ABSTRACT
Chlamydiae colonize the gastrointestinal tracts of both animals and humans. However, their medical significance remains unknown. We have previously shown that wild-type Chlamydia muridarum spreads to and establishes stable colonization of the gastrointestinal tract following intravaginal inoculation. In the present study, we found that C. muridarum with mutations in chromosomal genes tc0237 and/or tc0668 was defective in spreading to the mouse gastrointestinal tract, which correlated with its attenuated pathogenicity in the upper genital tract. This correlation was more consistent than that of chlamydial pathogenicity with ascending infection in the genital tract, since attenuated C. muridarum spread significantly less to the gastrointestinal tract but maintained robust ascending infection of the upper genital tract. Transcervical inoculation further confirmed the correlation between C. muridarum spreading to the gastrointestinal tract and its pathogenicity in the upper genital tract. Finally, defective spreading of C. muridarum mutants was due to their inability to colonize the gastrointestinal tract since intragastric inoculation did not rescue the mutants' colonization. Thus, promoting C. muridarum colonization of the gastrointestinal tract may represent a primary function of the TC0237 and TC0668 proteins. Correlation of chlamydial colonization of the gastrointestinal tract with chlamydial pathogenicity in the upper genital tract suggests a potential role for gastrointestinal chlamydiae in genital tract pathogenicity.
KEYWORDS: Chlamydia muridarum, mutations in tc0668 or tc0237, gut colonization, attenuation, hydrosalpinx, chlamydia, TC0237, TC0668, mutants, pathogenicity
INTRODUCTION
Sexually transmitted infection with Chlamydia trachomatis, if not treated appropriately, may lead to pathology such as hydrosalpinx, resulting in tubal infertility in women (1–3). Chlamydia muridarum infection of the mouse genital tract can cause hydrosalpinx and infertility (4–6), which has been used to investigate the mechanisms of C. trachomatis pathogenesis (7–12). Murine-model-based investigations have led to the conclusion that both chlamydial ascension to the upper genital tract (13–17) and tubal inflammation (7, 11, 18–24) may be required for chlamydial induction of hydrosalpinx. However, C. muridarum infections of the genital tracts of female mice are self-limited (cleared from the entire genital tract within 4 to 6 weeks) while the resultant upper genital tract pathologies such as tubal fibrosis and hydrosalpinx can be long lasting (when examined 8 to 10 weeks later) (6, 16, 25, 26). The question is how hydrosalpinx-causing tubal inflammation is maintained after a genital tract chlamydial infection is resolved.
C. muridarum is known to last for long periods of time in the mouse gastrointestinal (GI) tract (27–30). When C. muridarum was introduced into mouse mucosae, it readily colonized the GI tract (28). The genital tract C. muridarum organisms efficiently spread to and established long-lasting colonization of the GI tract (26), suggesting a potential link between long-lasting C. muridarum infection of the GI tract and long-term C. muridarum-induced pathology in the upper genital tract. Spreading was independent of the oral-fecal route since singly housed mice restrained from taking up mouse secretions still showed C. muridarum spreading from the genital to the GI tract (26, 28). A hematogenous route might mediate the spreading because C. muridarum survived in the blood and hematogenous C. muridarum established long-lasting colonization restricted to the GI tract (31). In addition, C. muridarum failed to autoinoculate the genital tract after colonization of the GI tract of the same mouse for 70 days (32), suggesting that GI tract C. muridarum may have to use an indirect mechanism to impact chlamydial pathogenicity in the genital tract. However, it is worth noting that complex human behaviors may provide many pathways for C. trachomatis to spread between the GI and genital tracts. It has been proposed that long-term colonization by chlamydial organisms in the GI tract may serve as a reservoir for autoinoculation of the genital tract (30, 33). Thus, there may be multiple pathways/mechanisms for GI tract chlamydiae to potentially promote pathogenicity in the upper genital tract.
We previously demonstrated that C. muridarum clones carrying mutations in the chromosomal genes tc0237 and/or tc0668 were highly attenuated in the induction of hydrosalpinx in the mouse upper genital tract (14, 15). However, these C. muridarum mutants maintained similar live-organism shedding courses in the lower genital tract. The precise mechanisms of the attenuation remain unknown. In the present study, we simultaneously monitored both vaginal and rectal swabs of mice infected with these mutants. The attenuated C. muridarum mutants consistently decreased their spreading to the GI tract but maintained robust ascension to the upper genital tract. Transcervical inoculation for further promotion of ascending infection failed to enhance either the mutants' pathogenicity in the upper genital tract or their spreading to the GI tract, further confirming the correlation of chlamydial pathogenicity in the upper genital tract with its spreading to the GI tract. The deficient spreading of C. muridarum mutants to the GI tract is likely due to their reduced ability to colonize the GI tract since direct intragastric inoculation failed to rescue their colonization. Defective colonization of the GI tract may represent a primary phenotype of these C. muridarum mutants, which correlates with their attenuated pathogenicity in the genital tract. Thus, we have presented the first experimental evidence both demonstrating a primary function of TC0237 and TC0668 in facilitation of chlamydial colonization of the GI tract and linking chlamydial colonization of the GI tract to chlamydial pathogenicity in the upper genital tract. The present study has provided a foundation for further investigation of the mechanisms by which GI tract chlamydiae may promote chlamydial pathogenicity in the genital tract.
RESULTS
C. muridarum mutants that fail to induce hydrosalpinx are defective in spreading to the GI tract following intravaginal inoculation.
We previously reported that a series of C. muridarum mutants were highly attenuated in induction of hydrosalpinx in the mouse upper genital tract (14, 15). However, the mechanisms of attenuation remained unknown. In the present study, we evaluated whether these C. muridarum mutants could spread to the GI tract since wild-type C. muridarum that induced hydrosalpinx effectively spread from the mouse genital tract to the GI tract (26). When evaluated in C3H/HeJ mice (Fig. 1), all of our mutants displayed a reduced ability to induce hydrosalpinx. A significant reduction was found in mutant clone G13.11.1, which carries a premature termination codon in tc0668 (designated a single mutant) and two clones (G28.54.1 and G28.51.1) each with mutations in both tc0237 and tc0668 (double mutants). We then compared their shedding courses in both the genital and GI tracts (Fig. 2). We found that both wild-type and mutant C. muridarum displayed similar courses of live-organism shedding from the lower genital tract regardless of their genotypes, confirming previous findings (15). However, all of our C. muridarum mutants showed decreased live-organism shedding in rectal swabs. Live C. muridarum organisms became detectable in the rectal swabs of mice infected with wild-type C. muridarum as early as day 3 after intravaginal inoculation and reached significant levels on day 7, while single mutants became detectable in rectal swabs until day 14 (mutants G13.59.2 and G13.11.1 with a substitution mutation and premature termination in TC0668, respectively) or 21 (mutant G40.50.2 with a substitution mutation in TC0237). The overall rectal shedding courses of C. muridarum single mutants were significantly decreased compared to that of wild-type C. muridarum. These single mutants also induced lower levels of hydrosalpinx, although a significant reduction was found with G13.11.1 only. These observations suggest a correlation between reduced spreading to the GI tract and decreased induction of hydrosalpinx. This correlation is strengthened by the observation that mice intravaginally infected with the double mutant G28.54.1 or G28.51.1 did not shed any significant live organisms in their rectal swabs and failed to show any significant hydrosalpinx. Statistically, the Spearman coefficient of correlation between rectal swab inclusion-forming unit (IFU) counts and hydrosalpinx scores was 0.6353, while that between the vaginal swab IFU counts and hydrosalpinx scores was 0.076 (see Table S1 in the supplemental material). The two coefficients were significantly different when measurements from individual mice were compared (see Table S1 and the paragraph on statistical analyses in Materials and Methods for more information).
FIG 1.
