ABSTRACT
Biofilms are multicellular communities of microorganisms living as a quorum rather than as individual cells. The bacterial human pathogen Staphylococcus aureus uses oxygen as a terminal electron acceptor during respiration. Infected human tissues are hypoxic or anoxic. We recently reported that impaired respiration elicits a programmed cell lysis (PCL) phenomenon in S. aureus leading to the release of cellular polymers that are utilized to form biofilms. PCL is dependent upon the AtlA murein hydrolase and is regulated, in part, by the SrrAB two-component regulatory system (TCRS). In the current study, we report that the SaeRS TCRS also governs fermentative biofilm formation by positively influencing AtlA activity. The SaeRS-modulated factor fibronectin-binding protein A (FnBPA) also contributed to the fermentative biofilm formation phenotype. SaeRS-dependent biofilm formation occurred in response to changes in cellular respiratory status. Genetic evidence presented suggests that a high cellular titer of phosphorylated SaeR is required for biofilm formation. Epistasis analyses found that SaeRS and SrrAB influence biofilm formation independently of one another. Analyses using a mouse model of orthopedic implant-associated biofilm formation found that both SaeRS and SrrAB govern host colonization. Of these two TCRSs, SrrAB was the dominant system driving biofilm formation in vivo. We propose a model wherein impaired cellular respiration stimulates SaeRS via an as yet undefined signal molecule(s), resulting in increasing expression of AtlA and FnBPA and biofilm formation.
KEYWORDS: AtlA, biofilm, FnBPA, SaeSR, SrrAB, Staphylococcus aureus, fermentation, oxygen, respiration, teichoic acids
INTRODUCTION
Staphylococcus aureus is a commensal bacterium that colonizes between 20 and 50% of the human population (1–5). S. aureus is capable of causing aggressive infections (6). A recent study found that nearly 92% of cases manifesting as invasive S. aureus infections in the United States required hospitalization (6). Treatment of S. aureus infections is problematic due to the increasing prevalence of multiantibiotic resistance. S. aureus strains that are resistant to nearly all known antibiotics, including the last-line antibiotics linezolid and daptomycin, have been isolated (7, 8).
Persistent bacterial infections in humans are associated with the ability of the bacterium to establish biofilms (9, 10). Biofilms are architecturally complex communities wherein bacteria are attached to a surface or each other and embedded in a polymeric matrix (10–12). The matrix provides protection from innate immunity and imparts therapeutic recalcitrance (10–12). When living in biofilms, bacteria make lifestyle choices as a quorum in a manner reminiscent of that for multicellular organisms (10–12). Biofilms of infectious agents are well characterized to form upon biomedical devices, such as prosthetic heart valves, catheters, and contact lenses (10–12).
The ability of S. aureus to cause biomedical device-associated infections is intimately connected to its ability to form biofilms (13, 14). S. aureus is also capable of attaching to and colonizing human tissues, causing diseases such as osteomyelitis (13, 14). Reflective of their clinical significance, biofilms are considered the etiologic agents of recurrent staphylococcal infections (13, 14). Biofilm formation in S. aureus is multifactorial and, consequently, is a highly regulated and deterministic process. S. aureus biofilm formation is responsive to signals as varied as nutrient limitation to quorum sensing (13, 14).
Oxygen concentrations vary between healthy and infected or necrotic tissues, as well as in wounds, where they are estimated to be below 1% (hypoxic) or completely lacking (anoxic) (15–17). Oxygen is thought to be the primary terminal electron acceptor supporting the respiratory growth of S. aureus in or on the human body. In the absence of oxygen or upon its limitation, S. aureus utilizes fermentative pathways to generate energy (18, 19).
A study by Cramton et al. found that decreased oxygen concentrations result in increased biofilm formation by S. aureus (20). However, the mechanisms underlying the influence of oxygen upon biofilm formation have yet to be fully described. We recently reported that oxygen impacts S. aureus biofilm formation in its capacity as a terminal electron acceptor for cellular respiration (21). Decreased oxygen concentrations lead to diminished respiration, which serves as a signal that elicits programmed cell lysis (PCL), culminating in increased biofilm formation (21). Chemical or physiological conditions that diminish respiration prompt increased biofilm formation. Likewise, heme (hemB::Tn) or menaquinone (menF::Tn) auxotrophs that are incapable of respiration form increased biofilms aerobically. PCL occurs via increased expression of a peptidoglycan hydrolase (AtlA) and decreased expression of the AtlA-inhibiting cell surface glycopolymer wall teichoic acid (WTA) (21). Increased AltA activity results in the release of high-molecular-weight DNA and cytosolic proteins into the extracellular milieu. The DNA and proteins that are released are incorporated into the biofilm matrix and serve as integral structural elements (21).
Two-component regulatory systems (TCRSs) are widespread regulatory systems utilized by organisms to adapt to their environments (22, 23). S. aureus TCRSs consist of two proteins: (i) a histidine kinase (HK) and (ii) a DNA binding response regulator (RR). The HK senses the stimulus. Upon stimulation, the HK undergoes autophosphorylation and subsequently phosphorylates the RR (22, 23). The phosphorylated RR binds to target DNA sequences and regulates transcription, resulting in a tailored physiological response. PCL and fermentative biofilm formation are governed, in part, by the SrrAB TCRS and occur in response to the accumulation of reduced menaquinone (21). SrrAB-dependent biofilm formation is silenced in the absence of menaquinone; however, a menF::Tn strain still forms increased biofilms in the presence of oxygen by an undefined mechanism (21).
The S. aureus SaeRS TCRS is a critical modulator of toxin and exoprotein expression and has crucial roles in circumventing innate immunity and in pathogenesis (24–26). SaeS is the HK, and SaeR is the RR. SaeRS activity is responsive to multiple host and nonhost signals (27–29). SaeRS is encoded by the saePQRS operon, and saeR and saeS are cotranscribed from an autoregulated promoter (27, 30, 31). SaeR is a member of the OmpR family of response regulators and binds to DNA in its phosphorylated state. Phosphorylated SaeR (SaeR∼P) displays a variable affinity for its target promoter regions; therefore, the SaeRS regulon consists of 2 classes of genes: class I genes are low-affinity targets that are activated in the presence of a large pool of SaeR∼P (such as fnbpA and coa), while class II genes are high-affinity targets that are activated even when SaeR∼P titers are low (32). SaeS displays polymorphisms between S. aureus isolates. In the Newman strain, SaeS contains Pro18 (SaeSP18) instead of Leu18 (SaeSL18), which is typically found in S. aureus strains (32–34). SaeSP18 imparts constitutive kinase activity, leading to an increased pool of SaeR∼P and the transcription of class I genes (32–34). SaeP and SaeQ form a membrane-associated complex that activates the phosphatase activity of SaeS, aiding the return of SaeS to a prestimulus state (35).
The goal of the current study was to expand our understanding of the molecular and regulatory mechanism(s) driving fermentative biofilm formation. We report that SaeRS positively governs fermentative biofilm formation. Data presented suggest that the influence of SaeRS upon biofilm formation is exerted via increased expression of the AltA murein hydrolase and fibronectin-binding protein A. Further, SaeRS-dependent biofilm formation occurs in response to alterations in respiratory status and during the presence of high titers of SaeR∼P. Epistatic analyses found that SrrAB and SaeRS govern biofilm formation independently of one another. We also report that SrrAB and SaeRS influence staphylococcal pathogenesis in the context of a mouse model of biofilm-associated infection.
RESULTS
Fermentative biofilm formation is dependent upon the SaeRS two-component system.
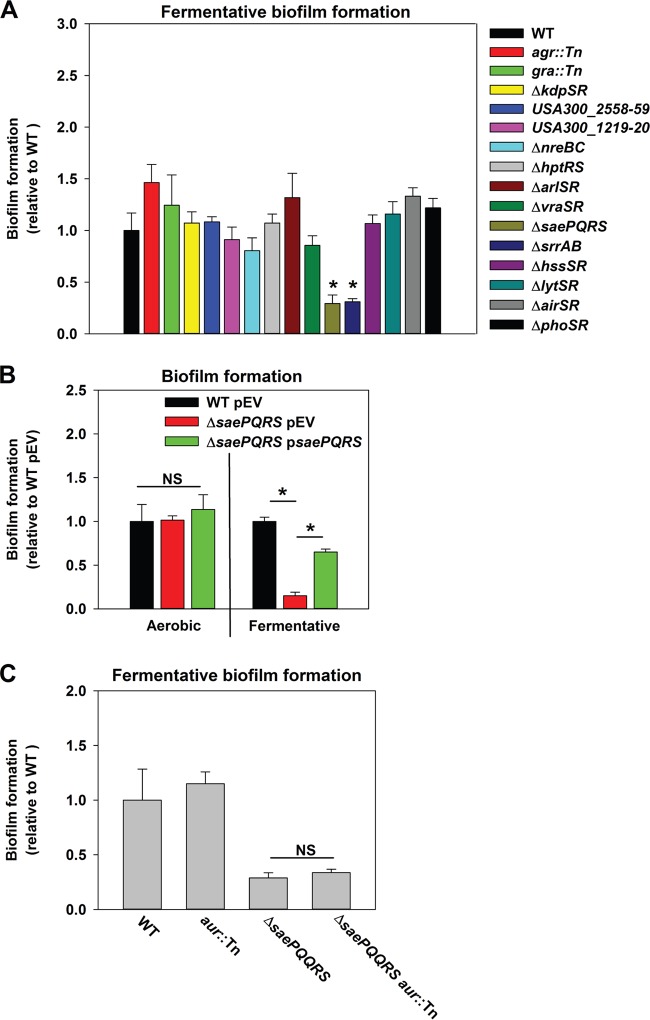
Cellular respiration is dependent upon membrane-associated factors. We reasoned that a regulatory system(s) utilized by the cell to perceive the respiratory status was likely to be membrane associated. The S. aureus genome encodes 16 two-component regulatory systems (TCRSs), and the histidine kinases (HKs) for 14 of these are predicted to be membrane associated. Fermentative biofilm formation was examined in strains that each lacked one individual TCRS (except WalKR, which is essential). The ΔsrrAB and ΔsaePQRS mutant strains were attenuated in fermentative biofilm formation (Fig. 1A). Reintroduction of saePQRS into the ΔsaePQRS strain upon a multicopy plasmid restored fermentative biofilm formation (Fig. 1B). The phenotype of the ΔsrrAB mutant was previously described (21).
