS
Bioinformatic analyses.
Nucleotide sequence data were analyzed with Sequencher 5 (Gene Codes Corporation) and Vector NTI (ThermoFisher Scientific). Bioinformatic analyses were performed using online tools at the ExPASy Bioinformatics Resource Portal (http://www.expasy.org). Signal sequence cleavage sites were detected using SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP). Helical regions and β-strands were determined using PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred). Conserved domains were identified with the NCBI CDD (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). Comparative sequence analyses and searches to identify batA orthologs in the genomes of B. pseudomallei and B. mallei isolates were performed with NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Strains, plasmids, tissue culture cell lines, and growth conditions.
The strains and plasmids used in the study are listed in Table 1. B. mallei was routinely grown at 37°C using brucella medium (BD) supplemented with glycerol at a final concentration of 5% (vol/vol). The following antibiotics were added to the medium for selection of recombinant strains: 7.5 μg/ml zeocin, 5 μg/ml kanamycin, and/or 7.5 μg/ml polymyxin B. For mouse infection experiments, B. mallei bacteria were cultured on agar plates for 40 h and suspended in phosphate-buffered saline (PBS) to a concentration of 1 × 109 CFU per ml. The suspensions were then serially diluted and used to inoculate mice; 100-μl portions were also spread onto agar plates to determine the number of CFU in the inoculum. B. pseudomallei was cultured on tryptic soy agar for 20 h at 37°C. Plate-grown bacteria were treated as described above to prepare the inoculum for challenge experiments. B. thailandensis was cultured at 37°C using Luria-Bertani (LB) medium supplemented with 50 μg/ml kanamycin when indicated. E. coli was propagated in low-salt Luria-Bertani (LSLB) medium at 37°C. For selection purposes, antibiotics were added to the LSLB medium at the following concentrations: 100 μg/ml ampicillin, 15 μg/ml chloramphenicol, 50 μg/ml kanamycin, and/or 50 μg/ml zeocin. Murine macrophages (J774A.1; ATCC TIB-67) were cultured as described by Balder et al. (92).
Recombinant-DNA methods.
Standard molecular biology techniques were performed as outlined by Sambrook and Russell (142). The Easy-DNA genomic DNA (gDNA) purification kit (ThermoFisher Scientific) was used to purify genomic DNA. Plasmid DNA was isolated using the QIAprep Spin Miniprep kit (Qiagen). PCR was performed with Platinum Pfx DNA polymerase (ThermoFisher Scientific) and a MasterAmp Extra-Long PCR kit (Epicenter/Illumina) following the manufacturers' recommendations. Insertion of DNA fragments into plasmids was performed with restriction endonucleases and T4 DNA ligase from New England BioLabs Inc. and using TransforMax EPI300 electrocompetent E. coli cells (Epicenter/Illumina).
A PCR product of 2.3 kb containing the batA gene was obtained from the genome of B. pseudomallei 1026b with primers P1 (5′-CAA CCG TGA TTC CCG ACC CG-3′) and P2 (5′-GTC CTG TCG GCC GCG AAT CT-3′). The DNA fragment was inserted into the vector pCC1 using the CopyControl PCR cloning kit (Epicenter/Illumina), generating plasmid pCCbatA. The construct was digested with the endonuclease AatII to delete a 1.3-kb DNA fragment internal to the batA ORF, treated with the End-It DNA end repair kit (Epicenter/Illumina), and ligated with a blunt-ended 0.4-kb zeocin resistance marker from plasmid pEM7/ZEO, producing plasmid pCCbatA.zeo. The latter was digested with BamHI, and a 1.4-kb DNA fragment corresponding to the batA ORF disrupted with the zeocin resistance cassette was gel purified with the High Pure PCR product purification kit (Roche Life Science), end repaired, and inserted into the EcoRV site of the gene replacement vector pKAS46, yielding construct pKASbatA.zeo.
A 3-kb PCR product containing the batA gene was amplified from the genome of B. pseudomallei K96243 with primers P3 (5′-CGG AAT TCA ACG GTT CGC CGC GCA TTT-3′ [the EcoRI site is underlined]) and P4 (5′-GGC ATG TCG CTC GTG TAC TA-3′). The DNA fragment was purified, digested with EcoRI, and ligated into the EcoRI and ScaI sites of the broad-host-range vector pBHR1, yielding plasmid pBatA. An amplicon encoding amino acids 30 to 307 of the batA ORF product was generated from genomic DNA isolated from B. mallei ATCC 23344 using oligonucleotides P5 (5′-CCC AAG CTT GGC GAC AGC CTG ACC GAC AAT-3′ [the HindIII site is underlined]) and P6 (5′-GGT TAA TTA AAG CCC AAG CAC CGG CGC GGT CGA-3′ [the PacI site is underlined]). The PCR product was purified, digested with HindIII and PacI, and ligated into the corresponding sites of the vector pETcoco-1. This approach created plasmid pHisBatA, which carries the batA gene fragment fused to an N-terminal His tag. A PCR product specifying the same portion of the batA ORF was amplified with primers P7 (5′-CGG GAT CCG GCG ACA GCC TGA CCG ACA AT-3′ [the BamHI site is underlined]) and P8 (5′-CCG CTC GAG TCC CAA GCA CCG GCG CGG TCG A-3′ [the XhoI site is underlined]) and inserted into the vector pGEX4T-2. The resulting plasmid, pGSTBatA, encodes amino acids 30 to 307 of BatA joined to a glutathione S-transferase (GST) tag at its N terminus.
A 1-kb amplicon corresponding to the genomic sequence located upstream of the B. mallei ATCC 23344 ilvB gene (locus tag BMA1848) was amplified with primers P9 (5′-CCC GCT AGC CAT CTT TGA CCT TTC GAA-3′ [the NheI site is underlined; the translational start codon of the ilvB ORF is in boldface and italicized]) and P10 (5′-TTG ACG CTG CCT TCG GAG CA-3′). The PCR product was inserted in the vector pCC1 using the CopyControl PCR cloning kit, yielding plasmid pCCilvUP. Similarly, a 1-kb amplicon that encompassed the genomic sequence downstream of ilvB was generated with oligonucleotides P11 (5′-CCC GCT AGC CTG TAA CGG CGC GAT GC-3′ [the NheI site is underlined; the translational stop codon of the ilvB ORF is in boldface and italicized]) and P12 (5′-AGC ATC ATC ACG ACG TCC GC-3′) and inserted into pCC1, producing plasmid pCCilvDOWN. The plasmid pCCilvUP was subsequently digested with SphI and NheI. The 1-kb insert, corresponding to the DNA sequence upstream of the ilvB gene, was gel purified and inserted into the SphI and NheI sites of the pCCilvDOWN plasmid. These experiments produced the plasmid pCCilvΔ, which specifies the upstream and downstream genomic sequences of the ilvB gene joined by a unique NheI site. The plasmid pCCilvΔ was digested with NheI, end repaired, and ligated with a blunt-ended 1.3-kb kanamycin resistance marker from plasmid pUC4K, yielding plasmid pCCilv.kan. To facilitate the construction of a B. mallei isogenic ilvB mutant strain containing the kanamycin resistance marker in its genome, we modified the vector pKAS46. The plasmid was digested with EcoRV and MfeI to remove its kanamycin resistance gene, purified from agarose gel slices, end repaired, and ligated with a blunt-ended 0.4-kb zeocin resistance marker. These experiments produced plasmid pKAS46-ZEO, which was linearized with EcoRI, end repaired, and ligated with a blunt-ended 3.3-kb insert from plasmid pCCilv.kan, generating the gene replacement plasmid pKASilv.kan.