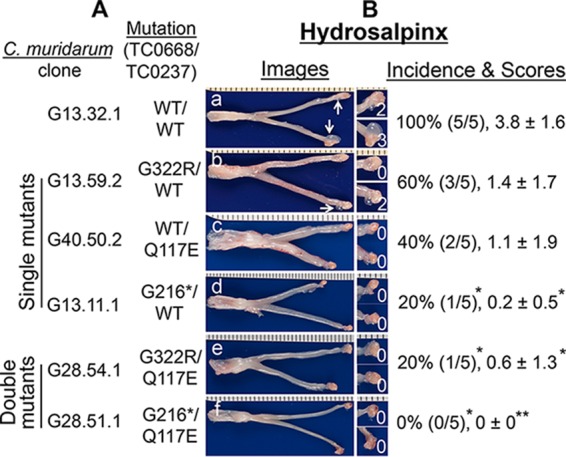
Ability of C. muridarum with or without mutations in tc0668 and/or tc0237 to induce hydrosalpinx in C3H/HeJ mice following intravaginal inoculation. (A) Wild-type (WT) C. muridarum (clone G13.32.1 [TC0668wt/TC0237wt], n = 5, image a) or C. muridarum with a substitution mutation in tc0668 (G13.59.2 [TC0668G322R], n = 5, image b) or tc0237 (G40.50.2 [TC0237Q117E], n = 5, image c), a premature stop codon in tc0668 (G13.11.1 [TC0668G216*], n = 5, image d), substitution mutations in both tc0668 and tc0237 (G28.54.1 [TC0668G322R/TC0237Q117E], n = 5, image e), or a premature stop codon in tc0668 plus the tc0237 substitution mutation (G28.51.1 [TC0668G216*/TC0237Q117E], n = 5, image f) was intravaginally inoculated into female C3H/HeJ mice at 2 × 105 IFUs per mouse. (B) All mice were sacrificed for evaluation of upper genital tract hydrosalpinx pathology (as described in Materials and Methods) on day 56 after inoculation. One representative genital tract image was chosen from each group. The entire genital tract is displayed with the vagina on the left and the oviduct/ovaries on the right. Oviducts with hydrosalpinges are indicated by white arrows. The oviduct/ovary from each side is shown magnified on the right. The hydrosalpinx scores are shown in the magnified images. Hydrosalpinx was scored for severity, and mice with hydrosalpinx were counted for calculation of the incidence rate in each group. Note that all of our mutant C. muridarum organisms produced reduced hydrosalpinx severity and incidence, with a significant reduction for clones G13.11.1, G28.54.1, and G28.51.1 (*, P < 0.05; **, P < 0.01; Fisher's exact test for incidence and Wilcoxon rank sum test for score comparison).
FIG 2.
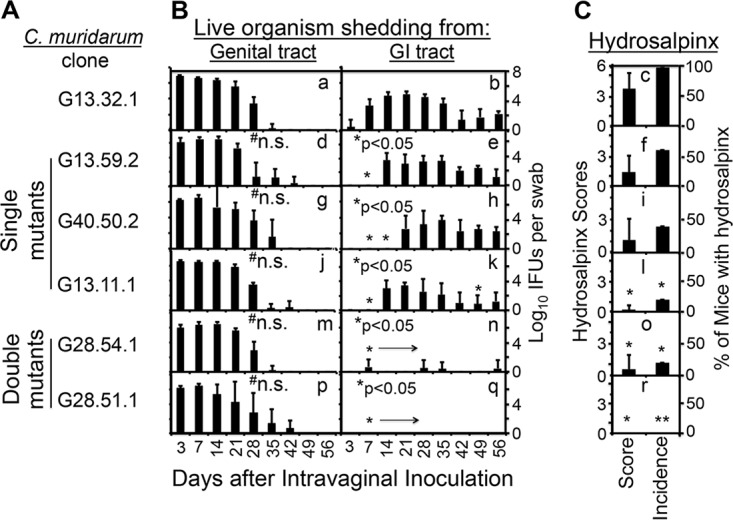
Abilities of C. muridarum with or without mutations in tc0668 and/or tc0237 to spread to the GI tract and induce hydrosalpinx in the genital tract. (A) The same C. muridarum organisms were used to infect C3H/HeJ mice as described in the legend to Fig. 1. (B) At various time points postinoculation, as indicated on the x axis, both vaginal (graphs a, d, g, j, m, and p) and rectal (graphs b, e, h, k, n, and q) swabs were taken for titration of live organisms from the genital and GI tracts, respectively, and the recovery of live organisms is expressed as log10 IFU counts per swab, as displayed on the y axis. (C) The hydrosalpinx score and incidence data are from Fig. 1. Note that although wild-type and mutant C. muridarum organisms displayed similar courses of live-organism shedding from the mouse genital tract, mutant C. muridarum organisms developed significantly delayed/reduced courses of shedding from the same mouse GI tracts, the latter of which correlated with reduced hydrosalpinx severity and incidence. n.s. stands for not significant compared to the wild-type clone, *, P < 0.05; **, P < 0.01 (for pathology comparison, see the legend to Fig. 1; for IFU comparison, b versus e, h, k, n, or q, area under the curve, or individual time points, Wilcoxon rank sum test).
C. muridarum mutants maintain robust ascending infection despite their reduced pathogenicity in the upper genital tract and defective spreading to the GI tract.
Since C. muridarum ascending infection to the upper genital tract is necessary for C. muridarum to induce hydrosalpinx, we next evaluated whether C. muridarum mutants were defective in ascending infection. We compared the live-organism loads of wild-type and attenuated C. muridarum mutant clones in different mouse organs and tissue sections from both the genital and GI tracts of C3H/HeJ mice on day 14 after intravaginal inoculation (Fig. 3). Wild-type organisms spread to most mouse tissues, including both the GI tract and non-GI tract organs/tissues in addition to the genital tract tissues, which is consistent with previous observations (26, 28). Interestingly, the attenuated mutant clones all achieved robust ascending infection to the upper genital tract tissues, including the uterine horn and oviduct/ovary. There were no significant differences in the yields of live organisms recovered from the upper genital tract tissues of mice infected with wild-type C. muridarum versus any of the attenuated C. muridarum mutants. Thus, the attenuated pathogenicity of the mutants in the upper genital tract was unlikely to have been caused by a lack of adequate ascending infection. On the other hand, the loads of attenuated C. muridarum mutants were consistently lighter in the GI tract tissues. The loads of two single mutants were consistently lighter in the small intestine, cecum, and colon tissues. More dramatically, no live organisms were detected in the GI tracts of mice infected with the double mutant G28.51.1. Clearly, the significantly reduced or lack of recovery of live organisms from the GI tract tissues correlated with attenuated pathogenicity in the upper genital tract. As a control, both the wild-type and attenuated C. muridarum clones were recovered at similar levels from the kidney tissues of the same mice, suggesting that reduced spreading of mutants to the GI tract was a specific but not a random event. A further comparison of the coefficients of correlation between C. muridarum pathogenicity (expressed as a hydrosalpinx index, which is equal to the group's mean hydrosalpinx score times the same group's hydrosalpinx incidence; pathology data from Fig. 1) and C. muridarum live-organism loads in different tissues revealed a stronger correlation of C. muridarum pathogenicity with live organisms recovered from the GI tract tissues than any other tissues, including upper genital tract tissues. We further evaluated the GI tract spreading of attenuated C. muridarum mutant G13.11.1 following transcervical inoculation (Fig. 4). We found that even with enhanced ascending infection, G13.11.1 still showed significantly reduced spreading to the GI tract. The enhanced ascending infection did not restore the spreading of the attenuated C. muridarum to the GI tract to the level of wild-type C. muridarum.
FIG 3.
