Abstract
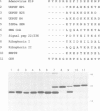
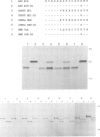
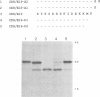
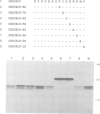
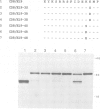
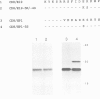
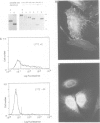
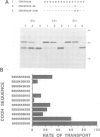
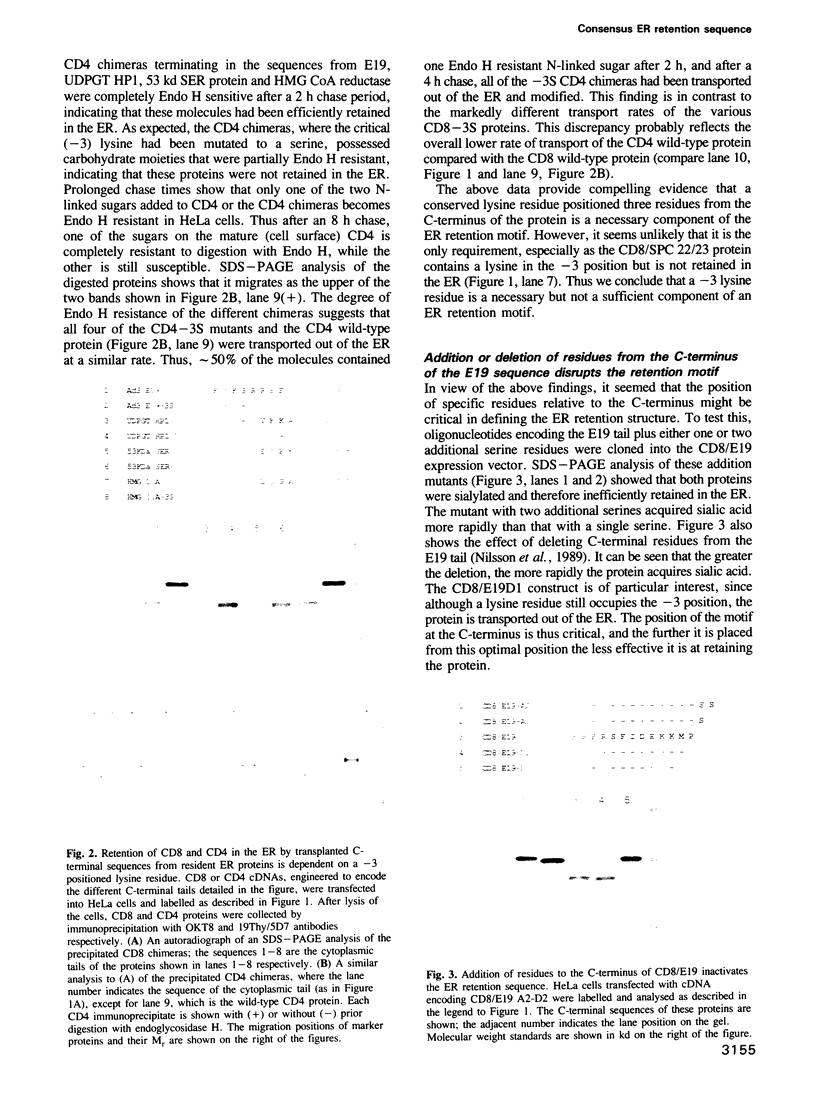
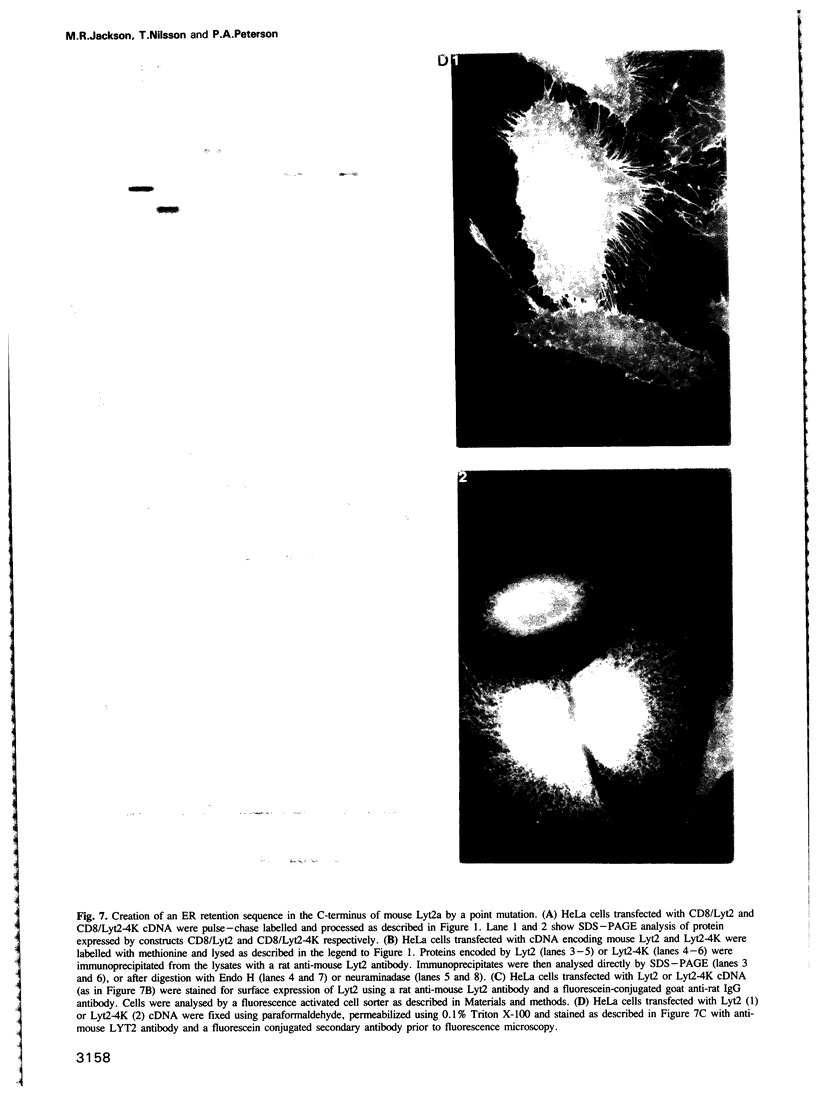
Several families of transmembrane endoplasmic reticulum (ER) proteins contain retention motifs in their cytoplasmically exposed tails. Mutational analyses demonstrated that two lysines positioned three and four or five residues from the C-terminus represent the retention motif. The introduction of a lysine preceding the lysine that occurs three residues from the terminus of Lyt2 renders this cell surface protein a resident of the ER. Likewise, the appropriate positioning of two lysine residues in a poly-serine sequence confines marker proteins to the ER. Arginines or histidines cannot replace lysines, suggesting that simple charge interactions are not sufficient to explain the retention. The identified consensus motif may serve as a retrieval signal that brings proteins back from a sorting compartment adjacent to the ER.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann J. E., Fusco P. J. The G protein of vesicular stomatitis virus has free access into and egress from the smooth endoplasmic reticulum of UT-1 cells. J Cell Biol. 1990 Mar;110(3):625–635. doi: 10.1083/jcb.110.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Chin D. J., Gil G., Russell D. W., Liscum L., Luskey K. L., Basu S. K., Okayama H., Berg P., Goldstein J. L., Brown M. S. Nucleotide sequence of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, a glycoprotein of endoplasmic reticulum. Nature. 1984 Apr 12;308(5960):613–617. doi: 10.1038/308613a0. [DOI] [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Anderson R. G., Faust J. R., Goldstein J. L., Brown M. S. Appearance of crystalloid endoplasmic reticulum in compactin-resistant Chinese hamster cells with a 500-fold increase in 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1185–1189. doi: 10.1073/pnas.79.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cladaras C., Wold W. S. DNA sequence of the early E3 transcription unit of adenovirus 5. Virology. 