Plasmids were introduced into B. thailandensis and E. coli by electroporation using a BTX Transporator Plus apparatus. The plasmids pKASbatA.zeo, pKASilv.kan, pBHR1ΔDra, and pBatA were transferred from E. coli S17 to B. mallei by conjugation as previously outlined (77, 92, 93, 143). All the plasmids were sequenced to ensure that PCR did not introduce mutations that would result in amino acid substitutions in the cloned gene products.
Construction of B. mallei ATCC 23344 batA and ilv isogenic mutant strains.
Upon conjugative transfer of plasmid pKASbatA.zeo, B. mallei colonies were selected for resistance to polymyxin B (which inhibits the growth of E. coli S17, used for conjugation), resistance to zeocin (to identify colonies containing the mutated copy of batA in their genomes), and sensitivity to kanamycin (to identify colonies that did not contain the vector pKAS46 integrated into their genomes). Individual colonies were analyzed by PCR with oligonucleotides P13 (5′-AAG AGG GCA TCA ACA TCC AG-3′) and P14 (5′-GAC GCC CGT CAT CTA TCT GT-3′), which produced an amplicon of 5 kb in the batA KO mutant strain and a larger DNA fragment of 6 kb in WT B. mallei ATCC 23344 (data not shown). This 1-kb difference in size is consistent with deletion of the above-mentioned 1.3-kb AatII fragment internal to the batA ORF and insertion of the 0.4-kb zeocin resistance marker in its place. Allelic exchange was confirmed by sequencing the PCR product and by Southern blot analysis (data not shown).
After conjugation to introduce plasmids pBHR1ΔDra and pBatA into the B. mallei batA KO mutant strain, polymyxin B-resistant colonies were screened for resistance to zeocin (to identify colonies that maintained the inactivated copy of the batA gene in their genomes) and resistance to kanamycin (to identify colonies that harbored episomal copies of pBHR1ΔDra and pBatA). Plasmid DNA was isolated from selected strains and analyzed with restriction endonucleases to verify constructs.
After conjugative transfer of plasmid pKASilv.kan, B. mallei colonies were selected for resistance to polymyxin B, resistance to kanamycin (to identify colonies containing the mutated copy of the ilvB gene in their genomes), and sensitivity to zeocin (to identify colonies that did not contain the vector pKAS46-ZEO integrated into their genomes). Individual colonies were analyzed by Southern blotting to verify allelic exchange, yielding the isogenic ilv KO mutant strain (data not shown).
Animal experiments.
Specific-pathogen-free (SPF) female BALB/c and C57BL/6 mice were purchased from Envigo and allowed to acclimate for at least 1 week prior to use. After administration of anesthetic, the mice were inoculated intratracheally using a Microsprayer nebulizing device (PennCentury) as previously reported (77, 93, 144). The infected animals were monitored daily, food and water were provided ad libitum, and humane endpoints were strictly observed. Mice exhibiting signs of intermediate to severe discomfort were euthanized in compliance with the AVMA Guidelines for the Euthanasia of Animals. Survival data were analyzed with Prism 6 (GraphPad Software, Inc.) using the Kaplan-Meier method. LD50s were calculated according to the method of Reed and Muench (145). Upon euthanasia, tissues were harvested using standard necropsy methods, homogenized with disposable grinders, serially diluted, and plated onto agar medium to determine the number of viable organisms. Inoculation with bacteria and euthanasia procedures were performed under anesthesia.
For passive-transfer experiments, antibody preparations were administered intraperitoneally (≤1 ml) 48 h prior to infection with WT organisms. For back-challenge experiments, mice were first inoculated intratracheally with 104 CFU of the batA KO mutant or 105 CFU of the ilv KO live attenuated strain using the Microsprayer device. Thirty to 45 days postinoculation, the animals were infected with WT bacteria (using a Microsprayer). For BALB/c mice challenged with WT B. mallei ATCC 23344 and B. pseudomallei 1026b, inoculating doses of approximately 8,000 (10 LD50) and 25,000 (5 LD50) CFU were used, respectively. These values were previously established while developing the Microsprayer aerosol delivery platform (144). For BALB/c mice challenged with WT B. pseudomallei K96243, an inoculating dose of ∼300 CFU was used. This inoculum was determined to be the equivalent of 5 LD50 in the model (data not shown). For C57BL/6 mice challenged with WT B. mallei ATCC 23344 and B. pseudomallei 1026b, inoculating doses of approximately 9,000 (10 LD50) and 16,000 (5 LD50) CFU were used, respectively. These values were determined using the method of Reed and Muench (unpublished data).
Antigen preparation and analysis.
Protein lysates were prepared from B. thailandensis and B. mallei strains using the ReadyPrep protein extraction kit (Bio-Rad). E. coli TUNER carrying the plasmids pHisBatA and pGSTBatA was used to produce recombinant forms of the BatA protein joined to His and GST tags, respectively. Both proteins were extracted from inclusion bodies and purified under denaturing conditions as previously described (98, 146). Purified Burkholderia oligosaccharide chains of LPS (OPS) and CPS were kindly provided by Donald E. Woods (University of Calgary).
For Western blot analyses, equal amounts of protein were resolved by SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes (EMD Millipore), and probed with antibodies as outlined previously (77). Antibody reactivity with protein bands was visualized by chemiluminescence using the substrate Luminata Crescendo Western HRP (EMD Millipore) and a Foto/Analyst Luminary/FX imaging system (Fotodyne Inc.). For ELISA, duplicate wells of Immulon 2HB plates (ThermoFisher Scientific) were coated with purified antigens (OPS, CPS, and recombinant BatA protein) or paraformaldehyde-fixed bacteria. Antibody reactivity to these preparations was determined according to the method of Zimmerman et al. (77). The binding of antibodies to the surfaces of bacteria was measured by flow cytometry, as previously outlined (77).
Antibodies.
Polyclonal antibodies against BatA were obtained by immunizing BALB/c mice with purified His-tagged BatA mixed with Freund's adjuvant, as described previously (147). The antibodies were shown to specifically react with BatA by ELISA and Western blotting using purified GST-tagged BatA protein (data not shown). BatA-MAb 1 was generated by fusing splenocytes (from a mouse immunized with His-tagged BatA) with Sp2/mIL6 cells (ATCC CRL 2016) as previously reported (77). Hybridomas secreting antibodies specific for BatA were identified by ELISA using GST-tagged BatA protein.