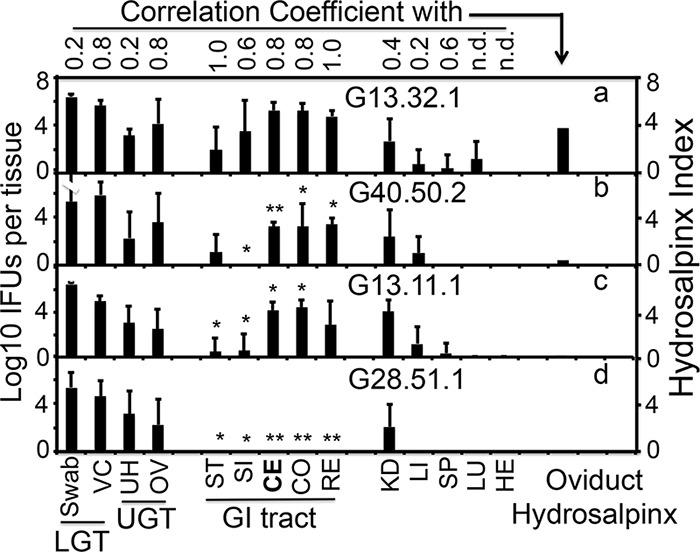
Abilities of C. muridarum with or without mutations in tc0668 and/or tc0237 to ascend to the upper genital tract and spread to tissues outside the genital tract following intravaginal inoculation. Wild-type G13.32.1 (n = 7; panel a) or mutant C. muridarum G40.50.2 (n = 5; panel b), G13.11.1 (n = 7; panel c), or G28.51.1 (n = 5; panel d) was intravaginally inoculated into female C3H/HeJ mice as described in the legend to Fig. 1. On day 14 after inoculation, mice were sacrificed for harvesting of genital tract tissues, which were divided into the vagina/cervix (VC), uterine horn (UH), and oviduct/ovary (OV), and GI tract tissues, which were divided into the stomach (ST), small intestine (SI), cecum (CE), colon (CO), rectum (RE), kidneys (KD), liver (LI), spleen (SP), lungs (LU), and heart (HE), as displayed on the x axis, for measurement of live organisms. Vaginal swabs taken prior to mouse sacrifice and VC samples were designated the lower genital tract (LGT), while UH and OV samples were designated the upper genital tract (UGT). Live-organism titers, expressed as log10 IFU counts per tissue sample, are displayed on the y axis. The hydrosalpinx data displayed in the last column for analysis are from Fig. 1. IFU counts from each tissue type of mutant-infected mice were compared with those from the corresponding tissues of wild-type C. muridarum-infected mice. The IFU counts recovered from most tissues of the GI tract but not the non-GI tract tissues of mutant C. muridarum-infected mice were significantly lower than those recovered from wild-type C. muridarum-infected mice (*, P < 0.05; **, P < 0.01; a versus b, c, or d, Wilcoxon rank sum test). The IFU counts recovered from the GI tract tissues positively correlated with reduced hydrosalpinges in mutant C. muridarum-infected mice (Spearman rank-order correlation; the corresponding coefficients are shown at the top).
FIG 4.
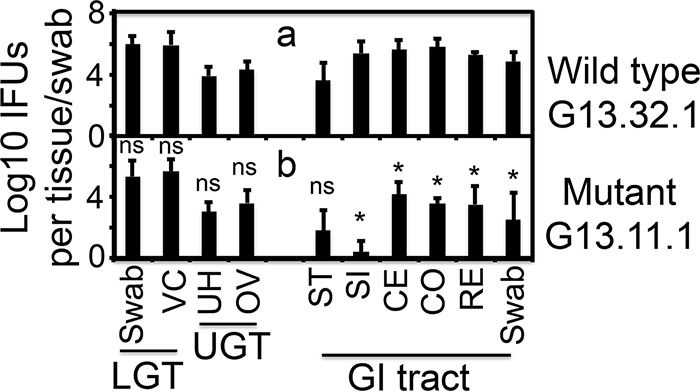
Abilities of C. muridarum with or without mutations in tc0668 to ascend to the oviduct and spread to GI tract tissues following transcervical inoculation. The wild-type (G13.32.1, n = 4, panel a) or attenuated mutant (G13.11.1, n = 4, c) C. muridarum clone was transcervically inoculated into female C3H/HeJ mice. On day 14 after inoculation, mice were sacrificed for harvesting of genital tract tissues, which were divided into the vagina/cervix (VC), uterine horn (UH), and oviduct/ovary (OV), and GI tract tissues, which were divided into the stomach (ST), small intestine (SI), cecum (CE), colon (CO), and anus-rectum (RE), as displayed on the x axis, for measurement of live organisms. Vaginal and rectal swabs taken prior to mouse sacrifice were also monitored. Live-organism titers, expressed as log10 IFU counts per tissue sample, are displayed on the y axis. Live organisms from vaginal swabs along with VC are designated the lower genital tract (LGT), while those from UH and OV are designated the upper genital tract (UGT). *, P < 0.05 (a versus b in corresponding tissues/swabs, Wilcoxon rank sum test); ns, not significant.
The correlation of C. muridarum pathogenicity in the upper genital tract with its spreading to the GI tract is reproduced in C57BL/6J mice.
The above-described experiments done to demonstrate the correlation of C. muridarum pathogenicity in the upper genital tract with its spreading to the GI tract were all done with C3H/HeJ mice. We next evaluated the correlation in C57BL/6J mice (Fig. 5 and 6). Since C. muridarum spreading from the genital to the GI tract was not affected by enhanced ascending infection (see above), we used transcervical inoculation in the current experiment. The purpose of using transcervical infection here was to minimize the impact of ascending infection on chlamydial induction of hydrosalpinx (6). In this way, we can evaluate whether, with sufficient ascending infection for all of our C. muridarum clones, the mutant C. muridarum clones are still defective in both spreading to the GI tract and induction of hydrosalpinx in the upper genital tract. We found that all three C. muridarum clones developed similar courses of live-organism shedding from vaginal swabs regardless of their genotype (Fig. 6). However, the single mutant C. muridarum G13.11.1 showed significantly reduced shedding from rectal swabs while the double mutant G28.51.1 completely failed to develop any significant live-organism shedding from rectal swabs. These shedding phenotypes are consistent with those observed in intravaginally infected C3H/HeJ mice. More importantly, the two mutants showed a significantly reduced ability to induce hydrosalpinx (Fig. 5). Thus, we have reproduced the correlation of C. muridarum pathogenicity in the upper genital tract with its spreading to the GI tract in C57BL/6J mice.
FIG 5.
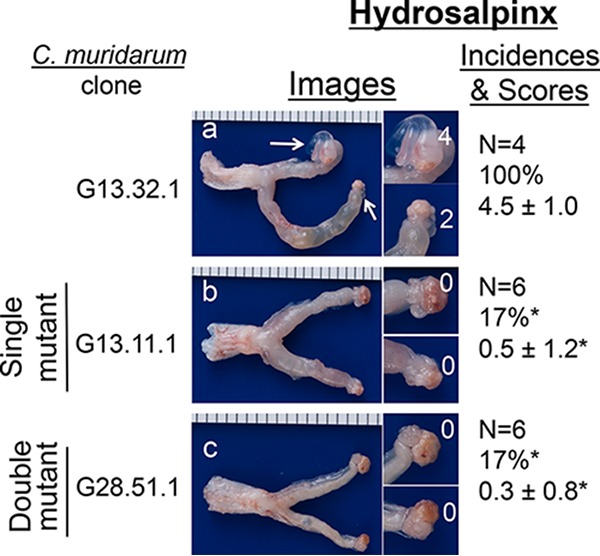
Ability of C. muridarum with or without mutations in tc0668 and/or tc0237 to induce hydrosalpinx in C57BL/6J mice following transcervical inoculation. As shown on the left side, wild-type (G13.32.1, n = 4, panel a) or mutant (G13.11.1, n = 6, b; G28.51.1, n = 6, c) C. muridarum organisms were inoculated into female C57BL/6J mice at 2 × 105 IFUs/mouse via transcervical inoculation. All mice were sacrificed for evaluation of upper genital tract hydrosalpinx pathology (as described in Materials and Methods) on day 63 after inoculation. One representative genital tract image was chosen from each group. The entire genital tract is displayed, with the vagina on the left and the oviduct/ovaries on the right. Oviducts with hydrosalpinges are indicated by white arrows. The magnified oviduct/ovary from each side is shown on the right. The hydrosalpinx scores are shown in the magnified images. Hydrosalpinx severity was scored, and mice with hydrosalpinx were counted for calculation of the incidence rate in each group. Note that the hydrosalpinx severity and incidence produced by both mutant C. muridarum organisms were significantly reduced (*, P < 0.05; Fisher's exact test for incidence and Wilcoxon rank sum test for score comparison).