FIG 1.
Fermentative biofilm formation is dependent upon the SaeRS two-component system. (A) A strain lacking SaeRS is attenuated in fermentative biofilm formation. The biofilm formation of the LAC (JMB 1100; here the wild-type [WT] strain), agr::Tn (JMB 1333), gra::Tn (JMB 1330), ΔkdpSR (JMB 1223), ΔUSA300_2558-59 (JMB 1232), ΔUSA300_1219-20 (JMB 1219), ΔnreBC (JMB 1145), ΔhptRS (JMB 1148), ΔarlSR (JMB 1383), ΔvraSR (JMB 1377), ΔsaePQRS::spc (JMB 1335), ΔsrrAB (JMB 467), ΔhssSR (JMB 1359), ΔlytSR (JMB 1357), ΔairSR (JMB 1241), and ΔphoSR (JMB 1359) strains following fermentative (anaerobic) growth is displayed. (B) The fermentative biofilm formation defect of the ΔsaePQRS::spc strain can be genetically complemented. The biofilm formation of the WT strain carrying pCM28 (pEV) or the ΔsaePQRS::spc strain carrying either pCM28 (pEV) or pCM28_saePQRS (psaePQRS) following aerobic or fermentative growth is displayed. (C) The biofilm formation defect of a ΔsaePQRS strain is not an outcome of increased Aur expression. The biofilm formation of the WT, ΔsaePQRS::spc, aur::Tn (JMB 6620), and ΔsaePQRS::spc aur::Tn (JMB 6618) strains following fermentative growth is displayed. The data represent the average values for eight wells, and error bars represent standard deviations. Error bars are displayed for all data, but on occasion they may be too small to see. Statistical significance was calculated using a two-tailed Student's t test, and P values of >0.05 were considered not significant. *, P < 0.05; NS, not significant.
Proteins are integral matrix components in fermenting biofilms. SaeRS negatively modulates expression of the secreted protease aureolysin (Aur), and Aur is the most abundant protein in the exoproteome of a LAC sae mutant (18). The introduction of an aur::Tn mutation into the ΔsaePQRS strain did not alter the fermentative biofilm formation phenotype of the ΔsaePQRS strain, suggesting that increased Aur expression does not contribute to the decreased biofilm phenotype of this strain (Fig. 1C).
SaeRS influences fermentative biofilm formation via the AtlA murein hydrolase and fibronectin-binding protein A.
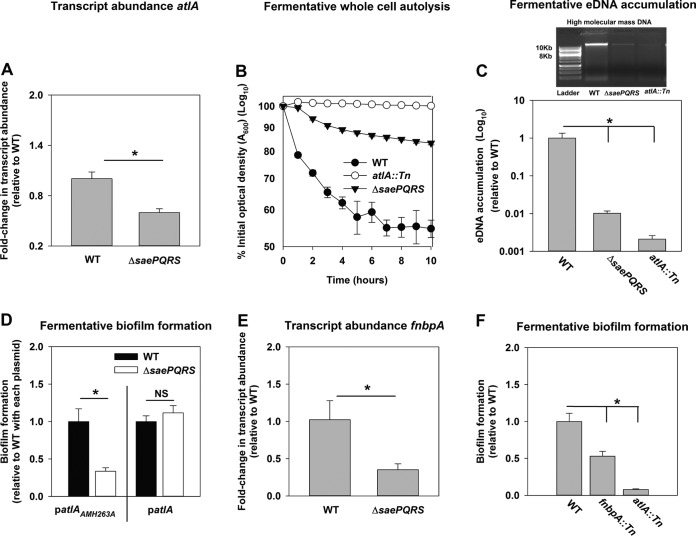
AtlA is a dominant factor required for the formation of fermentative biofilms (21). Phosphorylated SaeR preferentially binds a direct repeat sequence (GTTAAN6GTTAA) (31). The guanines in the binding sequence are dispensable (31, 36). A putative SaeR binding site was present ∼180 bp upstream of the predicted transcriptional start site for atlA, in the negative strand, suggesting that SaeR modulates atlA transcription (see Fig. S1 in the supplemental material). The binding site sequence differed from the consensus sequence by one guanine and one adenine (36). SaeR binding site sequences in the promoter regions of alternate SaeR regulon members, such as tst and nuc, also differ from the consensus sequence by 2 bp (37, 38). Consistent with the in silico prediction, atlA transcript levels were decreased in the ΔsaePQRS strain during fermentative growth (Fig. 2A).
FIG 2.
SaeRS influences fermentative biofilm formation via the AtlA murein hydrolase and fibronectin-binding protein A. (A) Transcript levels corresponding to atlA are decreased in a ΔsaePQRS strain. Biofilms of the WT (JMB 1100) and ΔsaePQRS::spc (JMB 1335) strains were cultured fermentatively, mRNA was extracted, and the abundance of the atlA transcript was quantified. Data were normalized to 16S rRNA levels and thereafter to the levels observed in the WT. (B) Autolysis of fermenting S. aureus is decreased in a strain lacking Sae. The WT, ΔsaePQRS::spc, and atlA::Tn (JMB 6625) strains were cultured fermentatively, and autolysis was examined in intact whole cells. (C) High-molecular-mass DNA (eDNA) accumulation is decreased in the biofilm matrix of a ΔsaePQRS strain. Biofilms of the WT, ΔsaePQRS::spc, and atlA::Tn strains were cultured fermentatively, and eDNA was extracted and analyzed using agarose gel electrophoresis (photograph at the top). The data were normalized to the viable cell count and thereafter to the level of eDNA accumulation in the fermenting WT. (D) atlA in multiple copies suppresses the biofilm formation defect of the ΔsaePQRS strain. The fermentative biofilm formation of the WT and ΔsaePQRS::spc strains carrying either patlAAM H263A or patlA is displayed. (E) Transcripts corresponding to fnbpA are decreased in a ΔsaePQRS strain. fnbpA transcripts levels were quantified from the same cDNA libraries used for the assay whose results are presented in panel A. The data were normalized to 16S rRNA levels and thereafter to the levels observed in the WT. (F) A strain lacking a functional fibronectin-binding protein A is deficient in the formation of fermentative biofilms. The biofilm formation of the WT, fnbpA::Tn (JMB 8403), and atlA::Tn (JMB 6625) strains following fermentative growth is displayed. The data presented represent the average values for eight wells (D and F) or from biological triplicates (A, C, and E). The data in panel B represent the average value from technical duplicates from one set of autolysis assays. The autolysis assays were conducted on least three separate occasions, and similar results were obtained. Error bars in all panels represent standard deviations. Error bars are displayed for all data but on occasion might be too small to see. Statistical significance was calculated using a two-tail Student's t test, and P values of >0.05 were considered not significant. *, P < 0.05.
A strain lacking SaeRS displayed phenotypes consistent with decreased expression of AtlA. The activity for AtlA was measured within the context of intact whole cells using autolysis assays (39). Fermentatively cultured ΔsaePQRS and atlA::Tn (positive-control) strains were deficient in autolysis (Fig. 2B). One outcome of S. aureus autolysis is the release of high-molecular-mass genomic DNA into the extracellular milieu (40, 41). The ΔsaePQRS and atlA::Tn (positive-control) strains displayed decreased accumulation of high-molecular-mass DNA in their biofilm matrices following fermentative growth (Fig. 2C). AtlA has also been implicated in the release of cytosolic proteins into the extracellular milieu (42). The activity of catalase (Kat), an abundant intracellular protein (43, 44), was decreased by ∼4-fold in the spent medium supernatant from the fermentatively cultured ΔsaePQRS strain (Fig. S2). These data were normalized to the intracellular Kat activity to negate for potential changes in Kat expression.
AtlA is a bifunctional enzyme that is proteolytically cleaved into a N-acetylmuramyl-l-alanine amidase (AM) and endo-β-N-acetylglucosaminidase (GL) (45). Full-length AtlA or AM is required for cleavage of S. aureus cells, while GL is dispensable for this activity (39). We further examined the influence of AtlA upon SaeRS-dependent biofilm formation by introducing multicopy plasmids with alleles encoding either full-length AtlA (patlA) or an enzymatically inactivated AM with the H263A mutation (patlAAM H263A) into the ΔsaePQRS strain and examined fermentative biofilm formation. The presence of patlA suppressed the fermentative biofilm formation defect of the ΔsaePQRS strain, whereas the defect remained in the strain carrying patlAAM H263A (Fig. 2D).
Fibronectin-binding protein A (FnBPA) is involved in aerobic biofilm formation (46). SaeR regulates the transcription of fnbpA. We found that the transcript levels corresponding to fnbpA were decreased in a ΔsaePQRS strain upon fermentative growth, suggesting that FnBPA may also have a role in fermentative biofilm formation (Fig. 2E). Consistent with this premise, an fnbpA::Tn strain was attenuated in fermentative biofilm formation (Fig. 2F). Interactions between AtlA and FnBPA facilitate biofilm formation, but it is unclear how (46). The fnbpA::Tn strain was not deficient in autolysis (data not shown), suggesting that the cell wall-cleaving activity of AtlA operates independently of FnBPA.