Immune serum was produced by first inoculating BALB/c mice with 104 CFU of the batA KO strain using a Microsprayer. Thirty to 45 days postinoculation, the animals were exsanguinated under anesthesia. After clotting, sera were collected, pooled, and filter sterilized with 0.22-μm Millex-GV filter units (EMD Millipore). Naive sera, obtained from SPF BALB/c mice, were purchased from Charles River Laboratories. Serum samples (immune and naive) were adsorbed with plate-grown B. mallei ATCC 23344 bacteria to remove antibodies binding to antigens constitutively expressed by the organism under routine laboratory growth conditions. Briefly, WT B. mallei cells were cultured on agar plates for 40 h and suspended to a concentration of 1 × 109 CFU/ml in 45 ml of PBS. The bacteria were pelleted, suspended in serum (≤10 ml), and incubated at room temperature with mixing for 30 min. Following this, the bacteria were pelleted, and the serum was collected and readsorbed with fresh bacterial suspensions twice more. After the third adsorption, the serum was collected, filter sterilized, and stored at −80°C. Of note, the final volumes of adsorbed serum preparations were the same as before adsorption. The same volumes of whole and adsorbed serum preparations were passively transferred to mice. Serum samples (immune and naive) were also treated with Pierce Protein A/G Plus Agarose beads (ThermoFisher Scientific) to purify IgG antibodies. Antibodies bound to the agarose beads were eluted, and the IgG-containing fractions were adjusted to physiologic pH, dialyzed against PBS, filter sterilized, and stored at −80°C. Immunoglobulin concentrations were determined by measuring absorbance at a wavelength of 280 nm.
The serum samples from mice that survived aerosol infection with B. mallei ATCC 23344 are described elsewhere (144). The serum samples from horses experimentally infected with B. mallei ATCC 23344 were obtained in the context of a separate study (unpublished data) (NIH-NIAID award U01AI077764).
Lipolytic enzyme assays.
Lipolytic enzyme activity was quantified using pNPs as outlined previously (76, 94, 98). The bacterial suspensions used in these experiments were standardized to an optical density at a wavelength of 600 nm (OD600) of 0.1. After mixing bacteria and p-nitrophenyl esters, the absorbance of each sample at a wavelength of 410 nm was measured after a 30-min incubation at room temperature.
Macrophage assays.
Experiments with macrophages were carried out as previously described by our group with a few modifications (77, 92, 93). B. mallei cells were grown on agar plates for 40 h and suspended to a concentration of 109 CFU/ml in PBS. Portions of the suspension were mixed with antibody preparations and incubated at 37°C with mixing for 30 min. These mixtures were then used to inoculate wells of duplicate 24-well tissue culture plates seeded with J774 macrophages. After incubation at 37°C for 1 h, the medium covering the monolayers was removed and replaced with fresh medium containing 50 μg/ml streptomycin, and the tissue culture plates were incubated at 37°C for 2 h to kill extracellular bacteria. Following this, the wells of one plate were washed with PBS and treated with a solution containing saponin to lyse macrophages, and serial dilutions of the well contents were spread on agar medium to calculate the number of bacteria phagocytosed by J774 cells. The wells of the second plate were washed, fresh medium without antibiotics was added, and the infected cells were incubated for 7 h at 37°C. After this incubation, the wells were treated as described above to determine the number of intracellular bacteria.
Compliance and animal research ethics statements.
The University of Georgia's Institutional Biosafety Committee approved the experiments in this study. All experiments with live B. mallei and B. pseudomallei were performed inside a class II biosafety cabinet in a biosafety level 3 (BSL3) laboratory in compliance with the rules and regulations of the U.S. Federal Select Agent Program. Infected animals were housed in an Innorack IVC dual-HEPA-filtered ventilated system (Innovive) located in an animal BSL3 (ABSL3) laboratory.
The University of Georgia's Institutional Animal Care and Use Committee approved the animal experiments in this study. All animal experiments were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Every effort was made to minimize animal suffering.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by DTRA contract HDTRA1-12-C-0081 to R.J.H. and E.R.L.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00102-17.
REFERENCES
- 1.Limmathurotsakul D, Golding N, Dance DAB, Messina JP, Pigott DM, Moyes CL, Rolim DB, Bertherat E, Day NPJ, Peacock SJ, Hay SI. 2016. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 1:15008. doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 2.Vietri NJ, Deshazer D. 2007. Melioidosis, p 147–166. In Dembek ZF. (ed), Medical aspects of biological warfare. Textbooks of biological warfare series. Borden Institute, Walter Reed Army Medical Center, Falls Church, VA. [Google Scholar]
- 3.Cheng AC, Currie BJ. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol 4:272–282. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 5.Stone JK, DeShazer D, Brett PJ, Burtnick MN. 2014. Melioidosis: molecular aspects of pathogenesis. Expert Rev Anti Infect Ther 12:1487–1499. doi: 10.1586/14787210.2014.970634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiersinga WJ, Currie BJ, Peacock SJ. 2012. Melioidosis. N Engl J Med 367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 7.Galyov EE, Brett PJ, Deshazer D. 2010. Molecular Insights into Burkholderia pseudomallei and Burkholderia mallei pathogenesis. Annu Rev Microbiol 64:495–517. doi: 10.1146/annurev.micro.112408.134030. [DOI] [PubMed] [Google Scholar]
- 8.Dance DA. 2015. Editorial commentary: melioidosis in Puerto Rico: the iceberg slowly emerges. Clin Infect Dis 60:251–253. doi: 10.1093/cid/ciu768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopalakrishnan R, Sureshkumar D, Thirunarayan MA, Ramasubramanian V. 2013. Melioidosis: an emerging infection in India. J Assoc Physicians India 61:612–614. [PubMed] [Google Scholar]
- 10.Carr-Gregory B, Waag DM. 2007. Glanders, p 121–146. In Dembek ZF. (ed), Medical aspects of biological warfare. Textbooks of biological warfare series. Borden Institute, Walter Reed Army Medical Center, Falls Church, VA. [Google Scholar]
- 11.Waag DM, Deshazer D. 2004. Glanders: new insights into an old disease. In Lindler LE, Korch GW, Lebeda FJ (ed), Biological weapons defense: infectious diseases and counterbioterrorism. Humana Press Inc., Totowa, NJ. [Google Scholar]
- 12.Khan I, Wieler LH, Melzer F, Elschner MC, Muhammad G, Ali S, Sprague LD, Neubauer H, Saqib M. 2013. Glanders in animals: a review on epidemiology, clinical presentation, diagnosis and countermeasures. Transbound Emerg Dis 60:204–221. doi: 10.1111/j.1865-1682.2012.01342.x. [DOI] [PubMed] [Google Scholar]