FIG 6.
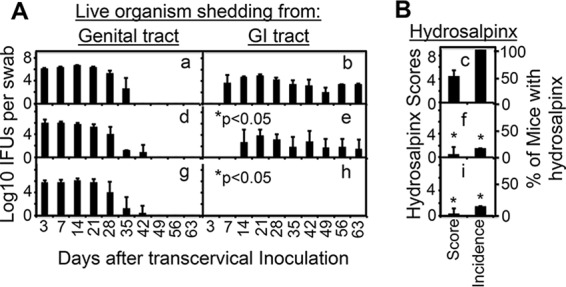
Comparison of C. muridarum with or without mutations in tc0668 and/or tc0237 for live-organism recovery from vaginal and rectal swabs and upper genital tract pathology following transcervical inoculation. The same C. muridarum organisms were used to infect C57BL/6J mice via transcervical inoculation as described in the legend to Fig. 5. (A) At various time points postinoculation, as indicated on the x axis, both vaginal (graphs a, d, and g) and rectal (graphs b, e, and h) swabs were taken for titration of live organisms from the genital and GI tracts, respectively, and live-organism recovery is expressed as log10 IFU counts per swab, as displayed on the y axis. (B) The hydrosalpinx data were obtained on day 63 as described in the legend to Fig. 5. Note that although both WT and mutant C. muridarum organisms displayed similar courses of live-organism shedding from the genital tract, mutant C. muridarum developed significantly delayed/reduced courses of shedding from the same mouse GI tracts, the latter of which correlated with reduced hydrosalpinx severity and incidence. *, P < 0.05 (Wilcoxon rank sum test for IFU comparison in graph b versus graph e or h; see the legend to Fig. 5 for a pathology comparison).
C. muridarum mutants are unable to colonize the GI tract.
To determine whether the inefficient spreading of C. muridarum mutants to the GI tract was due to an inability of the mutants to colonize the GI tract, we compared C. muridarum with or without mutations for colonization of the mouse GI tract following intragastric inoculation (Fig. 7). Wild-type C. muridarum organisms developed a robust time course of live-organism shedding in rectal swabs but without any significant spreading to the genital tract, which is consistent with a previous observation that GI tract C. muridarum organisms do not autoinoculate the genital tract of the same mouse (32). Importantly, with the same inoculation dose (2 × 105 IFUs), the two single mutants (G40.50.2 and G13.11.1) developed significantly delayed/reduced courses of shedding from the GI tract. Furthermore, the double mutant G28.51.1 completely failed to shed any detectable live organisms. This lack of colonization of the GI tract by G28.51.1 was maintained even at an inoculation dose of 1 × 107 IFUs per mouse. These observations have demonstrated that C. muridarum mutants are defective in colonization of the mouse GI tract, suggesting that the reduced or lack of spreading to the GI tract by the mutants is due to a reduced or lack of ability to colonize the GI tract.
FIG 7.
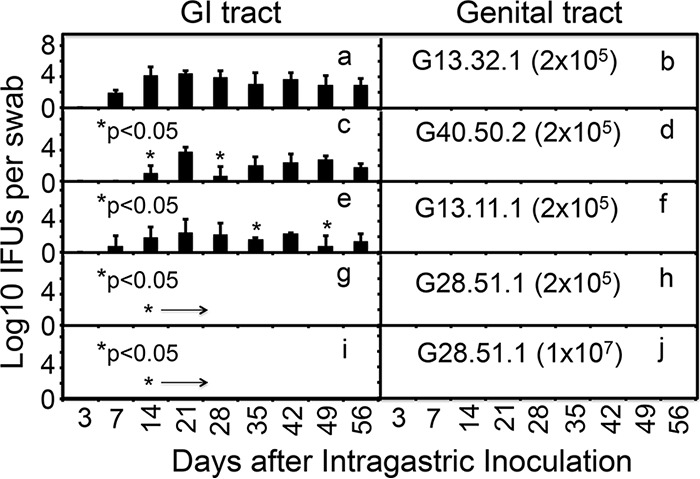
Ability of C. muridarum with or without mutations in tc0668 and/or tc0237 to colonize the mouse GI tract following intragastric inoculation. Wild-type (G13.32.1, n = 5, panels a and b) or mutant (G40.50.2, n = 5, panels c and d; G13.11.1, n = 5, panels e and f; G28.51.1, n = 5, panels g and j) C. muridarum organisms were inoculated intragastrically into female C57BL/6J mice at 2 × 105 IFUs (panels a to h) or 1 × 107 IFUs (panels i and j) per mouse. At various time points postinoculation, as indicated on the x axis, rectal (left panels) and vaginal (right panels) swabs were taken for titration of live C. muridarum organisms. The recovery of live organisms is expressed as log10 IFUs per swab, as displayed on the y axis. Note that C. muridarum organisms with single mutations in either tc0237 (G40.50.2) or tc0668 (G13.11.1) developed significantly delayed/reduced courses of shedding from the mouse GI tract at an inoculation dose of 1 × 105 IFUs per mouse (panels c or e; *, P < 0.05, area under the curve or individual time points, Wilcoxon rank sum test), while the double mutant (G28.51.1) failed to shed any detectable live organisms even at an inoculation dose of 1 × 107 IFUs per mouse (panel I; P < 0.01, area under the curve, Wilcoxon rank sum test). No significant live organisms were detected in any of the vaginal swabs.
To further determine whether C. muridarum mutants developed compensatory mutations during their replication in mouse tissues, we isolated C. muridarum G13.11.1 organisms from mouse cecum/colon tissues on day 56 after intragastric inoculation and G28.51.1 organisms from uterine horn/oviduct tissues on day 28 after intravaginal inoculation (no live G28.51.1 organisms can be recovered from the GI tract). Analyses of the next-generation sequencing (NGS) reads from both the G13.11.1 and G28.51.1 mixtures revealed that four mutations in G13.11.1 and three in G28.51.1 reached >5% of the population but all were <10% (Table S2). Since we have previously defined the threshold of this method as 5% (15), we further analyzed the seven mutations that are >5%. Of the four mutations accumulated in G13.11.1 genomes, three are in the intergenic region and the remaining one is located in the coding region of hypothetical protein TC0268. This nonsense mutation caused truncation of the protein at the 233rd tryptophan residue. A plaque-purified clone carrying this mutation but isogenic to its parental G13.11.1 clone was isolated and designated G13.11.1a (Table S2). Of the three mutations accumulated in G28.51.1 genomes, one was in the intergenic region, the second was a silent mutation in the coding sequence for OmpA, and the third was a missense mutation in the coding sequence for the FliI flagellum ATP synthase, resulting in a G203V substitution mutation. A plaque-purified clone carrying this mutation but isogenic to its parental G28.51.1 clone was isolated and designated G28.51.1a (Table S2). The analysis results indicate that the mutations did accumulate in the genomes during G13.11.1 and G28.51.1 replication in mouse cecum/colon and uterine/oviduct tissues, respectively. However, these mutations might not affect the defective colonization phenotype of either G13.11.1 or G28.51.1 after a primary inoculation. This is because the defective colonization of either the GI or genital tract by G13.11.1 or G28.51.1 was not altered during the courses of the corresponding experiments. To further test whether these new mutations can affect the C. muridarum phenotype when they occurred in 100% of the population, we compared the G13.11.1a and G28.51.1a variants with the corresponding parental clones, respectively, for colonization of both the genital and GI tracts (Fig. 8). We found that the variants (G13.11.1a and G28.51.1a) behaved exactly the same as their parental clones, regardless of the route used for inoculation and the samples detected. Thus, it is unlikely that compensatory mutations accumulated in the genomes of C. muridarum mutants after a primary inoculation. Nevertheless, it is possible that compensatory mutations may occur and be enriched by repeated passage through mouse tissues.