1985 Jan 15;140(1):28–43. doi: 10.1016/0042-6822(85)90443-x. [DOI] [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimaudo C., Hortsch M., Gausepohl H., Meyer D. I. Human ribophorins I and II: the primary structure and membrane topology of two highly conserved rough endoplasmic reticulum-specific glycoproteins. EMBO J. 1987 Jan;6(1):75–82. doi: 10.1002/j.1460-2075.1987.tb04721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Russ G., Yewdell J. W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989 Jul;109(1):61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J. N., Conibear E., Pearse B. M. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989 Apr;8(4):1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989 May;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding D., Fournel-Gigleux S., Jackson M. R., Burchell B. Cloning and substrate specificity of a human phenol UDP-glucuronosyltransferase expressed in COS-7 cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8381–8385. doi: 10.1073/pnas.85.22.8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. A., Kung P. C., Hansen W. P., Goldstein G. Simple and rapid measurement of human T lymphocytes and their subclasses in peripheral blood. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4914–4917. doi: 10.1073/pnas.77.8.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M., Meyer D. I. Immunochemical analysis of rough and smooth microsomes from rat liver. Segregation of docking protein in rough membranes. Eur J Biochem. 1985 Aug 1;150(3):559–564. doi: 10.1111/j.1432-1033.1985.tb09057.x. [DOI] [PubMed] [Google Scholar]
- Hussey R. E., Richardson N. E., Kowalski M., Brown N. R., Chang H. C., Siliciano R. F., Dorfman T., Walker B., Sodroski J., Reinherz E. L. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988 Jan 7;331(6151):78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- Hérissé J., Courtois G., Galibert F. Nucleotide sequence of the EcoRI D fragment of adenovirus 2 genome. Nucleic Acids Res. 1980 May 24;8(10):2173–2192. doi: 10.1093/nar/8.10.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyanagi T., Haniu M., Sogawa K., Fujii-Kuriyama Y., Watanabe S., Shively J. E., Anan K. F. Cloning and characterization of cDNA encoding 3-methylcholanthrene inducible rat mRNA for UDP-glucuronosyltransferase. J Biol Chem. 1986 Nov 25;261(33):15607–15614. [PubMed] [Google Scholar]
- Jackson M. R., Burchell B. The full length coding sequence of rat liver androsterone UDP-glucuronyltransferase cDNA and comparison with other members of this gene family. Nucleic Acids Res. 1986 Jan 24;14(2):779–795. doi: 10.1093/nar/14.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. R., McCarthy L. R., Harding D., Wilson S., Coughtrie M. W., Burchell B. Cloning of a human liver microsomal UDP-glucuronosyltransferase cDNA. Biochem J. 1987 Mar 1;242(2):581–588. doi: 10.1042/bj2420581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Owens I. S. Mouse UDP glucuronosyltransferase. cDNA and complete amino acid sequence and regulation. Eur J Biochem. 1987 Nov 2;168(3):515–521. doi: 10.1111/j.1432-1033.1987.tb13448.x. [DOI] [PubMed] [Google Scholar]