- 13.Malik P, Khurana SK, Dwivedi SK. 2010. Re-emergence of glanders in India: report of Maharashtra State. Indian J Microbiol 50:345–348. doi: 10.1007/s12088-010-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khaki P, Mosavari N, Khajeh NS, Emam M, Ahouran M, Hashemi S, Taheri MM, Jahanpeyma D, Nikkhah S. 2012. Glanders outbreak at Tehran Zoo, Iran. Iran J Microbiol 4:3–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Dvorak GD, Spickler AR. 2008. Glanders. J Am Vet Med Assoc 233:570–577. doi: 10.2460/javma.233.4.570. [DOI] [PubMed] [Google Scholar]
- 16.Mota RA, da Fonseca Oliveira AA, da Silva AM, Junior JW, da Silva LB, de Farias Brito M, Rabelo SS. 2010. Glanders in donkeys (Equus asinus) in the state of Pernambuco, Brazil: a case report. Braz J Microbiol 41:146–149. doi: 10.1590/S1517-83822010000100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik P, Singha H, Goyal SK, Khurana SK, Tripathi BN, Dutt A, Singh D, Sharma N, Jain S. 2015. Incidence of Burkholderia mallei infection among indigenous equines in India. Vet Rec Open 2:e000129. doi: 10.1136/vetreco-2015-000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Zandt KE, Greer MT, Gelhaus HC. 2013. Glanders: an overview of infection in humans. Orphanet J Rare Dis 8:131. doi: 10.1186/1750-1172-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arun S, Neubauer H, Gurel A, Ayyildiz G, Kuscu B, Yesildere T, Meyer H, Hermanns W. 1999. Equine glanders in Turkey. Vet Rec 144:255–258. doi: 10.1136/vr.144.10.255. [DOI] [PubMed] [Google Scholar]
- 20.Neubauer H, Meyer H, Finke EJ. 1997. Human glanders. Rev Int Services Sante Forces Armees 70:258–265. [Google Scholar]
- 21.Howe C. 1950. Glanders, p 185–202. In Christian HA. (ed), The Oxford Medicine. Oxford University Press, New York, NY. [Google Scholar]
- 22.Howe C, Miller WR. 1947. Human glanders: report of six cases. Ann Intern Med 26:93–115. doi: 10.7326/0003-4819-26-1-93. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan A, Kraus CN, DeShazer D, Becker PM, Dick JD, Spacek L, Bartlett JG, Byrne WR, Thomas DL. 2001. Glanders in a military research microbiologist. N Engl J Med 345:256–258. doi: 10.1056/NEJM200107263450404. [DOI] [PubMed] [Google Scholar]
- 24.Losada L, Ronning CM, DeShazer D, Woods D, Fedorova N, Kim HS, Shabalina SA, Pearson TR, Brinkac L, Tan P, Nandi T, Crabtree J, Badger J, Beckstrom-Sternberg S, Saqib M, Schutzer SE, Keim P, Nierman WC. 2010. Continuing evolution of Burkholderia mallei through genome reduction and large-scale rearrangements. Genome Biol Evol 2:102–116. doi: 10.1093/gbe/evq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song H, Hwang J, Yi H, Ulrich RL, Yu Y, Nierman WC, Kim HS. 2010. The early stage of bacterial genome-reductive evolution in the host. PLoS Pathog 6:e1000922. doi: 10.1371/journal.ppat.1000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holden MT, Titball RW, Peacock SJ, Cerdeno-Tarraga AM, Atkins T, Crossman LC, Pitt T, Churcher C, Mungall K, Bentley SD, Sebaihia M, Thomson NR, Bason N, Beacham IR, Brooks K, Brown KA, Brown NF, Challis GL, Cherevach I, Chillingworth T, Cronin A, Crossett B, Davis P, DeShazer D, Feltwell T, Fraser A, Hance Z, Hauser H, Holroyd S, Jagels K, Keith KE, Maddison M, Moule S, Price C, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Simmonds M, Songsivilai S, Stevens K, Tumapa S, Vesaratchavest M, Whitehead S, Yeats C, Barrell BG, Oyston PC, Parkhill J. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci U S A 101:14240–14245. doi: 10.1073/pnas.0403302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nierman WC, DeShazer D, Kim HS, Tettelin H, Nelson KE, Feldblyum T, Ulrich RL, Ronning CM, Brinkac LM, Daugherty SC, Davidsen TD, Deboy RT, Dimitrov G, Dodson RJ, Durkin AS, Gwinn ML, Haft DH, Khouri H, Kolonay JF, Madupu R, Mohammoud Y, Nelson WC, Radune D, Romero CM, Sarria S, Selengut J, Shamblin C, Sullivan SA, White O, Yu Y, Zafar N, Zhou L, Fraser CM. 2004. Structural flexibility in the Burkholderia mallei genome. Proc Natl Acad Sci U S A 101:14246–14251. doi: 10.1073/pnas.0403306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schell MA, Lipscomb L, DeShazer D. 2008. Comparative genomics and an insect model rapidly identify novel virulence genes of Burkholderia mallei. J Bacteriol 190:2306–2313. doi: 10.1128/JB.01735-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeShazer D, Brett PJ, Woods DE. 1998. The type II O-antigenic polysaccharide moiety of Burkholderia pseudomallei lipopolysaccharide is required for serum resistance and virulence. Mol Microbiol 30:1081–1100. doi: 10.1046/j.1365-2958.1998.01139.x. [DOI] [PubMed] [Google Scholar]
- 30.Burtnick MN, Brett PJ, Woods DE. 2002. Molecular and physical characterization of Burkholderia mallei O antigens. J Bacteriol 184:849–852. doi: 10.1128/JB.184.3.849-852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schell MA, Ulrich RL, Ribot WJ, Brueggemann EE, Hines HB, Chen D, Lipscomb L, Kim HS, Mrazek J, Nierman WC, Deshazer D. 2007. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol Microbiol 64:1466–1485. doi: 10.1111/j.1365-2958.2007.05734.x. [DOI] [PubMed] [Google Scholar]
- 32.Burtnick MN, Brett PJ, Harding SV, Ngugi SA, Ribot WJ, Chantratita N, Scorpio A, Milne TS, Dean RE, Fritz DL, Peacock SJ, Prior JL, Atkins TP, Deshazer D. 2011. The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect Immun 79:1512–1525. doi: 10.1128/IAI.01218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulrich RL, DeShazer D. 2004. Type III secretion: a virulence factor delivery system essential for the pathogenicity of Burkholderia mallei. Infect Immun 72:1150–1154. doi: 10.1128/IAI.72.2.1150-1154.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez MG, Pfeffer TL, Warawa JM. 2015. Type 3 secretion system cluster 3 is a critical virulence determinant for lung-specific melioidosis. PLoS Negl Trop Dis 9:e3441. doi: 10.1371/journal.pntd.0003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutierrez MG, Yoder-Himes DR, Warawa JM. 2015. Comprehensive identification of virulence factors required for respiratory melioidosis using Tn-seq mutagenesis. Front Cell Infect Microbiol 5:78. doi: 10.3389/fcimb.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens MP, Haque A, Atkins T, Hill J, Wood MW, Easton A, Nelson M, Underwood-Fowler C, Titball RW, Bancroft GJ, Galyov EE. 2004. Attenuated virulence and protective efficacy of a Burkholderia pseudomallei bsa type III secretion mutant in murine models of melioidosis. Microbiology 150:2669–2676. doi: 10.1099/mic.0.27146-0. [DOI] [PubMed] [Google Scholar]
- 37.Warawa J, Woods DE. 2005. Type III secretion system cluster 3 is required for maximal virulence of Burkholderia pseudomallei in a hamster infection model. FEMS Microbiol Lett 242:101–108. doi: 10.1016/j.femsle.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 38.DeShazer D, Waag DM, Fritz DL, Woods DE. 2001. Identification of a Burkholderia mallei polysaccharide gene cluster by subtractive hybridization and demonstration that the encoded capsule is an essential virulence determinant. Microb Pathog 30:253–269. doi: 10.1006/mpat.2000.0430. [DOI] [PubMed] [Google Scholar]