FIG 8.
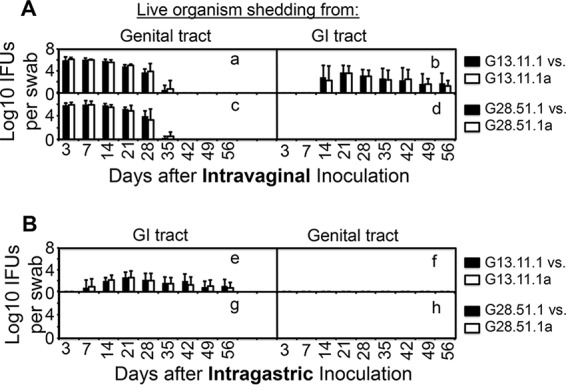
Abilities of mutant C. muridarum parental and variant clones isolated from mouse tissues to infect the mouse genital tract and spread to or colonize the GI tract. Mutant clones G13.11.1 and G28.51.1 (solid bar) and their corresponding variants isolated from C57BL/6J mouse tissues (designated G13.11.1a or G28.51.1a, open bars) were each inoculated intravaginally (A; n = 5) or intragastrically (B; n = 5) at 2 × 105 IFUs per C57BL/6J mouse. At various time points postinoculation, as indicated on the x axis, rectal (A, right side, B, left side) and vaginal (A, left side, B, right side) swabs were taken for titration of live C. muridarum organisms. The recovery of live organisms is expressed as log10 IFU counts per swab, as displayed on the y axis. Note that there was no significant difference in the number of IFUs between the parental mutant clones and the corresponding variant clones, regardless of the samples used for detection (rectal or vaginal swabs) or the route used for inoculation (intravaginal or intragastric).
DISCUSSION
Although chlamydial organisms have been detected in the GI tracts of both human and animal hosts (26–28, 30, 31, 33–44) and C. muridarum is known to colonize the GI tract for long periods of time (26, 30–33), the medical significance of these phenomena remains unclear. Here, we present the first experimental evidence of a correlation of C. muridarum spreading to and colonization of the GI tract with its pathogenicity in the upper genital tract. First, intravaginal inoculation with wild-type C. muridarum led to robust courses of live-organism shedding from both vaginal and rectal swabs of female C3H/HeJ mice and these mice all developed hydrosalpinx. However, C. muridarum mutants, although they maintained robust live-organism shedding from mouse vaginal swabs, showed significantly reduced live-organism shedding from rectal swabs and induction of hydrosalpinx. This was especially obvious in the two double mutants, which essentially failed to spread to the GI tract and were no longer able to induce any significant hydrosalpinx. Second, since ascending infection is necessary for C. muridarum induction of hydrosalpinx (6, 45), we simultaneously compared both C. muridarum ascending infection along the genital tract and spreading to tissues outside the genital tract between wild-type and mutant C. muridarum clones. All of our mutants with attenuated pathogenicity in the upper genital tract maintained robust ascending infection of upper genital tract tissues but decreased spreading to GI tract tissues, indicating a correlation of C. muridarum pathogenicity with its spreading to the GI tract but not with its ascension to the upper genital tract. Third, after transcervical inoculation into female C57BL/6J mice, C. muridarum mutants still significantly reduced their ascension to the GI tract and decreased their pathogenicity in the upper genital tract, both validating the correlation of chlamydial genital tract pathogenicity with chlamydial spread to the GI tract in a different mouse strain and demonstrating that the correlation is independent of ascending infection. Although transcervical inoculation enabled all of our mutants to reach the mouse oviducts, the mutants were still defective in induction of hydrosalpinx and spreading to the GI tract. Finally, intragastric inoculation with wild-type C. muridarum led to robust colonization of the GI tract while the same inoculation with the mutants failed to rescue their colonization. This observation suggests that the reduced spreading of the mutants from the genital to the GI tract was probably due to their inability to colonize the GI tract, thus further correlating chlamydial pathogenicity in the upper genital tract with the ability of chlamydiae to colonize the GI tract.
It is likely that defective colonization of the GI tract is the primary phenotype of the mutants assessed in the present study. This is because the functions of TC0668 and/or TC0237 appear to be more important for C. muridarum colonization of the GI tract than for its infection of the genital tract. The mechanistic question is how these “virulence” or “colonization” factors promote C. muridarum colonization of the GI tract. TC0668 is a 408-amino-acid hypothetical protein predicted to associate with the outer membrane complex of chlamydiae (46). The N-terminal 70 amino acids consist of a series of transmembrane domains, and the remaining region contains the domain-of-unknown-function 1207 (DUF1207) motif. Phylogenetic analysis of TC0668 indicates that C. muridarum inherited the DUF1207 motif from a distant chlamydial progenitor (15). However, the role of TC0668 in chlamydial pathogenesis remains unclear. Tertiary protein structure prediction and a homology search of TC0668 with the I-TASSER suite revealed that TC0668 shows structural homology with various eukaryotic integrins, including alpha V beta 1 (α5β1, fibronectin receptor), alpha IIb beta 3 (αIIbβ3, fibrinogen receptor), and alpha V beta 3 (α5β3, vitronectin receptor) (15). These integrins engage in diverse cellular activities, which makes it difficult to predict the function of TC0668. Our hypothesis is that TC0668 may be an essential outer membrane component that is required for C. muridarum to colonize the GI tract. Apparently, further structural analyses and biochemical characterizations are necessary to define the function of TC0668.
Regardless of how TC0668 and/or TC0237 promotes C. muridarum colonization of the GI tract, the fact that C. muridarum organisms that carry loss-of-function mutations in tc0668 and/or tc0237 are defective in both colonization of the GI tract and the induction of long-lasting hydrosalpinx in the genital tract but maintain robust ascending infection to the oviduct suggests that ascending infection-induced tubal inflammation alone is not sufficient to induce long-term hydrosalpinx and that C. muridarum colonization of the GI tract may contribute to genital tract pathogenicity. Although we have previously shown that both ascending infection and tubal inflammation are necessary for C. muridarum to induce hydrosalpinx (6), ascending-infection-induced inflammation of the oviduct alone may not be sufficient to induce long-lasting hydrosalpinx, which is consistent with the following observations. Although the levels of ascending infection of both the uterine horn and oviduct tissues of G28.51.1 (with double mutations) and G13.32.1 (wild type) after intravaginal inoculation were not significantly different (Fig. 3D versus A), G28.51.1 failed to induce any significant hydrosalpinx while G13.32.1 induced robust hydrosalpinx (Fig. 1). It is worth noting that C. muridarum ascension along the genital tract and its spreading outside the genital tract seem to be two independent events. Not only did ascension not necessarily lead to spreading, as described above, but also spreading was not dependent on ascension since C. muridarum spread from the lower genital tract to the GI tract as early as day 3 (26, 31) while C. muridarum reached its peak ascension 1 week later (47). In addition, A/J mice inoculated transcervically with wild-type C. muridarum (to promote ascending infection) still failed to develop robust hydrosalpinx (6, 25). The failure of A/J mice to develop hydrosalpinx was explained by the lack of complement factor C5 (48) since mice depleted of C5 lost the ability to develop hydrosalpinx in response to C. muridarum infection (23). Thus, C5-mediated tubal inflammation seemed to play an essential role in the induction of hydrosalpinx by C. muridarum. The question is whether the failure of C. muridarum mutants G13.11.1 or G28.51.1 to induce hydrosalpinx is due to their inability to activate C5-mediated tubal inflammation, which is worth further investigation. Alternatively, the present study has correlated C. muridarum induction of hydrosalpinx with C. muridarum colonization of the GI tract. It will be interesting to discern the following two aspects of the correlation. Does the correlation suggest that the ability of C. muridarum to colonize GI tract tissues reflects its ability to induce pathogenicity in the upper genital tract or that C. muridarum in the GI tract mechanistically contributes to chlamydial pathogenicity in the upper genital tract? Finally, it has been hypothesized that long-term chlamydial colonization of the GI tract may serve as a reservoir for autoinoculation of the genital tract (30, 33). Although we have recently found that C. muridarum failed to autoinoculate the genital tract after colonization of the GI tract of the same mouse for 70 days (32), because of the complexity of human behavior, there may be multiple pathways by which C. trachomatis can spread between the GI and genital tracts, allowing GI tract chlamydiae to promote pathogenicity in the upper genital tract.