Wall teichoic acids (WTA) are cell wall glycopolymers that negatively influence AtlA activity (47, 48). S. aureus cultured fermentatively has decreased expression of WTA (21). One consequence of this is that the heat-killed fermenting wild-type (WT) strain undergoes AtlA-dependent lysis at a rate higher than that for the respiring WT strain (21). We examined whether SaeRS influences WTA expression. To this end, the WT strain was cultured aerobically (positive control) and fermentatively, while the ΔsaePQRS strain was cultured fermentatively. Subsequently, the cells were heat killed to inactivate native autolysins and provided as the substrates in murein hydrolase assays. Murein hydrolase assays were conducted using proteins detached from the cell walls of a ΔatlA strain (referred to as the cell wall extract [CW extract]) carrying either the empty vector or a plasmid encoding full-length AtlA (patlA). As reported previously, lysis was undetectable with CW extracts from the ΔatlA strain carrying the empty vector, verifying that bacteriolytic activity under the conditions examined was entirely dependent upon AtlA (data not shown) (21). Heat-killed ΔsaePQRS cells were lysed at the same rate as fermentatively cultured WT cells by CW extracts from the ΔatlA strain carrying patlA, while WT cells cultured aerobically displayed increased resistance to lysis (Fig. S3). These data suggested that SaeRS does not influence WTA expression during fermentative growth. From the results presented in Fig. 2 and S1 to S3, we concluded that the influence of SaeRS upon fermentative biofilm formation is exerted, at least in part, via altered expression of AtlA and FnBPA.
Increased SaeR∼P is required for fermentative biofilm formation, and the SaeS kinase activity inhibitor zinc attenuates fermentative biofilm formation.
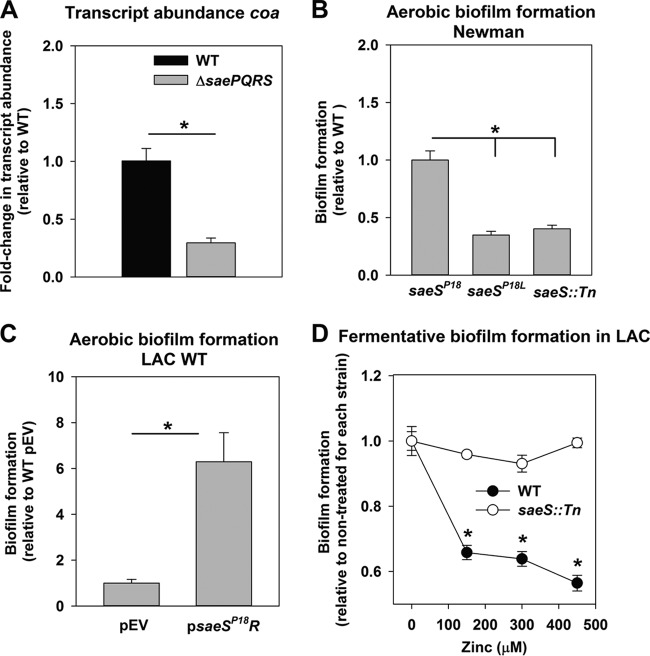
A high concentration of phosphorylated SaeR (SaeR∼P) is required for activation of the low-affinity class I target fnbpA (32); therefore, it was possible that fermentative growth results in an increased pool of SaeR∼P, which then promotes biofilm formation. The gene encoding coagulase (coa) is also a class I SaeR target. The transcript levels for coa were decreased in a ΔsaePQRS strain upon fermentative growth (Fig. 3A). Transcription of the saePQRS operon is controlled by the P3 and P1 promoters (30). The P3 promoter is constitutive and transcribes saeRS (30). The P1 promoter transcribes saePQRS and is autoinduced by SaeR; therefore, higher SaeS kinase activity leads to increased transcription of saePQRS (30). Transcript levels corresponding to saeQ, saeR, fnbpA, and coa were assessed and found to be increased in the WT upon fermentative growth (Fig. S4). Catalase (kat) is required to scavenge reactive oxygen species, and thus, kat transcription would be expected to be decreased upon anaerobic growth. Transcript levels for kat were decreased in fermentatively cultured cells, confirming appropriate transcription patterns (Fig. S4).
FIG 3.
Increased amounts of SaeR∼P are required for fermentative biofilm formation, and the SaeS kinase activity inhibitor zinc attenuates fermentative biofilm formation. (A) Transcript levels corresponding to coa are decreased in a ΔsaePQRS strain during fermentative growth. Biofilms of the WT (JMB 1100) and ΔsaePQRS::spc (JMB 1335) strains were cultured fermentatively, mRNA was extracted, and the abundance of the coa transcript was quantified. Data were normalized to 16S rRNA levels and thereafter to the levels observed in the WT. (B) The Newman strain of S. aureus forms SaeSP18-dependent biofilms during aerobic growth. Aerobic biofilm formation for the Newman strains containing the wild-type saeSP18 allele (JMB 1422), the saeSL18 allele (JMB 8263), or the saeS::Tn (JMB 7077) mutation is displayed. (C) Constitutive expression of the saeSP18 allele induces aerobic biofilm formation in the LAC WT strain. The biofilm formation of the LAC WT strain (JMB 1100) carrying pOS (pEV) or pOS_saeSP18R (psaeSP18R) following aerobic growth is displayed. (D) Supplementation of the growth medium with zinc, an inhibitor of SaeS kinase activity, results in a concentration-dependent decrease in fermentative biofilm formation. The biofilm formation of the LAC WT and the LAC saeS::Tn (JMB 7076) strains following fermentative growth in medium supplemented with various concentrations of zinc is displayed. The data represent the average values for eight wells (B to D) or from triplicate assays (A), and error bars represent standard deviations. Error bars are displayed for all data but on occasion may be too small to see. Statistical significance was calculated using a two-tailed Student's t test. *, P < 0.05.
The LAC strain of S. aureus carries the saeSL18 allele and does not form SaeRS-dependent biofilms aerobically (Fig. 1B). The saeSP18 allele in strain Newman confers constitutive SaeS kinase activity, resulting in an increased pool of SaeR∼P (32–34). We reasoned that strain Newman would display SaeSP18-dependent biofilm formation in the presence of oxygen. Aerobic biofilm formation in Newman was indeed attenuated when the saeSP18 allele was replaced with the saeSL18 allele (Fig. 3B). Biofilm formation was also attenuated in a Newman saeS::Tn strain (Fig. 3B). Moreover, expression of the saeSP18 allele under the transcriptional control of a constitutive promoter in the LAC WT strain increased aerobic biofilm formation (Fig. 3C).
Zinc (Zn) inhibits the autokinase activity of SaeS (26). If a high pool of SaeR∼P is associated with fermentative biofilm formation, then Zn would be expected to inhibit biofilm formation in an SaeRS-dependent manner (26). Consistent with this premise, Zn supplementation resulted in decreased biofilm formation in the LAC WT strain cultured fermentatively but not in the LAC ΔsaeS::Tn strain (Fig. 3D).
SaeRS-dependent biofilm formation is responsive to cellular respiratory status, and a menaquinone auxotroph forms Sae-dependent biofilms.
SrrAB modulates fermentative biofilm formation in response to changes in the cellular respiratory status (21). Similar to SrrB, SaeS is membrane spanning. We examined whether SaeRS-dependent biofilm formation also occurs in response to the altered respiratory status of the cell. In addition to oxygen, S. aureus can utilize nitrate as a terminal electron acceptor. Supplementation of anaerobic biofilms with nitrate resulted in diminished biofilm formation by both the WT and the ΔsaePQRS strains. However, ratiometric analyses revealed that this occurred to a lower degree in the ΔsaePQRS strain (Fig. 4A).
FIG 4.
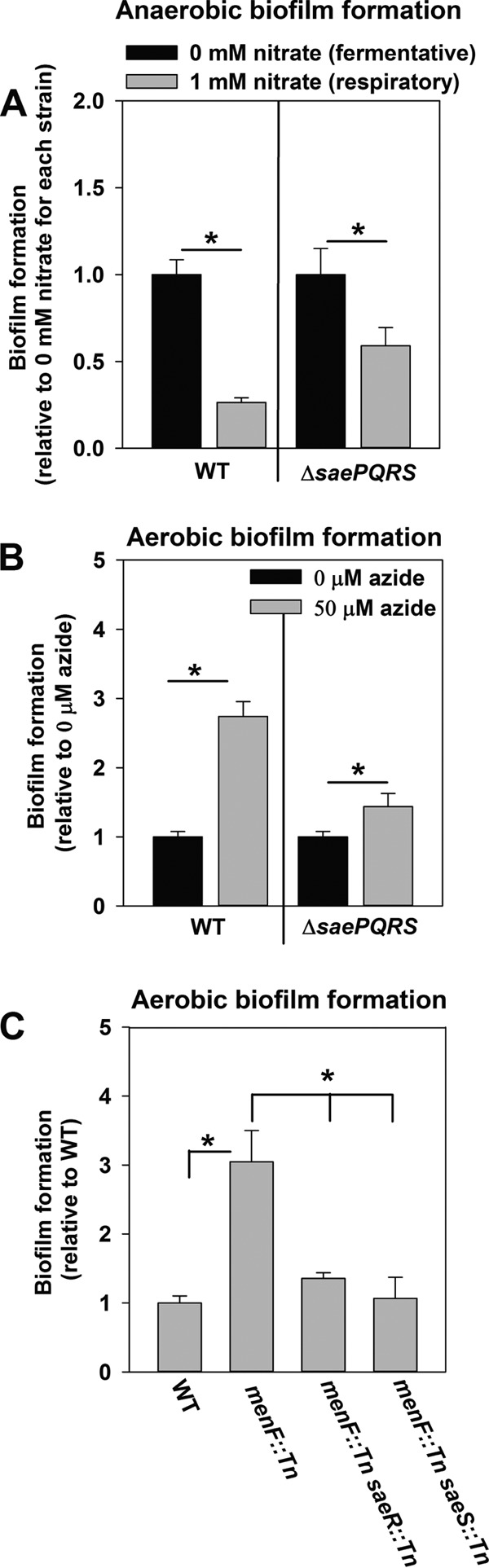
SaeRS-dependent biofilm formation is responsive to the cellular respiratory status, and a menaquinone auxotroph forms Sae-dependent biofilms. (A) The biofilm formation of a ΔsaePQRS strain is attenuated to a lower degree upon supplementation of anaerobic biofilms with the alternate electron acceptor nitrate. The biofilm formation of the WT (JMB 1100) and the ΔsaePQRS::spc (JMB 1335) strains following anaerobic growth in the presence or absence of 1 mM nitrate is displayed. (B) Induction of biofilm formation in a ΔsaePQRS strain is attenuated upon chemical inhibition of respiration. The biofilm formation of the WT and ΔsaePQRS::spc strains following aerobic growth in the presence or absence of 50 μM azide is displayed. (C) A menaquinone auxotroph forms SaeRS-dependent biofilms aerobically. The aerobic biofilm formation of the WT, menF::Tn (JMB 6219), saeR::Tn menF::Tn (JMB 7109), and saeS::Tn menF::Tn (JMB 7081) strains is displayed. The data represent the average values for eight wells, and error bars represent standard deviations. Error bars are displayed for all data but on occasion may be too small to see. Statistical significance was calculated using a two-tailed Student's t test. *, P < 0.05.