- 39.Warawa JM, Long D, Rosenke R, Gardner D, Gherardini FC. 2009. Role for the Burkholderia pseudomallei capsular polysaccharide encoded by the wcb operon in acute disseminated melioidosis. Infect Immun 77:5252–5261. doi: 10.1128/IAI.00824-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atkins T, Prior R, Mack K, Russell P, Nelson M, Prior J, Ellis J, Oyston PC, Dougan G, Titball RW. 2002. Characterisation of an acapsular mutant of Burkholderia pseudomallei identified by signature tagged mutagenesis. J Med Microbiol 51:539–547. doi: 10.1099/0022-1317-51-7-539. [DOI] [PubMed] [Google Scholar]
- 41.Reckseidler SL, DeShazer D, Sokol PA, Woods DE. 2001. Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect Immun 69:34–44. doi: 10.1128/IAI.69.1.34-44.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willcocks SJ, Denman CC, Atkins HS, Wren BW. 2016. Intracellular replication of the well-armed pathogen Burkholderia pseudomallei. Curr Opin Microbiol 29:94–103. doi: 10.1016/j.mib.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 43.David J, Bell RE, Clark GC. 2015. Mechanisms of disease: host-pathogen interactions between Burkholderia species and lung epithelial cells. Front Cell Infect Microbiol 5:80. doi: 10.3389/fcimb.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipsitz R, Garges S, Aurigemma R, Baccam P, Blaney DD, Cheng AC, Currie BJ, Dance DA, Gee JE, Larsen J, Limmathurotsakul D, Morrow MG, Norton R, O'Mara E, Peacock S, Pesik N, Rogers LP, Schweizer HP, Steinmetz I, Tan G, Tan P, Wiersinga WJ, Wuthiekanun V, Smith TL. 2012. Workshop on Treatment of and Postexposure Prophylaxis for Burkholderia pseudomallei and B. mallei infection, 2010. Emerg Infect Dis 18:e2. doi: 10.3201/eid1812.120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dance D. 2014. Treatment and prophylaxis of melioidosis. Int J Antimicrob Agents 43:310–318. doi: 10.1016/j.ijantimicag.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Podnecky NL, Rhodes KA, Schweizer HP. 2015. Efflux pump-mediated drug resistance in Burkholderia. Front Microbiol 6:305. doi: 10.3389/fmicb.2015.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schweizer HP. 2012. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol 7:1389–1399. doi: 10.2217/fmb.12.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thibault FM, Hernandez E, Vidal DR, Girardet M, Cavallo JD. 2004. Antibiotic susceptibility of 65 isolates of Burkholderia pseudomallei and Burkholderia mallei to 35 antimicrobial agents. J Antimicrob Chemother 54:1134–1138. doi: 10.1093/jac/dkh471. [DOI] [PubMed] [Google Scholar]
- 49.Viktorov DV, Zakharova IB, Podshivalova MV, Kalinkina EV, Merinova OA, Ageeva NP, Antonov VA, Merinova LK, Alekseev VV. 2008. High-level resistance to fluoroquinolones and cephalosporins in Burkholderia pseudomallei and closely related species. Trans R Soc Trop Med Hyg 102(Suppl 1):S103–S110. doi: 10.1016/S0035-9203(08)70025-7. [DOI] [PubMed] [Google Scholar]
- 50.Kenny DJ, Russell P, Rogers D, Eley SM, Titball RW. 1999. In vitro susceptibilities of Burkholderia mallei in comparison to those of other pathogenic Burkholderia spp. Antimicrob Agents Chemother 43:2773–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alibek K, Handelman S. 1999. Biohazard: the chilling true story of the largest covert biological weapons program in the world. Random House, New York, NY. [Google Scholar]
- 52.Lehavi O, Aizenstien O, Katz LH, Hourvitz A. 2002. Glanders: a potential disease for biological warfare in humans and animals. Harefuah 141(Spec no. 88–91):119 (In Hebrew.) [PubMed] [Google Scholar]
- 53.Wheelis M. 1999. Biological sabotage in World War I, p 35–72. In Geissler E, Moon JEC (ed), Biological and toxin weapons: research, development, and use from the Middle Ages to 1945. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 54.Wheelis M. 1998. First shots fired in biological warfare. Nature 395:213. [DOI] [PubMed] [Google Scholar]
- 55.Smart JK. 1997. History of chemical and biological warfare: an American perspective, p 16–64. In R Zajtchuk and RF Bellarmy (ed), Textbook of military medicine. Borden Institute and Office of the Army Surgeon General, Fort Sam Houston, TX. [Google Scholar]
- 56.Regis E. 1999. The biology of doom. Henry Holt and Company, New York, NY. [Google Scholar]
- 57.Bondi SK, Goldberg JB. 2008. Strategies toward vaccines against Burkholderia mallei and Burkholderia pseudomallei. Expert Rev Vaccines 7:1357–1365. doi: 10.1586/14760584.7.9.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatcher CL, Muruato LA, Torres AG. 2015. Recent advances in Burkholderia mallei and B. pseudomallei. Res Curr Trop Med Rep 2:62–69. doi: 10.1007/s40475-015-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choh LC, Ong GH, Vellasamy KM, Kalaiselvam K, Kang WT, Al-Maleki AR, Mariappan V, Vadivelu J. 2013. Burkholderia vaccines: are we moving forward? Front Cell Infect Microbiol 3:5. doi: 10.3389/fcimb.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarkar-Tyson M, Titball RW. 2010. Progress toward development of vaccines against melioidosis: a review. Clin Ther 32:1437–1445. doi: 10.1016/j.clinthera.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 61.Patel N, Conejero L, De Reynal M, Easton A, Bancroft GJ, Titball RW. 2011. Development of vaccines against Burkholderia pseudomallei. Front Microbiol 2:198. doi: 10.3389/fmicb.2011.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peacock SJ, Limmathurotsakul D, Lubell Y, Koh GC, White LJ, Day NP, Titball RW. 2012. Melioidosis vaccines: a systematic review and appraisal of the potential to exploit biodefense vaccines for public health purposes. PLoS Negl Trop Dis 6:e1488. doi: 10.1371/journal.pntd.0001488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silva EB, Dow SW. 2013. Development of Burkholderia mallei and pseudomallei vaccines. Front Cell Infect Microbiol 3:10. doi: 10.3389/fcimb.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aschenbroich SA, Lafontaine ER, Hogan RJ. 2016. Melioidosis and glanders modulation of the innate immune system: barriers to current and future vaccine approaches. Expert Rev Vaccines 15:1163–1181. doi: 10.1586/14760584.2016.1170598. [DOI] [PubMed] [Google Scholar]
- 65.Cotter SE, Surana NK, St Geme JW III. 2005. Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol 13:199–205. doi: 10.1016/j.tim.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Leyton DL, Rossiter AE, Henderson IR. 2012. From self sufficiency to dependence: mechanisms and factors important for autotransporter biogenesis. Nat Rev Microbiol 10:213–225. doi: 10.1038/nrmicro2733. [DOI] [PubMed] [Google Scholar]
- 67.Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala'Aldeen D. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev 68:692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henderson IR, Nataro JP. 2001. Virulence functions of autotransporter proteins. Infect Immun 69:1231–1243. doi: 10.1128/IAI.69.3.1231-1243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henderson IR, Navarro-Garcia F, Nataro JP. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol 6:370–378. doi: 10.1016/S0966-842X(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 70.Stathopoulos C, Hendrixson DR, Thanassi DG, Hultgren SJ, St Geme JW III, Curtiss R III. 2000. Secretion of virulence determinants by the general secretory pathway in gram-negative pathogens: an evolving story. Microbes Infect 2:1061–1072. doi: 10.1016/S1286-4579(00)01260-0. [DOI] [PubMed] [Google Scholar]