Regardless of how GI tract C. muridarum may promote genital pathogenicity, the current findings that C. muridarum with loss-of-function mutations in tc0668 and/or tc0237 is defective in both colonization of the GI tract and the induction of long-lasting hydrosalpinx in the genital tract but maintains robust ascending infection of the oviduct may have provided a platform for defining the roles of GI tract C. muridarum in genital pathogenicity and mechanisms by which GI tract C. muridarum contributes to genital pathogenicity.
Because of the difference between the interaction of C. muridarum with mice and that of C. trachomatis with humans, there is a big caveat against applying the knowledge learned from the murine system to humans. Like other animal chlamydial species, C. muridarum may have adapted to the mouse GI tract. However, C. trachomatis has been forced to adapt to the human genital tract since sexual transmission may be more efficient than oral-fecal route transmission between modern humans. Nevertheless, the genomes of C. muridarum and C. trachomatis are still largely conserved despite some significant differences (49, 50). For example, TC0668 shows 97% protein identity with its C. trachomatis serovar D counterpart, CT389. After all, C. trachomatis has been frequently detected in the human GI tract (51). Furthermore, like C. muridarum (27, 33), C. trachomatis has not been associated with any significant GI tract pathologies (52). Thus, further investigation of DUF1207-containing homologs may lead to useful information for combating highly prevalent urogenital C. trachomatis disease in humans.
MATERIALS AND METHODS
C. muridarum organism growth.
C. muridarum strain Nigg3 and its derived mutants, as well as variants isolated from mouse tissues, were all propagated and purified with HeLa cells (human cervical carcinoma epithelial cells, ATCC catalog number CCL2) as described previously (26, 53). C. muridarum mutant clones were generated by multiple in vitro passages as described previously (14, 15). The following six isogenic clones were used in the present study: G13.32.1 (retaining the wild-type Nigg3 genome sequence), G13.59.2 (carrying a substitution mutation in gene tc0668 resulting in replacement of the amino acid glycine [G] at the 322nd position of the TC0668 protein with arginine [R], designated TC0668G322R [TC0668R for short]), G40.50.2 (with a substitution mutation in gene tc0237 resulting in a mutation of Q [glutamine] 117 to E [glutamic acid] in the TC0237 protein, producing TC0237Q117E [TC0237E for short]), G13.11.1 (with a frameshift mutation in tc0668 resulting in a premature stop codon at the 216th codon [glycine], producing TC0668G216* [TC0668*, short]), G28.54.1 (with substitution mutations in both tc0668 and tc0237, resulting in TC0668G322R/TC0237Q117E [TC0668R/TC0237E for short]), and G28.51.1 (carrying both the premature stop codon in tc0668 and the substitution mutation in tc0237, resulting in TC0668G216*/TC0237Q117E [TC0668*/TC0237E for short]). In addition, in some experiments, new variants designated G13.11.1c and G28.51.1a isolated from tissues of mice infected with the corresponding parental clones (as described below) were also used. All C. muridarum organisms were purified as elementary bodies (EBs) that were stored in aliquots at −80°C until use.
Mouse infection.
Six- to 7-week-old female C3H/HeJ (stock number 000659) and C57BL/6J (stock number 000664; both from Jackson Laboratories, Inc., Bar Harbor, ME) mice were inoculated with C. muridarum EBs at 2 × 105 to 1 × 107 IFUs per mouse via different routes as described previously (6, 32) and as indicated for individual experiments. Briefly, 5 days prior to intravaginal and transcervical inoculations, mice were subcutaneously injected with 2.5 mg of colloidal depot medroxyprogesterone (Depo-Provera; Pharmacia & Upjohn Company LLC, Kalamazoo, MI) suspended in sterile phosphate-buffered saline. For intravaginal inoculation, an EB inoculum in 10 μl of SPG buffer (220 mM sucrose, 12.5 mM phosphate, 4 mM l-glutamic acid, pH 7.5) was delivered into each mouse's vagina with a 20-μl micropipette tip. For transcervical inoculation, a nonsurgical embryo transfer device (catalog number 60010; ParaTechs Corp., Lexington, KY) was used and the manufacturer's instructions were followed. After inoculation, mice were monitored for vaginal and rectal live-organism shedding or sacrificed for titration of live organisms in organs/tissues or for evaluation of pathology in the upper genital tract.
Animal experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Laboratory Animal Experiments of the University of Texas Health Science Center at San Antonio.
Titration of live chlamydial organisms from mouse swabs and tissues.
For monitoring of live-organism shedding from the genital and GI tracts, vaginal and rectal swabs were taken on day 3 postinoculation and weekly thereafter. Each swab was soaked in 0.5 ml of SPG buffer and vortexed with glass beads to release infectious EBs for quantitation. For titration of live chlamydial organisms recovered from mouse tissues, various organs/tissues were harvested on day 14 after inoculation. Each organ or tissue segment was transferred to a tube containing 0.5 ml (for each segment of genital tract tissue) or 2 ml (for each remaining tissue/organ) of SPG buffer. Each genital tract was cut into three segments or portions, including the vagina-cervix (CV), left and right uterine horns (UH), and left and right oviducts/ovaries (OV). Each GI tract was divided into five segments, including the stomach (ST), small intestine (SI), cecum (CE), colon (CO), and anus-rectum (RE). The kidneys (KD), liver (LI), spleen (SP), lungs (LU), and heart (HE) were also harvested. The organs and tissue segments were homogenized in cold SPG buffer. Live C. muridarum organisms released into swab suspensions or tissue supernatants were titrated on HeLa cells in duplicate as described previously (6, 19, 20, 26, 31, 32). The total number of IFUs per swab or tissue sample was converted into log10 for calculation of the mean and standard deviation across mice in the same group at each time point.
Isolation of C. muridarum from mouse tissues and whole-genome sequencing.
Uterine horn and oviduct tissues from five C57BL/6J mice were harvested on day 28 after intravaginal inoculation with G28.51.1 (with double mutations) or cecum/colon tissues from five C57BL/6J mice intragastrically inoculated with G13.11.1 (with single mutation) for 56 days to make homogenates in SPG buffer as described above. After sonication, the supernatants were inoculated onto HeLa cell monolayers in six-well plates for amplification. Both DEAE treatment and centrifugation were used to maximize the recovery of live organisms. Twenty-four hours later, the infected HeLa cells were harvested for purification of C. muridarum EBs as described previously (18). The purified EBs, after titration, were used for both whole-genome NGS (MiSeq; Illumina; University of Texas Health Science Center at San Antonio Sequencing Core) as described previously (14, 15) and infection of mice as described above. In addition, a plaque purification scheme was used to isolate individual clones from both G28.51.1 and G13.11.1 amplification mixtures as described previously (14). The isolated clones were first screened for mutations at designated sites by PCR amplicon sequencing, and the desired clones were then subjected to whole-genome sequencing by a combination of NGS and primer walking to fill in gaps (15). The whole-genome sequences were analyzed with in-house bioprofiling software as described previously (15).