We reasoned that SaeRS-dependent biofilms would be formed aerobically if respiratory processes were inhibited by either chemical or genetic means. Consistent with our premise, supplementation of aerobic cultures with the respiratory poison sodium azide increased biofilm formation in the WT. Ratiometric analyses revealed that biofilm induction was attenuated in the ΔsaePQRS strain (Fig. 4B). A menaquinone auxotroph (menF::Tn), which is incapable of respiring, displayed increased biofilm formation during aerobic growth. This phenotype was attenuated upon the introduction of the saeS::Tn or saeR::Tn mutation (Fig. 4C).
SaeRS and SrrAB influence fermentative biofilm formation independently of one another.
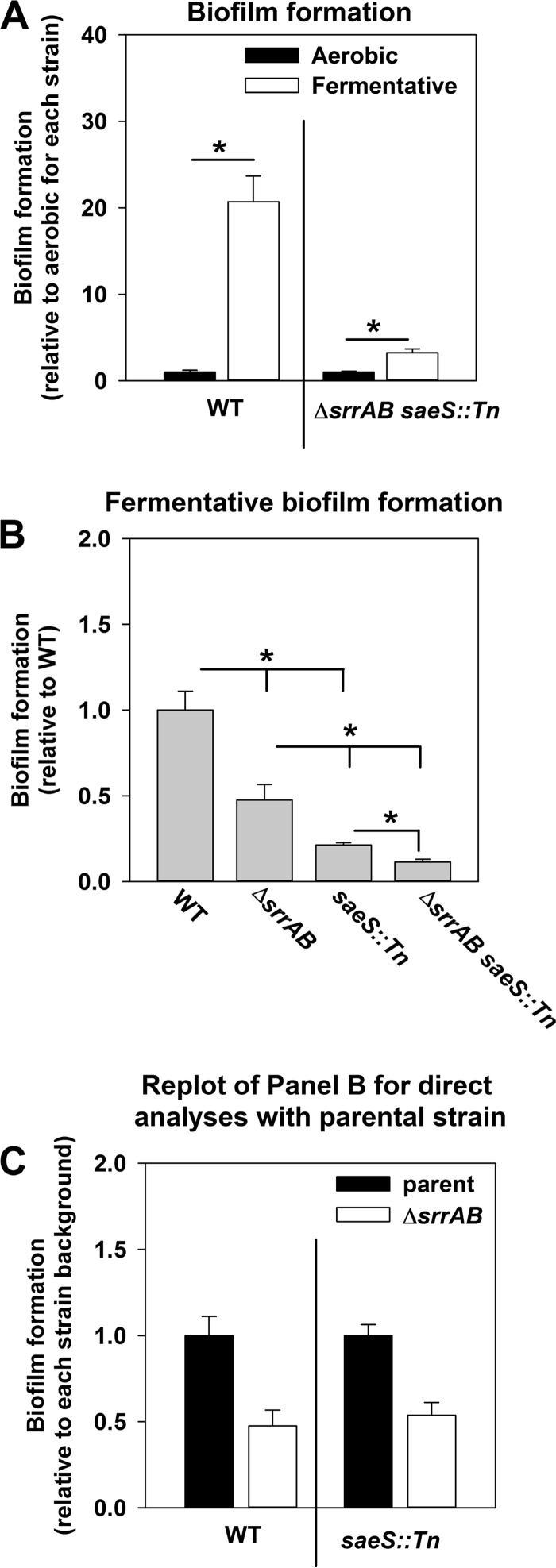
SrrAB-dependent biofilm formation is silenced in the menF::Tn strain (21). Aerobic biofilm formation in the menF::Tn saeS::Tn strain was similar to that in the WT strain. These data led us to reason that SrrAB and SaeRS are the dominant regulatory systems influencing fermentative biofilm formation and that they influence biofilm formation independently of each other. Consistent with this premise, biofilm formation was induced ∼20-fold in the WT following fermentative growth relative to the level of biofilm formation following aerobic growth; however, induction of biofilm formation was largely abrogated in the ΔsrrAB saeS::Tn double mutant strain (Fig. 5A). Further, the fermentative biofilm formation phenotype displayed by the ΔsrrAB saeS::Tn double mutant strain was more severe than that displayed by the ΔsrrAB and saeS::Tn strains (Fig. 5B). Ratiometric analyses of the data presented in Fig. 5B found that introduction of the ΔsrrAB mutation into either the WT or saeS::Tn strain resulted in a similar fold change decrease in biofilm formation (Fig. 5C). Moreover, the presence of srrAB upon a multicopy plasmid (psrrAB) was unable to suppress the fermentative biofilm formation defect of the ΔsaePQRS strain (Fig. S5).
FIG 5.
SaeRS and SrrAB influence fermentative biofilm formation independently of one another. (A) Induction of fermentative biofilm formation is nearly absent in the ΔsrrAB saeS::Tn strain. The biofilm formation of the WT (JMB 1100) and the ΔsrrAB saeS::Tn (JMB 7068) strains following aerobic and fermentative growth is displayed. To allow assessment of induction levels, the data were normalized to the biofilm levels displayed by each strain under aerobic growth. (B and C) The biofilm formation phenotypes of the ΔsaeS::Tn and ΔsrrAB mutations are additive. The biofilm formation of the WT, ΔsrrAB (JMB 1467), saeS::Tn (JMB 7076), and ΔsrrAB saeS::Tn strains following fermentative growth is displayed. Data were normalized with respect to the WT (B) or with respect to the parental strain into which the ΔsrrAB mutation was introduced (i.e., the WT or saeS::Tn strain) (C). The data represent the average values for eight wells, and error bars represent standard deviations. Error bars are displayed for all data but on occasion may be too small to see. Statistical significance was calculated using a two-tailed Student's t test. *, P < 0.05.
SaeRS and SrrAB influence biofilm formation in a murine model of orthopedic implant-associated infection.
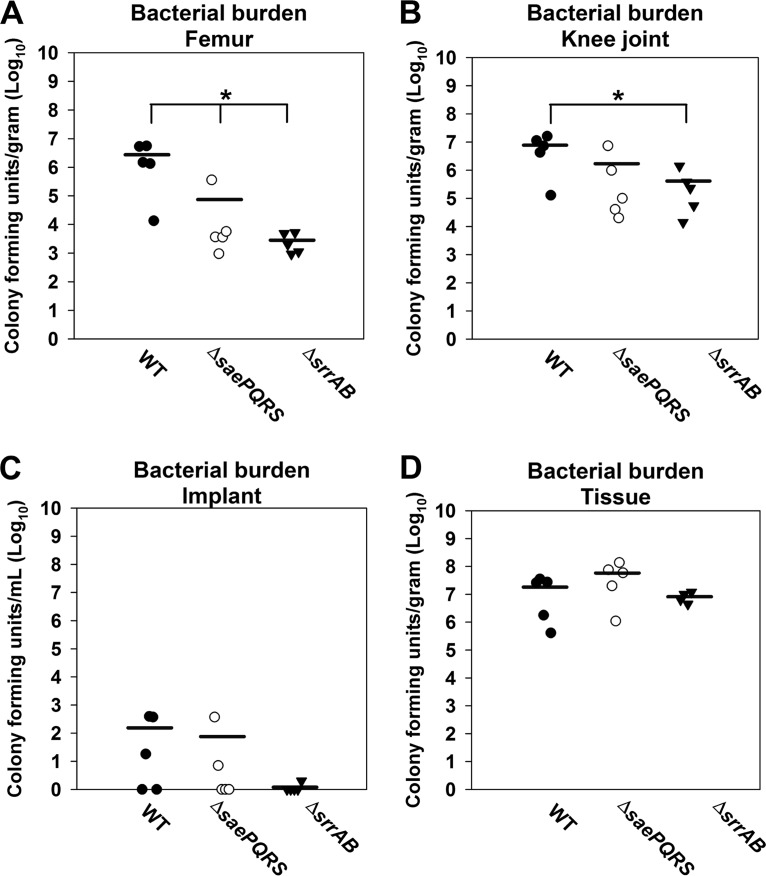
We examined whether the SrrAB and SaeRS regulatory systems were required for biofilm formation in vivo using an established mouse model of orthopedic implant-associated biofilm infection (49, 50). At 7 days postinfection, a ΔsrrAB strain displayed decreased bacterial burdens in the tissue surrounding the site of infection but not in the infected joint, the femur, or the implant (Fig. S6). The ΔsaePQRS strain was not attenuated in growth at day 7 in any of the tissues examined (Fig. S6). At 14 days postinfection, both the ΔsrrAB and the ΔsaePQRS strains displayed decreased bacterial burdens in the infected femur (Fig. 6), whereas the titers achieved with the ΔsrrAB strain were also reduced in the joint (Fig. 6). These findings suggest that SrrAB and SaeRS influence later stages of biofilm development in vivo, which might be regulated by limitations in oxygen availability as the infection progresses.
FIG 6.
SaeRS and SrrAB influence biofilm persistence in a murine model of orthopedic implant biofilm infection. (A to D) Male C57BL/6 mice were infected with the WT (JMB 1100), ΔsaePQRS::spc (JMB 1335), and ΔsrrAB (JMB 1467) strains (n = 5 mice/group). Animals were sacrificed at 14 days following infection, whereupon the implant was sonicated and host tissues surrounding the infected orthopedic implant site were homogenized to quantitate bacterial burdens. Results are expressed as the number of CFU per milliliter for the implant and the number of CFU per gram of tissue (for the soft tissue surrounding the knee, knee joint containing ligament and tendon structures, and femur) to normalize for differences in sampling size. Significant differences in bacterial burdens between mice are denoted by asterisks (*, P < 0.05). Statistics were conducted using a one-way analysis of variance (ANOVA), followed by Bonferroni's multiple-comparison test.