- 71.Linke D, Riess T, Autenrieth IB, Lupas A, Kempf VA. 2006. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol 14:264–270. doi: 10.1016/j.tim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 72.van Ulsen P, Rahman S, Jong WS, Daleke-Schermerhorn MH, Luirink J. 2014. Type V secretion: from biogenesis to biotechnology. Biochim Biophys Acta 1843:1592–1611. doi: 10.1016/j.bbamcr.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 73.Grijpstra J, Arenas J, Rutten L, Tommassen J. 2013. Autotransporter secretion: varying on a theme. Res Microbiol 164:562–582. doi: 10.1016/j.resmic.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 74.Dautin N, Bernstein HD. 2007. Protein secretion in gram-negative bacteria via the autotransporter pathway. Annu Rev Microbiol 61:89–112. doi: 10.1146/annurev.micro.61.080706.093233. [DOI] [PubMed] [Google Scholar]
- 75.Attia AS, Lafontaine ER, Latimer JL, Aebi C, Syrogiannopoulos GA, Hansen EJ. 2005. The UspA2 protein of Moraxella catarrhalis is directly involved in the expression of serum resistance. Infect Immun 73:2400–2410. doi: 10.1128/IAI.73.4.2400-2410.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Timpe JM, Holm MM, Vanlerberg SL, Basrur V, Lafontaine ER. 2003. Identification of a Moraxella catarrhalis outer membrane protein exhibiting both adhesin and lipolytic activities. Infect Immun 71:4341–4350. doi: 10.1128/IAI.71.8.4341-4350.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zimmerman SM, Michel F, Hogan RJ, Lafontaine ER. 2015. The autotransporter BpaB contributes to the virulence of Burkholderia mallei in an aerosol model of infection. PLoS One 10:e0126437. doi: 10.1371/journal.pone.0126437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lafontaine ER, Cope LD, Aebi C, Latimer JL, McCracken GH Jr, Hansen EJ. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J Bacteriol 182:1364–1373. doi: 10.1128/JB.182.5.1364-1373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balder R, Krunkosky TM, Nguyen CQ, Feezel L, Lafontaine ER. 2009. Hag mediates adherence of Moraxella catarrhalis to ciliated human airway cells. Infect Immun 77:4597–4608. doi: 10.1128/IAI.00212-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoiczyk E, Roggenkamp A, Reichenbecher M, Lupas A, Heesemann J. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J 19:5989–5999. doi: 10.1093/emboj/19.22.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alamuri P, Eaton KA, Himpsl SD, Smith SN, Mobley HL. 2009. Vaccination with proteus toxic agglutinin, a hemolysin-independent cytotoxin in vivo, protects against Proteus mirabilis urinary tract infection. Infect Immun 77:632–641. doi: 10.1128/IAI.01050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu DF, Mason KW, Mastri M, Pazirandeh M, Cutter D, Fink DL, St Geme JW III, Zhu D, Green BA. 2004. The C-terminal fragment of the internal 110-kilodalton passenger domain of the Hap protein of nontypeable Haemophilus influenzae is a potential vaccine candidate. Infect Immun 72:6961–6968. doi: 10.1128/IAI.72.12.6961-6968.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cutter D, Mason KW, Howell AP, Fink DL, Green BA, St Geme JW III. 2002. Immunization with Haemophilus influenzae Hap adhesin protects against nasopharyngeal colonization in experimental mice. J Infect Dis 186:1115–1121. doi: 10.1086/344233. [DOI] [PubMed] [Google Scholar]
- 84.Fusco WG, Choudhary NR, Routh PA, Ventevogel MS, Smith VA, Koch GG, Almond GW, Orndorff PE, Sempowski GD, Leduc I. 2014. The Haemophilus ducreyi trimeric autotransporter adhesin DsrA protects against an experimental infection in the swine model of chancroid. Vaccine 32:3752–3758. doi: 10.1016/j.vaccine.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitlock GC, Deeraksa A, Qazi O, Judy BM, Taylor K, Propst KL, Duffy AJ, Johnson K, Kitto GB, Brown KA, Dow SW, Torres AG, Estes DM. 2010. Protective response to subunit vaccination against intranasal Burkholderi mallei and B. pseudomallei challenge. Procedia Vaccinol 2. doi: 10.1016/j.provac.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bentancor LV, Routray A, Bozkurt-Guzel C, Camacho-Peiro A, Pier GB, Maira-Litran T. 2012. Evaluation of the trimeric autotransporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect Immun 80:3381–3388. doi: 10.1128/IAI.06096-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lazar Adler NR, Stevens JM, Stevens MP, Galyov EE. 2011. Autotransporters and their role in the virulence of Burkholderia pseudomallei and Burkholderia mallei. Front Microbiol 2:151. doi: 10.3389/fmicb.2011.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Campos CG, Borst L, Cotter PA. 2013. Characterization of BcaA, a putative classical autotransporter protein in Burkholderia pseudomallei. Infect Immun 81:1121–1128. doi: 10.1128/IAI.01453-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lazar Adler NR, Stevens MP, Dean RE, Saint RJ, Pankhania D, Prior JL, Atkins TP, Kessler B, Nithichanon A, Lertmemongkolchai G, Galyov EE. 2015. Systematic mutagenesis of genes encoding predicted autotransported proteins of Burkholderia pseudomallei identifies factors mediating virulence in mice, net intracellular replication and a novel protein conferring serum resistance. PLoS One 10:e0121271. doi: 10.1371/journal.pone.0121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Campos CG, Byrd MS, Cotter PA. 2013. Functional characterization of Burkholderia pseudomallei trimeric autotransporters. Infect Immun 81:2788–2799. doi: 10.1128/IAI.00526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stevens JM, Ulrich RL, Taylor LA, Wood MW, Deshazer D, Stevens MP, Galyov EE. 2005. Actin-binding proteins from Burkholderia mallei and Burkholderia thailandensis can functionally compensate for the actin-based motility defect of a Burkholderia pseudomallei bimA mutant. J Bacteriol 187:7857–7862. doi: 10.1128/JB.187.22.7857-7862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balder R, Lipski S, Lazarus JJ, Grose W, Wooten RM, Hogan RJ, Woods DE, Lafontaine ER. 2010. Identification of Burkholderia mallei and Burkholderia pseudomallei adhesins for human respiratory epithelial cells. BMC Microbiol 10:250. doi: 10.1186/1471-2180-10-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lafontaine ER, Balder R, Michel F, Hogan RJ. 2014. Characterization of an autotransporter adhesin protein shared by Burkholderia mallei and Burkholderia pseudomallei. BMC Microbiol 14:92. doi: 10.1186/1471-2180-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilhelm S, Tommassen J, Jaeger KE. 1999. A novel lipolytic enzyme located in the outer membrane of Pseudomonas aeruginosa. J Bacteriol 181:6977–6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilhelm S, Rosenau F, Kolmar H, Jaeger KE. 2011. Autotransporters with GDSL passenger domains: molecular physiology and biotechnological applications. Chembiochem 12:1476–1485. doi: 10.1002/cbic.201100013. [DOI] [PubMed] [Google Scholar]