Mouse genital tract gross pathology evaluation.
Upon euthanasia, mouse genital tracts were excised and the gross pathology of hydrosalpinx was documented by high-resolution digital photography. Hydrosalpinx was further scored on an ordinal scale where 0 indicates no hydrosalpinx, 1 indicates hydrosalpinx observable only under magnification, 2 indicates visible hydrosalpinx smaller than the ovary, 3 indicates hydrosalpinx roughly equal in size to the ovary, and 4 indicates hydrosalpinx larger than the ovary. Bilateral hydrosalpinx severity was calculated for each mouse as the sum of the scores of the left and right oviducts. Hydrosalpinx incidence was calculated as the number of mice with a bilateral score of ≥1 divided by the number of mice in the group.
Immunofluorescence assay.
An immunofluorescence assay was used for titration of live organisms as described previously (53, 54). Briefly, HeLa cells grown on coverslips were fixed with paraformaldehyde (Sigma) and permeabilized with saponin (Sigma). After washing and blocking, the cell samples were subjected to a combination of antibody and chemical staining. Hoechst dye (blue; Sigma) was used to visualize nuclear DNA. A rabbit antichlamydial antibody (raised by immunization with C. muridarum EBs; data not shown) and goat anti-rabbit IgG conjugated with Cy2 (green; Jackson Immuno Research Laboratories, Inc., West Grove, PA) were used to visualize chlamydial inclusions. After immunolabeling, the cell samples were used to count inclusions under an Olympus AX-70 fluorescence microscope equipped with multiple filter sets (Olympus, Melville, NY).
Statistical analyses.
The numbers of live organisms in groups (expressed as IFU counts), genome copies, and hydrosalpinx scores, were compared with the Wilcoxon rank sum test. Incidences of hydrosalpinx in groups were evaluated with Fisher's exact probability test (http://vassarstats.net/tab2x2.html). Correlations of chlamydial pathogenicity in the upper genital tract with chlamydial colonization of different tissues were analyzed by calculating Spearman rank-order correlation coefficients (http://vassarstats.net/corr_rank.html). The significance of the difference between two correlation coefficients was also calculated (http://vassarstats.net/rdiff.html).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants from the U.S. National Institutes of Health to G.Z. L.S. was supported by Tianjin Medical University General Hospital.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00321-17.
REFERENCES
- 1.Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. 2012. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol 119:1009–1016. doi: 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. doi: 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodgers AK, Wang J, Zhang Y, Holden A, Berryhill B, Budrys NM, Schenken RS, Zhong G. 2010. Association of tubal factor infertility with elevated antibodies to Chlamydia trachomatis caseinolytic protease P. Am J Obstet Gynecol 203:494.e7–494.e14. doi: 10.1016/j.ajog.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 5.de la Maza LM, Pal S, Khamesipour A, Peterson EM. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun 62:2094–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. doi: 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlcek KR, Li W, Manam S, Zanotti B, Nicholson BJ, Ramsey KH, Murthy AK. 2016. The contribution of Chlamydia-specific CD8(+) T cells to upper genital tract pathology. Immunol Cell Biol 94:208–212. doi: 10.1038/icb.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Maza LM, Peterson EM. 2002. Vaccines for Chlamydia trachomatis infections. Curr Opin Investig Drugs 3:980–986. [PubMed] [Google Scholar]
- 9.Morrison RP, Caldwell HD. 2002. Immunity to murine chlamydial genital infection. Infect Immun 70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu C, Peng B, Li Z, Lei L, Li Z, Chen L, He Q, Zhong G, Wu Y. 2013. Induction of protective immunity against Chlamydia muridarum intravaginal infection with the chlamydial immunodominant antigen macrophage infectivity potentiator. Microbes Infect 15:329–338. doi: 10.1016/j.micinf.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RM, Kerr MS, Slaven JE. 2014. An atypical CD8 T-cell response to Chlamydia muridarum genital tract infections includes T cells that produce interleukin-13. Immunology 142:248–257. doi: 10.1111/imm.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockey DD, Wang J, Lei L, Zhong G. 2009. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines 8:1365–1377. doi: 10.1586/erv.09.98. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, Chen C, Li Z, Liu Q, Wu Y, Baseman J, Zhong G. 2014. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun 82:5327–5335. doi: 10.1128/IAI.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Zhou Z, Conrad T, Yang Z, Dai J, Li Z, Wu Y, Zhong G. 2015. In vitro passage selects for Chlamydia muridarum with enhanced infectivity in cultured cells but attenuated pathogenicity in mouse upper genital tract. Infect Immun 83:1881–1892. doi: 10.1128/IAI.03158-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad TA, Gong S, Yang Z, Matulich P, Keck J, Beltrami N, Chen C, Zhou Z, Dai J, Zhong G. 2015. The chromosome-encoded hypothetical protein TC0668 is an upper genital tract pathogenicity factor of Chlamydia muridarum. Infect Immun 84:467–479. doi: 10.1128/IAI.01171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, Baseman J, Zhong G. 2014. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun 82:983–992. doi: 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Connell CM, Ingalls RR, Andrews CW Jr, Scurlock AM, Darville T. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 179:4027–4034. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 18.Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. 2008. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect Immun 76:515–522. doi: 10.1128/IAI.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Cheng W, Shivshankar P, Lei L, Zhang X, Wu Y, Yeh IT, Zhong G. 2009. Distinct roles of CD28- and CD40 ligand-mediated costimulation in the development of protective immunity and pathology during Chlamydia muridarum urogenital infection in mice. Infect Immun 77:3080–3089. doi: 10.1128/IAI.00611-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Lei L, Chang X, Li Z, Lu C, Zhang X, Wu Y, Yeh IT, Zhong G. 2010. Mice deficient in MyD88 develop a Th2-dominant response and severe pathology in the upper genital tract following Chlamydia muridarum infection. J Immunol 184:2602–2610. doi: 10.4049/jimmunol.0901593. [DOI] [PubMed] [Google Scholar]
- 21.Dai J, Tang L, Chen J, Yu P, Chen Z, Zhong G. 2016. The p47phox deficiency significantly attenuates the pathogenicity of Chlamydia muridarum in the mouse oviduct but not uterine tissues. Microbes Infect 18:190–198. doi: 10.1016/j.micinf.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Murthy AK, Li W, Chaganty BK, Kamalakaran S, Guentzel MN, Seshu J, Forsthuber TG, Zhong G, Arulanandam BP. 2011. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect Immun 79:2928–2935. doi: 10.1128/IAI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z, Conrad T, Zhou Z, Chen J, Dutow P, Klos A, Zhong G. 2014. Complement factor C5 but not C3 contributes significantly to hydrosalpinx development in mice infected with Chlamydia muridarum. Infect Immun 82:3154–3163. doi: 10.1128/IAI.01833-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manam S, Nicholson BJ, Murthy AK. 2013. OT-1 mice display minimal upper genital tract pathology following primary intravaginal Chlamydia muridarum infection. Pathog Dis 67:221–224. doi: 10.1111/2049-632X.