DISCUSSION
Biofilm formation is a crucial facet of staphylococcal infections (13, 14). In a recent study, we reported that growth conditions of diminished respiration elicit increased biofilm formation by S. aureus in a process dependent upon the AtlA murein hydrolase (21). The goal of the current study was to expand our understanding of the molecular and regulatory mechanisms driving fermentative biofilm formation. Herein we report that, in addition to SrrAB, the SaeRS TCRS also modulates fermentative biofilm formation. A strain lacking SaeRS was attenuated in biofilm formation, had decreased transcript levels corresponding to atlA during fermentative growth, and displayed phenotypes consistent with decreased AtlA-dependent autolysis. Moreover, the biofilm-deficient phenotype of the ΔsaePQRS strain was suppressed by the introduction of atlA in multiple copies. These data support a model wherein SaeRS exerts its effect upon biofilm formation, at least in part, via AtlA (Fig. 7). It is currently unclear whether SaeR directly influences AtlA transcription.
FIG 7.
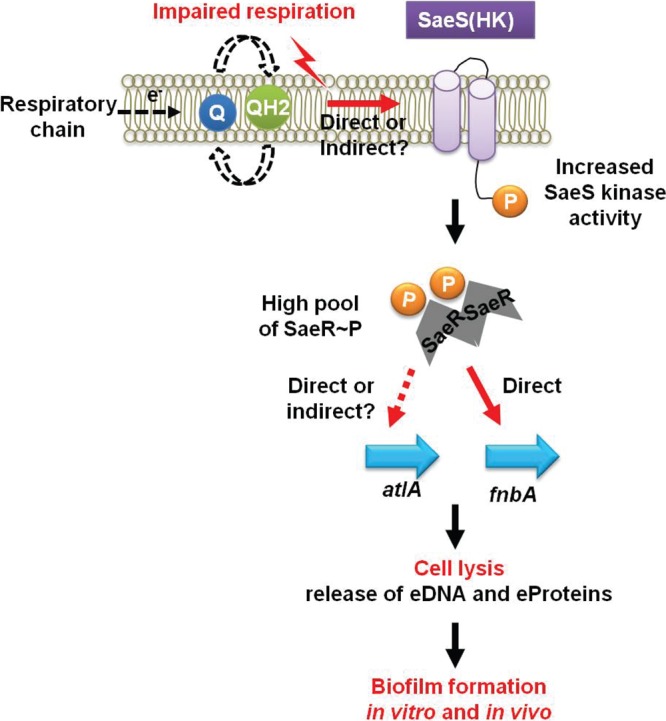
A working model for the influence of respiration upon SaeRS-dependent autolysis and biofilm formation in S. aureus. A decreased capacity to respire stimulates the SaeS histidine kinase in an as yet unknown manner and, subsequently, an increased concentration of phosphorylated SaeR (SaeR∼P). Increased titers of SaeR∼P result in greater expression of the AtlA murein hydrolase, facilitating autolysis and the release of DNA and proteins. Increased titers of SaeR∼P also result in increased expression of FnBPA. The increased amount of FnBPA and the accumulation of polymeric cellular molecules released during autolysis increase biofilm formation. While FnBPA is a direct SaeR binding target, the mechanism by which SaeR increases AtlA expression will require further biochemical analyses. eProteins, extracellular proteins.
Fibronectin-binding protein A (FnBPA) interacts with AtlA to facilitate aerobic biofilm formation (46). Transcription of fnbpA is modulated by SaeR (32). The ΔsaePQRS strain had decreased fnbpA transcript levels during fermentative growth, and a strain lacking FnBPA was attenuated in fermentative biofilm formation. The fnbpA::Tn strain was not attenuated for autolysis, suggesting that FnBPA exerts its influence independently of the ability of AtlA to lyse cells.
Increased levels of phosphorylated SaeR (SaeR∼P) result in increased levels of fnbpA transcription (32). Taken together with the data presented herein, one inference is that fermentative biofilm formation is a result of increased SaeRS activity and increased levels of SaeR∼P. In support of this hypothesis, the transcript levels of alternate class I SaeR regulon genes, such as coa, were increased during fermentative growth. Providing further support to this idea, strains carrying the saeSP18 allele, which increases the cellular titer of SaeR∼P, formed SaeRS-dependent biofilms during aerobic growth. Moreover, supplementation of the growth medium with zinc, an inhibitor of SaeS kinase activity (26), attenuated SaeRS-dependent fermentative biofilm formation.
Anaerobic SaeRS-dependent biofilm formation was suppressed upon supplementation of the growth medium with nitrate, which serves as an electron acceptor. This lent support to a model wherein SaeRS is responsive to the cellular respiratory status (Fig. 7). Further emphasizing this model, SaeRS-dependent biofilm formation could be triggered by chemical or genetic inhibition of respiration. It is currently unclear which cellular molecule(s) stimulates SaeS or is involved in transferring the stimulus to SaeS during fermentative growth. The SaeRS output is altered by a variety of extracellular stimuli, and these alterations result in increased or decreased kinase activity (26, 28, 34). The findings reported herein suggest that Sae activity may also be responsive to an intracellular signal resulting in increased kinase activity (Fig. 7). The mechanistic details underlying the integration of internal and external signals, as well as repressing and activating signals, by Sae are currently unclear. However, recent studies have postulated an intriguing theory wherein intramembrane HKs, such as SaeS, could achieve signal integration using a trip wire-like model (51, 52). In this model, the conformations adopted by the N-terminal domain govern the kinase activity of the histidine kinase. Thus, the conformations adopted by the N-terminal domain upon interaction with a stimulus would dictate whether the system output is increased or decreased.
SaeRS modulates expression of the major staphylococcal nuclease (nuc-1) during aerobic growth (38). It has been suggested that this regulation allows Sae to modulate biofilm maturation (53). DNA is an integral structural element in fermenting biofilms, and this polymer is released in a Sae- and AtlA-dependent manner (21). The manifest question was whether nuclease activity modulates fermentative biofilm formation. We found that strains lacking either of the staphylococcal nucleases (Nuc1 or Nuc2) did not have an altered fermentative biofilm phenotype (data not shown). Thus, if Sae is modulating Nuc1 expression during fermentative growth, it has no effect on the Sae-dependent biofilm phenotype that we investigated. It is interesting to note that both the guanine residues in the SaeR binding site within the nuc-1 promoter are replaced with adenines. To our knowledge, this sequence arrangement is unique among SaeR targets.
Clinical isolates of S. aureus that are incapable of respiration, termed small-colony variants (SCV), display increased resistance toward antibiotics and cause persistent infections (54, 55). SCV strains are typically heme or menaquinone auxotrophs (19, 54). We have previously shown that a heme auxotroph forms SrrAB-dependent biofilms and this phenotype requires the presence of menaquinone (21). Consequently, a menaquinone auxotroph forms biofilms in a manner independent of SrrAB (21). In the current study, we found that a menaquinone auxotroph forms SaeRS-dependent biofilms. The results presented herein, in conjunction with those of our prior studies, lend considerable insight into the regulatory mechanisms that may predominate within SCV strains.
One characteristic of the growth cycle of biofilms is the periodic detachment and shedding of bacterial cells (13). The detached cells aid in the dispersal of infection (13). Consequently, biofilm-associated cells are considered the etiologic agents of recurrent staphylococcal infections (13, 14). The regulatory and molecular mechanisms that drive biofilm formation or dispersal in vivo are largely unknown (13, 14). Our findings that both SrrAB and SaeRS are required for biofilm formation in a mouse model of infection shed light on the regulatory factors that may operate in vivo. Since both SrrAB and SaeRS modulate the expression of factors involved in cell lysis, it is tempting to speculate that these factors are also important for in vivo biofilm formation. However, further experimentation is necessary to draw this conclusion.
In summary, we report that SaeRS plays a role in fermentative biofilm formation. Sae is responsive to the cellular respiratory status, and decreased respiration elicits increased activity of SaeRS, resulting in increased transcription of atlA and fnbpA, which modulate biofilm formation. Further, SrrAB and SaeRS affect persistence in a murine model of orthopedic implant-associated infection.
MATERIALS AND METHODS
Materials.
Restriction enzymes, a quick DNA ligase kit, deoxynucleoside triphosphates, and Phusion DNA polymerase were purchased from New England BioLabs. A plasmid miniprep kit, gel extraction kit, and the RNAprotect reagent were purchased from Qiagen. DNase I was purchased from Ambion. Lysostaphin was purchased from Ambi Products. Oligonucleotides were purchased from Integrated DNA Technologies, and the sequences are listed in Table S1 in the supplemental material. TRIzol and high-capacity cDNA reverse transcription kits were purchased from Life Technologies. Tryptic soy broth (TSB) was purchased from MP Biomedicals. Unless otherwise specified, all chemicals were purchased from Sigma-Aldrich and were of the highest purity available.
Bacterial growth conditions.
Unless otherwise stated, the S. aureus strains used in this study (Table 1) were constructed in the community-associated S. aureus USA300_LAC strain that was cured of the native plasmid pUSA03 that confers erythromycin resistance (56). Overnight cultures of S. aureus were grown at 37°C in 10-ml culture tubes containing 1 ml of TSB or 30-ml culture tubes containing 5 ml TSB. Difco BiTek agar was added (15 g liter−1) for solid medium. When selecting for or against plasmids, antibiotics where added to the following concentrations: 150 μg ml−1 ampicillin, 30 μg ml−1 chloramphenicol (Cm), and 10 μg ml−1 erythromycin (Erm).
TABLE 1.