- Koch G. L., Macer D. R., Wooding F. B. Endoplasmin is a reticuloplasmin. J Cell Sci. 1988 Jul;90(Pt 3):485–491. doi: 10.1242/jcs.90.3.485. [DOI] [PubMed] [Google Scholar]
- Kuroki K., Russnak R., Ganem D. Novel N-terminal amino acid sequence required for retention of a hepatitis B virus glycoprotein in the endoplasmic reticulum. Mol Cell Biol. 1989 Oct;9(10):4459–4466. doi: 10.1128/mcb.9.10.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E., Charuk J. H., Clarke D. M., Green N. M., Zubrzycka-Gaarn E., MacLennan D. H. Molecular cloning and expression of cDNA encoding the 53,000-dalton glycoprotein of rabbit skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1989 Feb 25;264(6):3484–3493. [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman D. R., Thomas Y., Maddon P. J., Chess L., Axel R. The isolation and sequence of the gene encoding T8: a molecule defining functional classes of T lymphocytes. Cell. 1985 Feb;40(2):237–246. doi: 10.1016/0092-8674(85)90138-2. [DOI] [PubMed] [Google Scholar]
- Luskey K. L., Stevens B. Human 3-hydroxy-3-methylglutaryl coenzyme A reductase. Conserved domains responsible for catalytic activity and sterol-regulated degradation. J Biol Chem. 1985 Aug 25;260(18):10271–10277. [PubMed] [Google Scholar]
- Mackenzie P. I. Rat liver UDP-glucuronosyltransferase. Sequence and expression of a cDNA encoding a phenobarbital-inducible form. J Biol Chem. 1986 May 5;261(13):6119–6125. [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Mazzarella R. A., Green M. ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94). J Biol Chem. 1987 Jun 25;262(18):8875–8883. [PubMed] [Google Scholar]
- Miyazawa S., Osumi T., Hashimoto T., Ohno K., Miura S., Fujiki Y. Peroxisome targeting signal of rat liver acyl-coenzyme A oxidase resides at the carboxy terminus. Mol Cell Biol. 1989 Jan;9(1):83–91. doi: 10.1128/mcb.9.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Nakauchi H., Nolan G. P., Hsu C., Huang H. S., Kavathas P., Herzenberg L. A. Molecular cloning of Lyt-2, a membrane glycoprotein marking a subset of mouse T lymphocytes: molecular homology to its human counterpart, Leu-2/T8, and to immunoglobulin variable regions. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5126–5130. doi: 10.1073/pnas.82.15.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Jackson M., Peterson P. A. Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell. 1989 Aug 25;58(4):707–718. doi: 10.1016/0092-8674(89)90105-0. [DOI] [PubMed] [Google Scholar]