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Zhou Z, Chen J, Wu G, Yang Z, Zhou Z, Baseman J, Zhang J, Reddick RL, Zhong G. 2014. Lack of long-lasting hydrosalpinx in A/J mice correlates with rapid but transient chlamydial ascension and neutrophil recruitment in the oviduct following intravaginal inoculation with Chlamydia muridarum. Infect Immun 82:2688–2696. doi: 10.1128/IAI.00055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Huang Y, Gong S, Yang Z, Sun X, Schenken R, Zhong G. 2015. In vivo and ex vivo imaging reveals a long-lasting chlamydial infection in the mouse gastrointestinal tract following genital tract inoculation. Infect Immun 83:3568–3577. doi: 10.1128/IAI.00673-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igietseme JU, Portis JL, Perry LL. 2001. Inflammation and clearance of Chlamydia trachomatis in enteric and nonenteric mucosae. Infect Immun 69:1832–1840. doi: 10.1128/IAI.69.3.1832-1840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry LL, Hughes S. 1999. Chlamydial colonization of multiple mucosae following infection by any mucosal route. Infect Immun 67:3686–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeruva L, Melnyk S, Spencer N, Bowlin A, Rank RG. 2013. Differential susceptibilities to azithromycin treatment of chlamydial infection in the gastrointestinal tract and cervix. Antimicrob Agents Chemother 57:6290–6294. doi: 10.1128/AAC.01405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeruva L, Spencer N, Bowlin AK, Wang Y, Rank RG. 2013. Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathog Dis 68:88–95. doi: 10.1111/2049-632X.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai J, Zhang T, Wang L, Shao L, Zhu C, Zhang Y, Failor C, Schenken R, Baseman J, He C, Zhong G. 2016. Intravenous inoculation with Chlamydia muridarum leads to a long-lasting infection restricted to the gastrointestinal tract. Infect Immun 84:2382–2388. doi: 10.1128/IAI.00432-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Zhang Q, Zhang T, Zhang Y, Zhu C, Sun X, Zhang N, Xue M, Zhong G. 2016. The Chlamydia muridarum organisms fail to auto-inoculate the mouse genital tract after colonization in the gastrointestinal tract for 70 days. PLoS One 11:e0155880. doi: 10.1371/journal.pone.0155880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rank RG, Yeruva L. 2014. Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect Immun 82:1362–1371. doi: 10.1128/IAI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bazan JA, Carr Reese P, Esber A, Lahey S, Ervin M, Davis JA, Fields K, Turner AN. 2015. High prevalence of rectal gonorrhea and chlamydia infection in women attending a sexually transmitted disease clinic. J Womens Health (Larchmt) 24:182–189. doi: 10.1089/jwh.2014.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dukers-Muijrers NH, Schachter J, van Liere GA, Wolffs PF, Hoebe CJ. 2015. What is needed to guide testing for anorectal and pharyngeal Chlamydia trachomatis and Neisseria gonorrhoeae in women and men? Evidence and opinion. BMC Infect Dis 15:533. doi: 10.1186/s12879-015-1280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dukers-Muijrers NH, Speksnijder AG, Morre SA, Wolffs PF, van der Sande MA, Brink AA, van den Broek IV, Werner MI, Hoebe CJ. 2013. Detection of anorectal and cervicovaginal Chlamydia trachomatis infections following azithromycin treatment: prospective cohort study with multiple time-sequential measures of rRNA, DNA, quantitative load and symptoms. PLoS One 8:e81236. doi: 10.1371/journal.pone.0081236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gratrix J, Singh AE, Bergman J, Egan C, McGinnis J, Drews SJ, Read R. 2014. Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics. Sex Transm Dis 41:589–591. doi: 10.1097/OLQ.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 38.Gratrix J, Singh AE, Bergman J, Egan C, Plitt SS, McGinnis J, Bell CA, Drews SJ, Read R. 2015. Evidence for increased chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clin Infect Dis 60:398–404. doi: 10.1093/cid/ciu831. [DOI] [PubMed] [Google Scholar]
- 39.Javanbakht M, Gorbach P, Stirland A, Chien M, Kerndt P, Guerry S. 2012. Prevalence and correlates of rectal chlamydia and gonorrhea among female clients at sexually transmitted disease clinics. Sex Transm Dis 39:917–922. doi: 10.1097/OLQ.0b013e31826ae9a2. [DOI] [PubMed] [Google Scholar]
- 40.Khosropour CM, Dombrowski JC, Barbee LA, Manhart LE, Golden MR. 2014. Comparing azithromycin and doxycycline for the treatment of rectal chlamydial infection: a retrospective cohort study. Sex Transm Dis 41:79–85. doi: 10.1097/OLQ.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musil K, Currie M, Sherley M, Martin S. 2016. Rectal chlamydia infection in women at high risk of chlamydia attending Canberra Sexual Health Centre. Int J STD AIDS 27:526–530. doi: 10.1177/0956462415586317. [DOI] [PubMed] [Google Scholar]
- 42.Peters RP, Dubbink JH, van der Eem L, Verweij SP, Bos ML, Ouburg S, Lewis DA, Struthers H, McIntyre JA, Morre SA. 2014. Cross-sectional study of genital, rectal, and pharyngeal chlamydia and gonorrhea in women in rural South Africa. Sex Transm Dis 41:564–569. doi: 10.1097/OLQ.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 43.Steedman NM, McMillan A. 2009. Treatment of asymptomatic rectal Chlamydia trachomatis: is single-dose azithromycin effective? Int J STD AIDS 20:16–18. doi: 10.1258/ijsa.2008.008211. [DOI] [PubMed] [Google Scholar]
- 44.Pospischil A, Borel N, Chowdhury EH, Guscetti F. 2009. Aberrant chlamydial developmental forms in the gastrointestinal tract of pigs spontaneously and experimentally infected with Chlamydia suis. Vet Microbiol 135:147–156. doi: 10.1016/j.vetmic.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Yang Z, Sun X, Tang L, Ding Y, Xue M, Zhou Z, Baseman J, Zhong G. 2015. Intrauterine infection with plasmid-free Chlamydia muridarum reveals a critical role of the plasmid in chlamydial ascension and establishes a model for evaluating plasmid-independent pathogenicity. Infect Immun 83:2583–2592. doi: 10.1128/IAI.00353-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Afrane M, Clemmer DE, Zhong G, Nelson DE. 2010. Identification of Chlamydia trachomatis outer membrane complex proteins by differential proteomics. J Bacteriol 192:2852–2860. doi: 10.1128/JB.01628-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell J, Huang Y, Liu Y, Schenken R, Arulanandam B, Zhong G. 2014. Bioluminescence imaging of Chlamydia muridarum ascending infection in mice. PLoS One 9:e101634. doi: 10.1371/journal.pone.0101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuite A, Elias M, Picard S, Mullick A, Gros P. 2005. Genetic control of susceptibility to Candida albicans in susceptible A/J and resistant C57BL/6J mice. Genes Immun 6:672–682. [DOI] [PubMed] [Google Scholar]
- 49.Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, Hickey EK, Peterson J, Utterback T, Berry K, Bass S, Linher K, Weidman J, Khouri H, Craven B, Bowman C, Dodson R, Gwinn M, Nelson W, DeBoy R, Kolonay J, McClarty G, Salzberg SL, Eisen J, Fraser CM. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res 28:1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov RL, Zhao Q, Koonin EV, Davis RW. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 51.Pabbaraju K, Wong S, Gill K, Severini A, Roy F, Gratrix J, Singh AE, Naidu P, Read R, Drews SJ. 2017. Use of the APTIMA Combo 2 assay and a secondary algorithm to detect and confirm Chlamydia trachomatis in rectal-only infections. Sex Transm Dis 44:118–119. doi: 10.1097/OLQ.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 52.Elliott PR, Forsey T, Darougar S, Treharne JD, Lennard-Jones JE. 1981. Chlamydiae and inflammatory bowel disease. Gut 22:25–27. doi: 10.1136/gut.22.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong G, Fan P, Ji H, Dong F, Huang Y. 2001. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J Exp Med 193:935–942. doi: 10.1084/jem.193.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