Microbial strains used in this study
| Strain | Genotype or descriptiona | Genetic background | Source or reference |
|---|---|---|---|
| S. aureus strains | |||
| JMB 1100 | Wild type, USA300_LAC (Erm sensitive), MRSA, CC8, SaeSL18 | LAC | 56 |
| RN4220 | Restriction minus, MSSA, CC8 | NCTC8325 | 57 |
| JMB 1467 | ΔsrrAB (SAUSA300_1441-42) | LAC | 71 |
| JMB 2047 | ΔsrrAB::tet | LAC | 72 |
| JMB 2078 | katA::Tn (ermB) (SAUSA300_1232) | LAC | V. Torres |
| JMB 1422 | Parent, Newman, MSSA, CC8, SaeSP18 | Newman | Eric Skaar and reference 73 |
| JMB 8263 | SaeSP18L chromosomal allelic replacement | Newman | 74 |
| JMB 7076 | saeS::Tn (ermB) | LAC | This work, BEI Resources, and reference 75 |
| JMB 7077 | saeS::Tn (ermB) | Newman | This work, BEI Resources, and reference 75 |
| JMB 7068 | ΔsrrAB saeS::Tn (ermB) | LAC | This work |
| JMB 6625 | atlA::Tn (ermB) | LAC | This work, BEI Resources, and reference 75 |
| JMB 6623 | ΔsaePQRS::spc atlA::Tn (ermB) | LAC | This work |
| JMB 6620 | aur::Tn (ermB) | LAC | This work, BEI Resources, and reference 75 |
| JMB 6618 | ΔsaePQRS::spc aur::Tn (ermB) | LAC | This work |
| JMB 6029 | menF::Tn (ermB) | LAC | This work, BEI Resources, and reference 75 |
| JMB 6219 | menF::Tn (tet) | LAC | 21 |
| JMB 7081 | saeS::Tn (ermB) menF::Tn (tet) | LAC | This work |
| JMB 7100 | saeR::Tn (ermB) | LAC | This work, BEI Resources, and reference 75 |
| JMB 7109 | saeR::Tn (ermB) menF::Tn (tet) | LAC | This work |
| JMB 6460 | ΔsrrAB::tet ΔsaePQRS::spc | LAC | This work |
| JMB 8403 | fnbA::Tn (ermB) | LAC | This work, BEI Resources, and reference 75 |
| JMB 1148 | ΔhptRS | LAC | 71 |
| JMB 1357 | ΔlytSR | LAC | 71 |
| JMB 1330 | graS::Tn (ermB) | LAC | 56 |
| JMB 1335 | ΔsaePQRS::spc | LAC | 31 |
| JMB 1219 | ΔSAUSA300_1219-1220 | LAC | 71 |
| JMB 1383 | ΔarlSR | LAC | 71 |
| JMB 1358 | ΔphoSR | LAC | 71 |
| JMB 1241 | ΔairSR | LAC | 71 |
| JMB 1377 | ΔvraSR | LAC | 71 |
| JMB 1333 | agr::Tn (ermB) | LAC | 76 |
| JMB 1223 | ΔkdpSR | LAC | 71 |
| JMB 1359 | ΔhssSR | LAC | 71 |
| JMB 1145 | ΔnreBC | LAC | 71 |
| JMB 1232 | ΔSAUSA300_2558-2559 | LAC | 71 |
| Other strains | |||
| Escherichia coli PX5 | Protein Express | ||
| Saccharomyces cerevisiae FY2 | William Belden |
MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
Growth model to assess biofilm formation.
Aerobic, overnight cultures were diluted into fresh TSB and incubated statically at 37°C. The cultures were grown in 96-well microtiter plates containing 200 μl in each well or 6-well plates containing 6 ml in each well. For aerobic growth, the plates were covered with an Aera seal (Excel Scientific), which allowed for uniform gas exchange. For anaerobic growth, cultures were inoculated aerobically, followed immediately by passage through an air lock (3 vacuum/gas exchange cycles) into a Coy anaerobic chamber equipped with a catalyst to maintain the oxygen concentrations below 1 ppm. Anaerobic growth in the presence of a terminal electron acceptor was achieved by supplementing the medium with sodium nitrate (prepared fresh daily) at the point of inoculation.
Static model of biofilm formation.
Biofilm formation was examined as described earlier, with minor changes (21, 44). Overnight cultures were diluted into fresh TSB to a final optical density (A590) of 0.05. Aliquots (200 μl) of diluted cultures were added to the wells of a 96-well microtiter plate (Corning 3268), and the plate was subsequently incubated statically at 37°C for 22 h. Prior to harvesting of the biofilm, the optical density (A590) of the cultures was determined. The plate was subsequently washed twice with water, biofilms were heat fixed at 60°C, and the plates were allowed to cool to room temperature. The biofilms were stained with 0.1% crystal violet, washed thrice with water, and destained with 33% acetic acid, and the absorbance of the resulting solution at 570 nm was recorded and standardized to an acetic acid blank and subsequently to the optical density of the culture upon harvest. Finally, the data were normalized with respect to those for the WT or as described in the figure legends to obtain relative biofilm formation.
Recombinant DNA and genetic techniques.
Escherichia coli DH5α was used as a cloning host for plasmid construction. All clones were passaged through S. aureus RN4220 (57) and subsequently transduced into the appropriate strains using bacteriophage 80α (58). All S. aureus mutant strains and plasmids were verified using PCR, sequencing of PCR products or plasmids (Genewiz, South Plainfield, NJ), or genetic/chemical complementation of phenotypes.
Construction of mutant strains and plasmids.
All plasmids utilized are listed in Table 2. The pCM28_saePQRS plasmid, containing saePQRS under the transcriptional control of the native promoter, was constructed using yeast recombinational cloning as previously described (59–61). The saePQRS alleles and the upstream promoter region were amplified from the LAC chromosome.
TABLE 2.
Plasmids used in this study
| Plasmid name | Insert locus or function | Source or reference |
|---|---|---|
| pCM28 | Insertless cloning vector | A. Horswill |
| pCM28_saePQRS | saePQRS-complementing vector | This work |
| pCM28_srrAB | srrAB-complementing vector | 72 |
| pOS-1 | Insertless cloning vector | Victor Torres |
| pOS_saeQRS P18 | Vector carrying a constitutively active SaeS allele cloned from Newman | Victor Torres |
| pTnTet | Construction of menF::Tn (Tet) | 77 |
| pJB141 | atlA-complementing vector | 39 |
| pJB122 | atlAAM H263A-complementing vector | 39 |
| pJB128 | Insertless cloning vector | 39 |
Quantitative real-time PCR assays.
Biofilms were cultured in the presence or absence of oxygen for 22 h. At the point of harvest, the spent medium was discarded, the remaining culture was immediately resuspended in RNAprotect reagent (Qiagen), and the suspension was treated according to the manufacturer's instructions. The treated culture was subjected to centrifugation, the supernatant was discarded, and the cell pellet was resuspended in RNase-free 50 mM Tris, pH 8. Cell extracts were generated as described earlier (44, 62). RNA was extracted using the TRIzol reagent per the manufacturer's instructions. Downstream treatments of the purified RNA and construction of cDNA libraries were as described earlier (62). Primers for PCR were designed manually or using Primer Express (version 3.0) software from Applied Biosystems (Table S1). Quantitative real-time PCRs were conducted as described earlier (62).
Quantification of high-molecular-mass eDNA.
Extracellular DNA (eDNA) was analyzed as described earlier (21). Overnight cultures were diluted into TSB to a final optical density (A600) of 0.05 in a final volume of 6 ml per well of a six-well plate. The cultures were incubated statically at 37°C for 22 h. At the point of harvest, the spent medium supernatant was aspirated out of each well. One milliliter of 1× phosphate-buffered saline (PBS) was immediately added to the wells, and a cell scraper was used to transfer the contents to an Eppendorf tube. The biomass was pelleted by centrifugation, and the supernatant was removed by aspiration. The pellets were thoroughly resuspended in 1× PBS and vortexed for 5 min using a Vortex Genie 2 mixer (Scientific Industries) at the highest speed possible and a vertical microtube adapter. Aliquots were removed for determination of the viable cell count (number of CFU), and samples were pelleted by centrifugation. Control experiments verified that the viable cell counts were not affected by the vortexing procedure (data not shown). Equal volumes of the supernatants were assessed for the presence of high-molecular-mass DNA (>10 kb) using agarose gel electrophoresis. To assess the extracellular DNA in a semiquantitative manner, the gels were photographed and the bands were subjected to density analysis using ImageJ software. For each sample, the spot densities were normalized to the viable cell count (number of CFU) and subsequently as mentioned in the figure legends.
Cytoplasmic protein release assays.
Strains were cultured as described above for the eDNA analyses (21). The samples were vortexed briefly, the biomass was transferred into a microcentrifuge tube, and cell pellets and spent medium supernatants were partitioned by centrifugation. The spent medium supernatant was retained for further analyses. The cell pellets were resuspended in lysis buffer (50 mM Tris, 150 mM NaCl, 4 μg lysostaphin, 8 μg DNase, pH 7.5) and incubated at 37°C until confluent lysis was observed. Cell lysates were clarified using centrifugation to obtain cell extracts. Catalase (Kat) activity in both the cell extracts and the spent medium supernatants was assayed as described elsewhere (44, 63). The ratio of extracellular to intracellular Kat activity was utilized to determine protein release. In control experiments, Kat activity was undetectable in a katA::Tn strain (data not shown).
Whole-cell autolysis assays.
Overnight cultures were diluted into TSB to a final optical density (A600) of 0.05 and cultured for 4 h (21). Whole-cell autolysis assays were conducted as described earlier (21). Briefly, the cultures were harvested by centrifugation, and the cell pellets were washed twice and resuspended in autolysis buffer (0.2 M sodium acetate, 150 mM NaCl, 0.01% Triton X-100, pH 5). The cell suspensions were then incubated at 37°C with shaking, and the optical densities were recorded periodically.
Murein hydrolase assays.
Biofilms were cultured for 4 h, and cells were harvested as mentioned above for the eDNA analyses (21). Thereafter, cell wall-associated protein extracts (CW extracts) were prepared and murein hydrolase activity was determined as described elsewhere, with minor changes (64). Briefly, cell pellets were washed and CW extracts were prepared by resuspension in 3 M lithium chloride and incubation for 25 min (64). The protein concentrations of the extracts were determined, and between 0.1 to 0.5 μg of an individual extract was combined with heat-killed cell substrates (optical density [A600], 0.35) in assay buffer (50 mM HEPES, 150 mM NaCl, 0.01% Triton X-100, pH 7.5). Samples were incubated with shaking at 37°C, and optical densities were recorded periodically.