- Orci L., Brown M. S., Goldstein J. L., Garcia-Segura L. M., Anderson R. G. Increase in membrane cholesterol: a possible trigger for degradation of HMG CoA reductase and crystalloid endoplasmic reticulum in UT-1 cells. Cell. 1984 Apr;36(4):835–845. doi: 10.1016/0092-8674(84)90033-3. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Sleight R. G. Defining lipid transport pathways in animal cells. Science. 1985 Sep 13;229(4718):1051–1057. doi: 10.1126/science.4035344. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Schekman R. Clathrin: a role in the intracellular retention of a Golgi membrane protein. Science. 1989 Sep 22;245(4924):1358–1365. doi: 10.1126/science.2675311. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988 Apr;7(4):913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Päbo S., Bhat B. M., Wold W. S., Peterson P. A. A short sequence in the COOH-terminus makes an adenovirus membrane glycoprotein a resident of the endoplasmic reticulum. Cell. 1987 Jul 17;50(2):311–317. doi: 10.1016/0092-8674(87)90226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Shelness G. S., Kanwar Y. S., Blobel G. cDNA-derived primary structure of the glycoprotein component of canine microsomal signal peptidase complex. J Biol Chem. 1988 Nov 15;263(32):17063–17070. [PubMed] [Google Scholar]
- Signäs C., Akusjärvi G., Pettersson U. Region E3 of human adenoviruses; differences between the oncogenic adenovirus-3 and the non-oncogenic adenovirus-2. Gene. 1986;50(1-3):173–184. doi: 10.1016/0378-1119(86)90322-7. [DOI] [PubMed] [Google Scholar]
- Stirzaker S. C., Both G. W. The signal peptide of the rotavirus glycoprotein VP7 is essential for its retention in the ER as an integral membrane protein. Cell. 1989 Mar 10;56(5):741–747. doi: 10.1016/0092-8674(89)90677-6. [DOI] [PubMed] [Google Scholar]
- Warren G. Protein transport. Signals and salvage sequences. Nature. 1987 May 7;327(6117):17–18. doi: 10.1038/327017a0. [DOI] [PubMed] [Google Scholar]
- Wieland F. T., Gleason M. L., Serafini T. A., Rothman J. E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987 Jul 17;50(2):289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Wold F. In vivo chemical modification of proteins (post-translational modification). Annu Rev Biochem. 1981;50:783–814. doi: 10.1146/annurev.bi.50.070181.004031. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989 Oct 5;341(6241):456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]