- 96.Tielen P, Rosenau F, Wilhelm S, Jaeger KE, Flemming HC, Wingender J. 2010. Extracellular enzymes affect biofilm formation of mucoid Pseudomonas aeruginosa. Microbiology 156:2239–2252. doi: 10.1099/mic.0.037036-0. [DOI] [PubMed] [Google Scholar]
- 97.Wilhelm S, Gdynia A, Tielen P, Rosenau F, Jaeger KE. 2007. The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J Bacteriol 189:6695–6703. doi: 10.1128/JB.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lipski SL, Akimana C, Timpe JM, Wooten RM, Lafontaine ER. 2007. The Moraxella catarrhalis autotransporter McaP is a conserved surface protein that mediates adherence to human epithelial cells through its N-terminal passenger domain. Infect Immun 75:314–324. doi: 10.1128/IAI.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Akimana C, Lafontaine ER. 2007. The Moraxella catarrhalis outer membrane protein CD contains two distinct domains specifying adherence to human lung cells. FEMS Microbiol Lett 271:12–19. doi: 10.1111/j.1574-6968.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 100.Deshazer D. 2007. Virulence of clinical and environmental isolates of Burkholderia oklahomensis and Burkholderia thailandensis in hamsters and mice. FEMS Microbiol Lett 277:64–69. doi: 10.1111/j.1574-6968.2007.00946.x. [DOI] [PubMed] [Google Scholar]
- 101.Brett PJ, Deshazer D, Woods DE. 1997. Characterization of Burkholderia pseudomallei and Burkholderia pseudomallei-like strains. Epidemiol Infect 118:137–148. doi: 10.1017/S095026889600739X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith MD, Angus BJ, Wuthiekanun V, White NJ. 1997. Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect Immun 65:4319–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ulett GC, Currie BJ, Clair TW, Mayo M, Ketheesan N, Labrooy J, Gal D, Norton R, Smith CA, Barnes J, Warner J, Hirst RG. 2001. Burkholderia pseudomallei virulence: definition, stability and association with clonality. Microbes Infect 3:621–631. doi: 10.1016/S1286-4579(01)01417-4. [DOI] [PubMed] [Google Scholar]
- 104.Gallagher LA, Ramage E, Patrapuvich R, Weiss E, Brittnacher M, Manoil C. 2013. Sequence-defined transposon mutant library of Burkholderia thailandensis. mBio 4:e00604-13. doi: 10.1128/mBio.00604-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burtnick MN, Brett PJ. 2013. Burkholderia mallei and Burkholderia pseudomallei cluster 1 type VI secretion system gene expression is negatively regulated by iron and zinc. PLoS One 8:e76767. doi: 10.1371/journal.pone.0076767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parthasarathy N, DeShazer D, England M, Waag DM. 2006. Polysaccharide microarray technology for the detection of Burkholderia pseudomallei and Burkholderia mallei antibodies. Diagn Microbiol Infect Dis 56:329–332. doi: 10.1016/j.diagmicrobio.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haque A, Chu K, Easton A, Stevens MP, Galyov EE, Atkins T, Titball R, Bancroft GJ. 2006. A live experimental vaccine against Burkholderia pseudomallei elicits CD4+ T cell-mediated immunity, priming T cells specific for 2 type III secretion system proteins. J Infect Dis 194:1241–1248. doi: 10.1086/508217. [DOI] [PubMed] [Google Scholar]
- 108.Atkins T, Prior RG, Mack K, Russell P, Nelson M, Oyston PC, Dougan G, Titball RW. 2002. A mutant of Burkholderia pseudomallei, auxotrophic in the branched chain amino acid biosynthetic pathway, is attenuated and protective in a murine model of melioidosis. Infect Immun 70:5290–5294. doi: 10.1128/IAI.70.9.5290-5294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ulrich RL, Amemiya K, Waag DM, Roy CJ, DeShazer D. 2005. Aerogenic vaccination with a Burkholderia mallei auxotroph protects against aerosol-initiated glanders in mice. Vaccine 23:1986–1992. doi: 10.1016/j.vaccine.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 110.Torres AG, Gregory AE, Hatcher CL, Vinet-Oliphant H, Morici LA, Titball RW, Roy CJ. 2015. Protection of non-human primates against glanders with a gold nanoparticle glycoconjugate vaccine. Vaccine 33:686–692. doi: 10.1016/j.vaccine.2014.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gregory AE, Judy BM, Qazi O, Blumentritt CA, Brown KA, Shaw AM, Torres AG, Titball RW. 2015. A gold nanoparticle-linked glycoconjugate vaccine against Burkholderia mallei. Nanomedicine 11:447–456. doi: 10.1016/j.nano.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burtnick MN, Heiss C, Roberts RA, Schweizer HP, Azadi P, Brett PJ. 2012. Development of capsular polysaccharide-based glycoconjugates for immunization against melioidosis and glanders. Front Cell Infect Microbiol 2:108. doi: 10.3389/fcimb.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scott AE, Burtnick MN, Stokes MG, Whelan AO, Williamson ED, Atkins TP, Prior JL, Brett PJ. 2014. Burkholderia pseudomallei capsular polysaccharide conjugates provide protection against acute melioidosis. Infect Immun 82:3206–3213. doi: 10.1128/IAI.01847-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Scott AE, Laws TR, D'Elia RV, Stokes MG, Nandi T, Williamson ED, Tan P, Prior JL, Atkins TP. 2013. Protection against experimental melioidosis following immunization with live Burkholderia thailandensis expressing a manno-heptose capsule. Clin Vaccine Immunol 20:1041–1047. doi: 10.1128/CVI.00113-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Romero CM, DeShazer D, Feldblyum T, Ravel J, Woods D, Kim HS, Yu Y, Ronning CM, Nierman WC. 2006. Genome sequence alterations detected upon passage of Burkholderia mallei ATCC 23344 in culture and in mammalian hosts. BMC Genomics 7:228. doi: 10.1186/1471-2164-7-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tuanyok A, Kim HS, Nierman WC, Yu Y, Dunbar J, Moore RA, Baker P, Tom M, Ling JM, Woods DE. 2005. Genome-wide expression analysis of iron regulation in Burkholderia pseudomallei and Burkholderia mallei using DNA microarrays. FEMS Microbiol Lett 252:327–335. doi: 10.1016/j.femsle.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 117.Schell MA, Zhao P, Wells L. 2011. Outer membrane proteome of Burkholderia pseudomallei and Burkholderia mallei from diverse growth conditions. J Proteome Res 10:2417–2424. doi: 10.1021/pr1012398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ooi WF, Ong C, Nandi T, Kreisberg JF, Chua HH, Sun G, Chen Y, Mueller C, Conejero L, Eshaghi M, Ang RM, Liu J, Sobral BW, Korbsrisate S, Gan YH, Titball RW, Bancroft GJ, Valade E, Tan P. 2013. The condition-dependent transcriptional landscape of Burkholderia pseudomallei. PLoS Genet 9:e1003795. doi: 10.1371/journal.pgen.1003795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sonnleitner E, Valentini M, Wenner N, Haichar FZ, Haas D, Lapouge K. 2012. Novel targets of the CbrAB/Crc carbon catabolite control system revealed by transcript abundance in Pseudomonas aeruginosa. PLoS One 7:e44637. doi: 10.1371/journal.pone.0044637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stuer W, Jaeger KE, Winkler UK. 1986. Purification of extracellular lipase from Pseudomonas aeruginosa. J Bacteriol 168:1070–1074. doi: 10.1128/jb.168.3.1070-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun 63:4231–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Camilli A, Tilney LG, Portnoy DA. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol 8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Birmingham CL, Canadien V, Gouin E, Troy EB, Yoshimori T, Cossart P, Higgins DE, Brumell JH. 2007. Listeria monocytogenes evades killing by autophagy during colonization of host cells. Autophagy 3:442–451. doi: 10.4161/auto.4450. [DOI] [PubMed] [Google Scholar]