Mouse model of S. aureus orthopedic implant biofilm infection.
A mouse model of S. aureus orthopedic implant infection was utilized as previously described (65–69). Male C57BL/6 mice (8 to 12 weeks old) were purchased from Charles River Laboratories (Frederick, MD), and studies were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council (70). The animal use protocol was approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. Briefly, mice were anesthetized with a ketamine (100 mg/kg of body weight; Hospira, Lake Forest, IL)-xylazine (5 mg/kg of body weight; Akorn, Decatur, IL) cocktail, and the surgical site was shaved and disinfected with povidone-iodine. A medial parapatellar arthrotomy with lateral displacement of the quadriceps-patella was performed to access the distal femur. Next, a burr hole was created in the femoral intercondylar notch extending into the intramedullary canal using a 26-gauge needle, whereupon a precut 0.8-cm-length, orthopedic-grade Kirschner (K) wire (diameter, 0.6 mm; nickel-titanium [Nitinol]; Custom Wire Technologies, Port Washington, WI) was inserted into the intramedullary canal, leaving approximately 1 mm protruding into the joint space. A total of 103 CFU of the S. aureus wild type, ΔsrrAB, or ΔsaePQRS strain was inoculated at the implant tip. Analgesia (buprenorphine [Buprenex] at 0.1 mg/kg subcutaneously; Reckitt Benckiser, Hull, UK) was administered immediately following infection and again 24 h later for pain relief. After this interval, all mice exhibited normal ambulation and no discernible pain behaviors.
Supplementary Material
ACKNOWLEDGMENTS
The J. M. Boyd lab is supported by Rutgers University, the Charles and Johanna Busch Foundation, and USDA MRF project NE-1028. A. A. Mashruwala is supported by the Douglas Eveleigh Fellowship from the Microbial Biology Graduate Program and an Excellence Fellowship from Rutgers University. The T. Kielian lab is supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases, grant P01 AI083211 (project 4 to T. Kielian). T. D. Scherr was supported by an American Heart Association predoctoral fellowship (14PRE20380400).
We thank William Belden for use of his real-time thermocycler. We thank Jeffrey Bose and Kenneth Bayles for kindly sharing the atlA plasmids and strains with us. We thank Alex Horswill, Ann Stock, Victor Torres, Eric Skaar, and Chia Lee for sharing S. aureus clinical isolates, strains, and plasmids.
A.A.M., J.M.B., T.K., C.M.G., and T.D.S. conceived and designed the experiments; A.A.M. acquired the data for all studies except the mouse studies; C.M.G. and T.D.S. acquired the data for the mouse studies; A.A.M. and J.M.B. wrote the original draft; A.A.M., J.M.B., and T.K. wrote, reviewed, and edited the manuscript; J.M.B. and T.K. obtained funding and acquired reagents and resources; and A.A.M., T.K., and J.M.B. supervised the study.
We declare that we do not have financial or nonfinancial competing interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00157-17.
REFERENCES
- 1.Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, Johnson SK, Vandenesch F, Fridkin S, O'Boyle C, Danila RN, Lynfield R. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 2.Graham PL III, Lin SX, Larson EL. 2006. A U.S. population-based survey of Staphylococcus aureus colonization. Ann Intern Med 144:318–325. doi: 10.7326/0003-4819-144-5-200603070-00006. [DOI] [PubMed] [Google Scholar]
- 3.Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci U S A 99:7687–7692. doi: 10.1073/pnas.122108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohara-Nemoto Y, Haraga H, Kimura S, Nemoto TK. 2008. Occurrence of staphylococci in the oral cavities of healthy adults and nasal oral trafficking of the bacteria. J Med Microbiol 57:95–99. doi: 10.1099/jmm.0.47561-0. [DOI] [PubMed] [Google Scholar]
- 5.Zafar U, Johnson LB, Hanna M, Riederer K, Sharma M, Fakih MG, Thirumoorthi MC, Farjo R, Khatib R. 2007. Prevalence of nasal colonization among patients with community-associated methicillin-resistant Staphylococcus aureus infection and their household contacts. Infect Control Hosp Epidemiol 28:966–969. doi: 10.1086/518965. [DOI] [PubMed] [Google Scholar]
- 6.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez Garcia M, De la Torre MA, Morales G, Pelaez B, Tolon MJ, Domingo S, Candel FJ, Andrade R, Arribi A, Garcia N, Martinez Sagasti F, Fereres J, Picazo J. 2010. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA 303:2260–2264. doi: 10.1001/jama.2010.757. [DOI] [PubMed] [Google Scholar]
- 8.Sass P, Berscheid A, Jansen A, Oedenkoven M, Szekat C, Strittmatter A, Gottschalk G, Bierbaum G. 2012. Genome sequence of Staphylococcus aureus VC40, a vancomycin- and daptomycin-resistant strain, to study the genetics of development of resistance to currently applied last-resort antibiotics. J Bacteriol 194:2107–2108. doi: 10.1128/JB.06631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedghizadeh PP, Kumar SK, Gorur A, Schaudinn C, Shuler CF, Costerton JW. 2009. Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. J Am Dent Assoc 140:1259–1265. doi: 10.14219/jada.archive.2009.0049. [DOI] [PubMed] [Google Scholar]
- 10.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 11.Costerton JW, Montanaro L, Arciola CR. 2005. Biofilm in implant infections: its production and regulation. Int J Artif Organs 28:1062–1068. [DOI] [PubMed] [Google Scholar]
- 12.Bispo PJ, Haas W, Gilmore MS. 2015. Biofilms in infections of the eye. Pathogens 4:111–136. doi: 10.3390/pathogens4010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joo HS, Otto M. 2012. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem Biol 19:1503–1513. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otto M. 2008. Staphylococcal biofilms. Curr Top Microbiol Immunol 322:207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. 2011. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogelberg KH, Konig M. 1993. Hypoxia of diabetic feet with abnormal arterial blood flow. Clin Invest 71:466–470. [DOI] [PubMed] [Google Scholar]
- 17.Arnold F, West D, Kumar S. 1987. Wound healing: the effect of macrophage and tumour derived angiogenesis factors on skin graft vascularization. Br J Exp Pathol 68:569–574. [PMC free article] [PubMed] [Google Scholar]
- 18.Cassat JE, Hammer ND, Campbell JP, Benson MA, Perrien DS, Mrak LN, Smeltzer MS, Torres VJ, Skaar EP. 2013. A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell Host Microbe 13:759–772. doi: 10.1016/j.chom.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammer ND, Reniere ML, Cassat JE, Zhang Y, Hirsch AO, Indriati Hood M, Skaar EP. 2013. Two heme-dependent terminal oxidases power Staphylococcus aureus organ-specific colonization of the vertebrate host. mBio 4:e00241-13. doi: 10.1128/mBio.00241-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cramton SE, Ulrich M, Gotz F, Doring G. 2001. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun 69:4079–4085. doi: 10.1128/IAI.69.6.4079-4085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mashruwala AA, van de Guchte A, Boyd JM. 2017. Impaired respiration elicits SrrAB-dependent programmed cell lysis and biofilm formation in Staphylococcus aureus. eLife 6:e23845. doi: 10.7554/eLife.23845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stock AM, Robinson VL, Goudreau PN. 2000. Two-component signal transduction. Annu Rev Biochem 69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 23.Stephenson K, Hoch JA. 2002. Two-component and phosphorelay signal-transduction systems as therapeutic targets. Curr Opin Pharmacol 2:507–512. doi: 10.1016/S1471-4892(02)00194-7. [DOI] [PubMed] [Google Scholar]
- 24.Voyich JM, Vuong C, DeWald M, Nygaard TK, Kocianova S, Griffith S, Jones J, Iverson C, Sturdevant DE, Braughton KR, Whitney AR, Otto M, DeLeo FR. 2009. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J Infect Dis 199:1698–1706. doi: 10.1086/598967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goerke C, Fluckiger U, Steinhuber A, Bisanzio V, Ulrich M, Bischoff M, Patti JM, Wolz C. 2005. Role of Staphylococcus aureus global regulators sae and sigmaB in virulence gene expression during device-related infection. Infect Immun 73:3415–3421. doi: 10.1128/IAI.73.6.3415-3421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho H, Jeong DW, Liu Q, Yeo WS, Vogl T, Skaar EP, Chazin WJ, Bae T. 2015. Calprotectin increases the activity of the SaeRS two component system and murine mortality during Staphylococcus aureus infections. PLoS Pathog 11:e1005026. doi: 10.1371/journal.ppat.1005026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novick RP, Jiang D. 2003. The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology 149:2709–2717. doi: 10.1099/mic.0.26575-0. [DOI] [PubMed] [Google Scholar]
- 28.Geiger T, Goerke C, Mainiero M, Kraus D, Wolz C. 2008. The virulence regulator Sae of Staphylococcus aureus: promoter activities and response to phagocytosis-related signals. J Bacteriol 190:3419–3428. doi: 10.1128/JB.01927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makgotlho PE, Marincola G, Schafer D, Liu Q, Bae T, Geiger T, Wasserman E, Wolz C, Ziebuhr W, Sinha B. 2013. SDS interferes with SaeS signaling of Staphylococcus aureus independently of SaePQ. PLoS One 8:e71644. doi: 10.1371/journal.pone.0071644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong DW, Cho H, Lee H, Li C, Garza J, Fried M, Bae T. 2011. Identification of the P3 promoter and distinct roles of the two promoters of the SaeRS two-component system in Staphylococcus aureus. J Bacteriol 193:4672–4684. doi: 10.1128/JB.00353-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nygaard TK, Pallister KB, Ruzevich P, Griffith S, Vuong C, Voyich JM. 2010. SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J Infect Dis 201:241–254. doi: 10.1086/649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mainiero M, Goerke C, Geiger T, Gonser C, Herbert S, Wolz C. 2010. Differential target gene activation by the Staphylococcus aureus two-component system SaeRS. J Bacteriol 192:613–623. doi: 10.1128/JB.01242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinhuber A, Goerke C, Bayer MG, Doring G, Wolz C. 2003. Molecular architecture of the regulatory locus sae of Staphylococcus aureus and its impact on expression of virulence factors. J Bacteriol 185:6278–6286. doi: 10.1128/JB.185.21.6278-6286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adhikari RP, Novick RP. 2008. Regulatory organization of the staphylococcal sae locus. Microbiology 154:949–959. doi: 10.1099/mic.0.2007/012245-0. [DOI] [PubMed] [Google Scholar]
- 35.Jeong DW, Cho H, Jones MB, Shatzkes K, Sun F, Ji Q, Liu Q, Peterson SN, He C, Bae T. 2012. The auxiliary protein complex SaePQ activates the phosphatase activity of sensor kinase SaeS in the SaeRS two-component system of Staphylococcus aureus. Mol Microbiol 86:331–348. doi: 10.1111/j.1365-2958.2012.08198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun F, Li C, Jeong D, Sohn C, He C, Bae T. 2010. In the Staphylococcus aureus two-component system Sae, the response regulator SaeR binds to a direct repeat sequence and DNA binding requires phosphorylation by the sensor kinase SaeS. J Bacteriol 192:2111–2127. doi: 10.1128/JB.01524-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baroja ML, Herfst CA, Kasper KJ, Xu SX, Gillett DA, Li J, Reid G, McCormick JK. 2016. The SaeRS two-component system is a direct and dominant transcriptional activator of toxic shock syndrome toxin 1 in Staphylococcus aureus. J Bacteriol 198:2732–2742. doi: 10.1128/JB.00425-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson ME, Nygaard TK, Ackermann L, Watkins RL, Zurek OW, Pallister KB, Griffith S, Kiedrowski MR, Flack CE, Kavanaugh JS, Kreiswirth BN, Horswill AR, Voyich JM. 2013. Staphylococcus aureus nuclease is an SaeRS-dependent virulence factor. Infect Immun 81:1316–1324. doi: 10.1128/IAI.01242-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bose JL, Lehman MK, Fey PD, Bayles KW. 2012. Contribution of the Staphylococcus aureus Atl AM and GL murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS One 7:e42244. doi: 10.1371/journal.pone.0042244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foulston L, Elsholz AK, DeFrancesco AS, Losick R. 2014. The extracellular matrix of Staphylococcus aureus biofilms comprises cytoplasmic proteins that associate with the cell surface in response to decreasing pH. mBio 5:e01667-14. doi: 10.1128/mBio.01667-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A 104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasztor L, Ziebandt AK, Nega M, Schlag M, Haase S, Franz-Wachtel M, Madlung J, Nordheim A, Heinrichs DE, Gotz F. 2010. Staphylococcal major autolysin (Atl) is involved in excretion of cytoplasmic proteins. J Biol Chem 285:36794–36803. doi: 10.1074/jbc.M110.167312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cosgrove K, Coutts G, Jonsson IM, Tarkowski A, Kokai-Kun JF, Mond JJ, Foster SJ. 2007. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J Bacteriol 189:1025–1035. doi: 10.1128/JB.01524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mashruwala AA, Bhatt S, Poudel S, Boyd ES, Boyd JM. 2016. The DUF59 containing protein SufT is involved in the maturation of iron-sulfur (FeS) proteins during conditions of high FeS cofactor demand in Staphylococcus aureus. PLoS Genet 12:e1006233. doi: 10.1371/journal.pgen.1006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oshida T, Sugai M, Komatsuzawa H, Hong YM, Suginaka H, Tomasz A. 1995. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-l-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc Natl Acad Sci U S A 92:285–289. doi: 10.1073/pnas.92.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houston P, Rowe SE, Pozzi C, Waters EM, O'Gara JP. 2011. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect Immun 79:1153–1165. doi: 10.1128/IAI.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlag M, Biswas R, Krismer B, Kohler T, Zoll S, Yu W, Schwarz H, Peschel A, Gotz F. 2010. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol Microbiol 75:864–873. doi: 10.1111/j.1365-2958.2009.07007.x. [DOI] [PubMed] [Google Scholar]
- 48.Biswas R, Martinez RE, Gohring N, Schlag M, Josten M, Xia G, Hegler F, Gekeler C, Gleske AK, Gotz F, Sahl HG, Kappler A, Peschel A. 2012. Proton-binding capacity of Staphylococcus aureus wall teichoic acid and its role in controlling autolysin activity. PLoS One 7:e41415. doi: 10.1371/journal.pone.0041415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, Cheung AL, Finerman GA, Lieberman JR, Adams JS, Miller LS. 2010. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One 5:e12580. doi: 10.1371/journal.pone.0012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Q, Cho H, Yeo WS, Bae T. 2015. The extracytoplasmic linker peptide of the sensor protein SaeS tunes the kinase activity required for staphylococcal virulence in response to host signals. PLoS Pathog 11:e1004799. doi: 10.1371/journal.ppat.1004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mascher T. 2014. Bacterial (intramembrane-sensing) histidine kinases: signal transfer rather than stimulus perception. Trends Microbiol 22:559–565. doi: 10.1016/j.tim.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Moormeier DE, Bose JL, Horswill AR, Bayles KW. 2014. Temporal and stochastic control of Staphylococcus aureus biofilm development. mBio 5:e01341-14. doi: 10.1128/mBio.01341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 55.Melter O, Radojevic B. 2010. Small colony variants of Staphylococcus aureus—review. Folia Microbiol (Praha) 55:548–558. doi: 10.1007/s12223-010-0089-3. [DOI] [PubMed] [Google Scholar]
- 56.Boles BR, Thoendel M, Roth AJ, Horswill AR. 2010. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 5:e10146. doi: 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kreiswirth BN, Lofdahl S, Betley MJ, O'Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 58.Novick RP. 1991. Genetic systems in staphylococci. Methods Enzymol 204:587–636. doi: 10.1016/0076-6879(91)04029-N. [DOI] [PubMed] [Google Scholar]
- 59.Mashruwala AA, Boyd JM. 2016. De novo assembly of plasmids using yeast recombinational cloning. Methods Mol Biol 1373:33–41. doi: 10.1007/7651_2015_275. [DOI] [PubMed] [Google Scholar]
- 60.Joska TM, Mashruwala A, Boyd JM, Belden WJ. 2014. A universal cloning method based on yeast homologous recombination that is simple, efficient, and versatile. J Microbiol Methods 100:46–51. doi: 10.1016/j.mimet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mashruwala AA, Roberts CA, Bhatt S, May KL, Carroll RK, Shaw LN, Boyd JM. 2016. Staphylococcus aureus SufT: an essential iron-sulphur cluster assembly factor in cells experiencing a high-demand for lipoic acid. Mol Microbiol 102:1099–1119. doi: 10.1111/mmi.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mashruwala AA, Pang YY, Rosario-Cruz Z, Chahal HK, Benson MA, Mike LA, Skaar EP, Torres VJ, Nauseef WM, Boyd JM. 2015. Nfu facilitates the maturation of iron-sulfur proteins and participates in virulence in Staphylococcus aureus. Mol Microbiol 95:383–409. doi: 10.1111/mmi.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beers RF Jr, Sizer IW. 1952. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140. [PubMed] [Google Scholar]
- 64.Mani N, Tobin P, Jayaswal RK. 1993. Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J Bacteriol 175:1493–1499. doi: 10.1128/jb.175.5.1493-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scherr TD, Lindgren KE, Schaeffer CR, Hanke ML, Hartman CW, Kielian T. 2014. Mouse model of post-arthroplasty Staphylococcus epidermidis joint infection. Methods Mol Biol 1106:173–181. doi: 10.1007/978-1-62703-736-5_16. [DOI] [PubMed] [Google Scholar]
- 66.Heim CE, Vidlak D, Kielian T. 2015. Interleukin-10 production by myeloid-derived suppressor cells contributes to bacterial persistence during Staphylococcus aureus orthopedic biofilm infection. J Leukoc Biol 98:1003–1013. doi: 10.1189/jlb.4VMA0315-125RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heim CE, Vidlak D, Scherr TD, Hartman CW, Garvin KL, Kielian T. 2015. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J Immunol 194:3861–3872. doi: 10.4049/jimmunol.1402689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heim CE, Vidlak D, Scherr TD, Kozel JA, Holzapfel M, Muirhead DE, Kielian T. 2014. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J Immunol 192:3778–3792. doi: 10.4049/jimmunol.1303408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scherr TD, Hanke ML, Huang O, James DB, Horswill AR, Bayles KW, Fey PD, Torres VJ, Kielian T. 2015. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. mBio 6:e01021-15. doi: 10.1128/mBio.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 71.Pang YY, Schwartz J, Bloomberg S, Boyd JM, Horswill AR, Nauseef WM. 2014. Methionine sulfoxide reductases protect against oxidative stress in Staphylococcus aureus encountering exogenous oxidants and human neutrophils. J Innate Immun 6:353–364. doi: 10.1159/000355915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mashruwala AA, Boyd JM. 2017. The Staphylococcus aureus SrrAB regulatory system modulates hydrogen peroxide resistance factors, which imparts protection to aconitase during aerobic growth. PLoS One 12:e0170283. doi: 10.1371/journal.pone.0170283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duthie ES, Lorenz LL. 1952. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol 6:95–107. [DOI] [PubMed] [Google Scholar]
- 74.Luong TT, Sau K, Roux C, Sau S, Dunman PM, Lee CY. 2011. Staphylococcus aureus ClpC divergently regulates capsule via sae and codY in strain Newman but activates capsule via codY in strain UAMS-1 and in strain Newman with repaired saeS. J Bacteriol 193:686–694. doi: 10.1128/JB.00987-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, Horswill AR. 2011. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One 6:e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bose JL, Fey PD, Bayles KW. 2013. Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl Environ Microbiol 79:2218–2224. doi: 10.1128/AEM.00136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.