- 124.Whitworth T, Popov VL, Yu XJ, Walker DH, Bouyer DH. 2005. Expression of the Rickettsia prowazekii pld or tlyC gene in Salmonella enterica serovar Typhimurium mediates phagosomal escape. Infect Immun 73:6668–6673. doi: 10.1128/IAI.73.10.6668-6673.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Radulovic S, Troyer JM, Beier MS, Lau AO, Azad AF. 1999. Identification and molecular analysis of the gene encoding Rickettsia typhi hemolysin. Infect Immun 67:6104–6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Renesto P, Dehoux P, Gouin E, Touqui L, Cossart P, Raoult D. 2003. Identification and characterization of a phospholipase D-superfamily gene in rickettsiae. J Infect Dis 188:1276–1283. doi: 10.1086/379080. [DOI] [PubMed] [Google Scholar]
- 127.Pascual DW, Suo Z, Cao L, Avci R, Yang X. 2013. Attenuating gene expression (AGE) for vaccine development. Virulence 4:384–390. doi: 10.4161/viru.24886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang L, Zhai B, Lin X. 2012. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog 8:e1002765. doi: 10.1371/journal.ppat.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang X, Thornburg T, Suo Z, Jun S, Robison A, Li J, Lim T, Cao L, Hoyt T, Avci R, Pascual DW. 2012. Flagella overexpression attenuates Salmonella pathogenesis. PLoS One 7:e46828. doi: 10.1371/journal.pone.0046828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mott TM, Vijayakumar S, Sbrana E, Endsley JJ, Torres AG. 2015. Characterization of the Burkholderia mallei tonB mutant and its potential as a backbone strain for vaccine development. PLoS Negl Trop Dis 9:e0003863. doi: 10.1371/journal.pntd.0003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bozue JA, Chaudhury S, Amemiya K, Chua J, Cote CK, Toothman RG, Dankmeyer JL, Klimko CP, Wilhelmsen CL, Raymond JW, Zavaljevski N, Reifman J, Wallqvist A. 2016. Phenotypic characterization of a novel virulence-factor deletion strain of Burkholderia mallei that provides partial protection against inhalational glanders in mice. Front Cell Infect Microbiol 6:21. doi: 10.3389/fcimb.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hatcher CL, Mott TM, Muruato LA, Sbrana E, Torres AG. 2016. Burkholderia mallei CLH001 attenuated vaccine strain is immunogenic and protects against acute respiratory glanders. Infect Immun 84:2345–2354. doi: 10.1128/IAI.00328-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Silva EB, Goodyear A, Sutherland MD, Podnecky NL, Gonzalez-Juarrero M, Schweizer HP, Dow SW. 2013. Correlates of immune protection following cutaneous immunization with an attenuated Burkholderia pseudomallei vaccine. Infect Immun 81:4626–4634. doi: 10.1128/IAI.00915-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Michaelsen TE, Kolberg J, Aase A, Herstad TK, Hoiby EA. 2004. The four mouse IgG isotypes differ extensively in bactericidal and opsonophagocytic activity when reacting with the P1.16 epitope on the outer membrane PorA protein of Neisseria meningitidis. Scand J Immunol 59:34–39. doi: 10.1111/j.0300-9475.2004.01362.x. [DOI] [PubMed] [Google Scholar]
- 135.Nieves W, Petersen H, Judy BM, Blumentritt CA, Russell-Lodrigue K, Roy CJ, Torres AG, Morici LA. 2014. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin Vaccine Immunol 21:747–754. doi: 10.1128/CVI.00119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nelson M, Prior JL, Lever MS, Jones HE, Atkins TP, Titball RW. 2004. Evaluation of lipopolysaccharide and capsular polysaccharide as subunit vaccines against experimental melioidosis. J Med Microbiol 53:1177–1182. doi: 10.1099/jmm.0.45766-0. [DOI] [PubMed] [Google Scholar]
- 137.Limmathurotsakul D, Funnell SG, Torres AG, Morici LA, Brett PJ, Dunachie S, Atkins T, Altmann DM, Bancroft G, Peacock SJ, Steering Group on Melioidosis Vaccine Development. 2015. Consensus on the development of vaccines against naturally acquired melioidosis. Emerg Infect Dis 21. doi: 10.3201/eid2106.141480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Trevino SR, Permenter AR, England MJ, Parthasarathy N, Gibbs PH, Waag DM, Chanh TC. 2006. Monoclonal antibodies passively protect BALB/c mice against Burkholderia mallei aerosol challenge. Infect Immun 74:1958–1961. doi: 10.1128/IAI.74.3.1958-1961.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bell G. 1923. Serum treatment of glanders. Can Med Assoc J 13:200–201. [PMC free article] [PubMed] [Google Scholar]
- 140.Burgess JF. 1936. Chronic glanders. Can Med Assoc J 34:258–262. [PMC free article] [PubMed] [Google Scholar]
- 141.Watson EA. 1924. Sur la serotherapie de la morve, dans l'espece humaine en particulier. Rec Med Vet 4:220–223. http://gallica.bnf.fr/ark:/12148/bpt6k6472738k/f28.image.r=morve%20serotherapie%20morve%20watson. [Google Scholar]
- 142.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 143.Burtnick M, Bolton A, Brett P, Watanabe D, Woods D. 2001. Identification of the acid phosphatase (acpA) gene homologues in pathogenic and non-pathogenic Burkholderia spp. facilitates TnphoA mutagenesis. Microbiology 147:111–120. doi: 10.1099/00221287-147-1-111. [DOI] [PubMed] [Google Scholar]
- 144.Lafontaine ER, Zimmerman SM, Shaffer TL, Michel F, Gao X, Hogan RJ. 2013. Use of a safe, reproducible, and rapid aerosol delivery method to study infection by Burkholderia pseudomallei and Burkholderia mallei in mice. PLoS One 8:e76804. doi: 10.1371/journal.pone.0076804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reed LJ, Muench H. 1938. A simple method for estimating fifty percent end points. Am J Hyg 27:793–497. [Google Scholar]
- 146.Shaffer TL, Balder R, Buskirk SW, Hogan RJ, Lafontaine ER. 2013. Use of the chinchilla model to evaluate the vaccinogenic potential of the Moraxella catarrhalis filamentous hemagglutinin-like proteins MhaB1 and MhaB2. PLoS One 8:e67881. doi: 10.1371/journal.pone.0067881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lafontaine ER, Wagner NJ, Hansen EJ. 2001. Expression of the Moraxella catarrhalis UspA1 protein undergoes phase variation and is regulated at the transcriptional level. J Bacteriol 183:1540–1551. doi: 10.1128/JB.183.5.1540-1551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.DeShazer D, Brett PJ, Carlyon R, Woods DE. 1997. Mutagenesis of Burkholderia pseudomallei with Tn5-OT182: isolation of motility mutants and molecular characterization of the flagellin structural gene. J Bacteriol 179:2116–2125. doi: 10.1128/jb.179.7.2116-2125.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Simon R, Priefer U, Puhler A. 1983. A broad host range mobilisation system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 150.Skorupski K, Taylor RK. 1996. Positive selection vectors for allelic exchange. Gene 169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.