Abstract
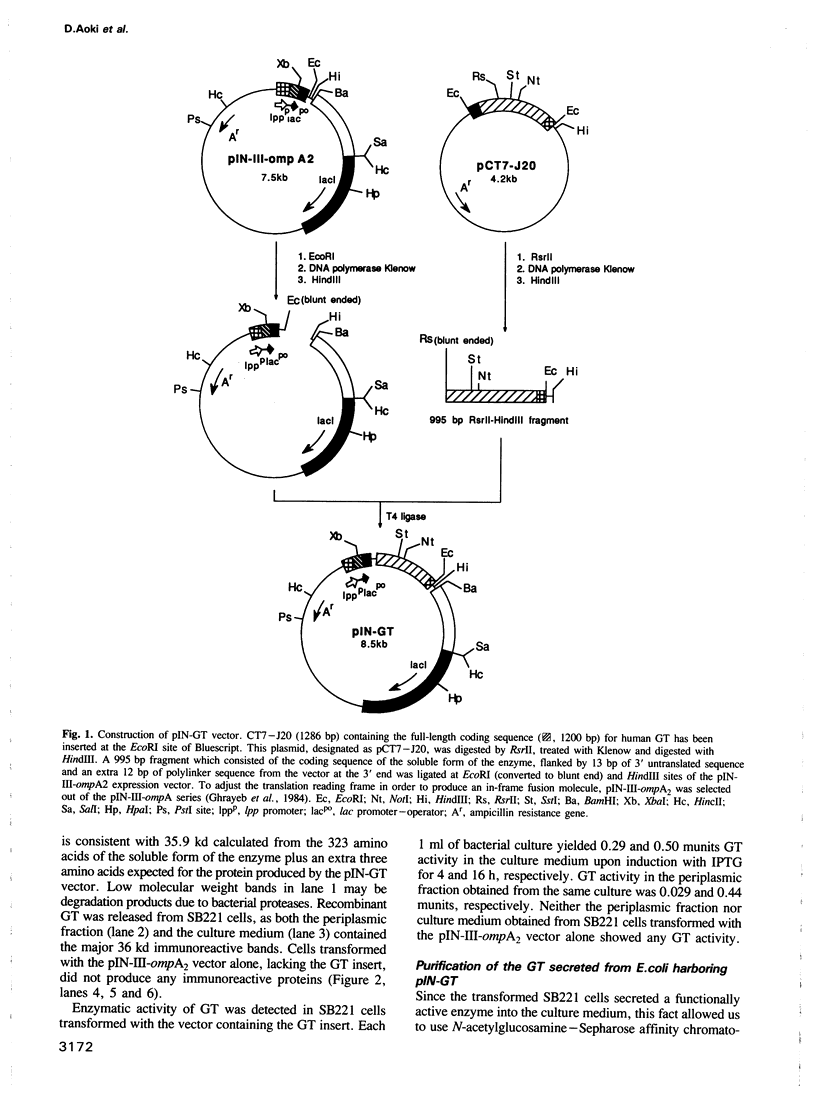
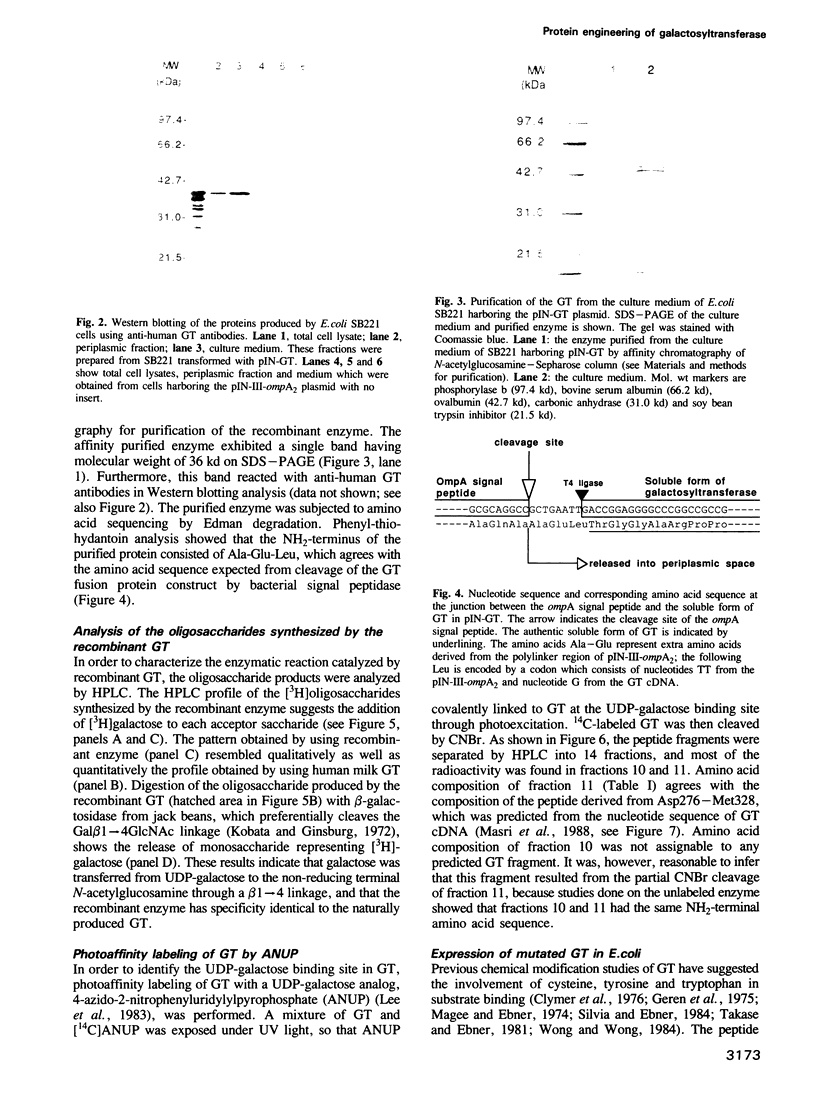
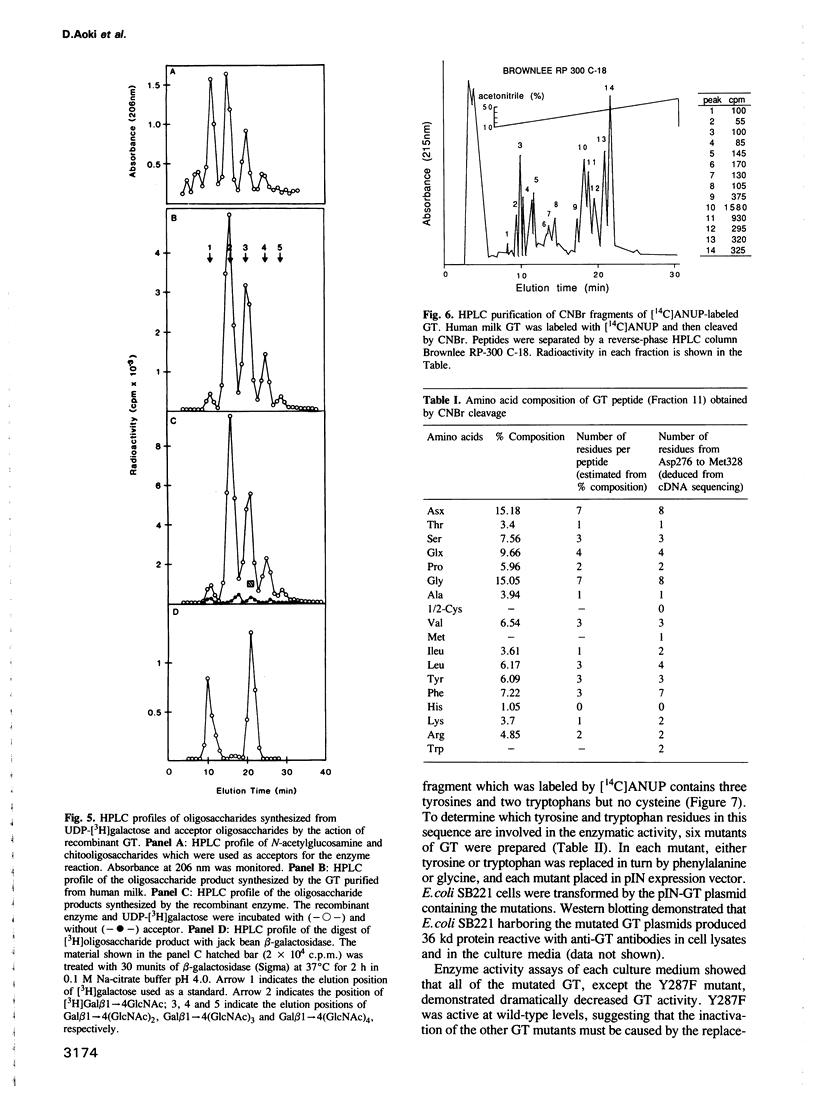
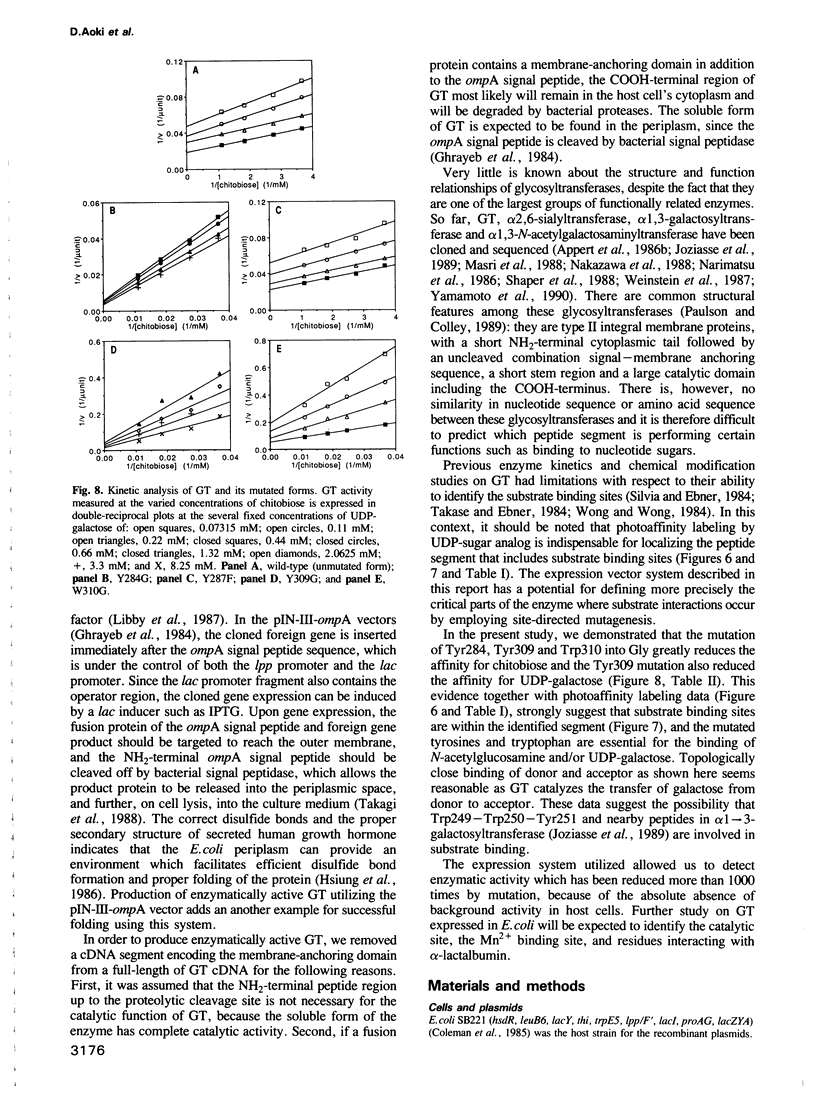
An expression vector, pIN-GT, encoding the soluble form of beta 1,4-galactosyltransferase (GT) has been constructed from human GT cDNAs and the pIN-III-ompA2 expression vector. Escherichia coli strain SB221 harboring the pIN-GT plasmid produces and secretes a fusion protein consisting of the ompA signal and GT. The expression of GT was detected by assaying enzymatic activity as well as by Western blotting using anti-GT antibodies. The recombinant GT was purified to homogeneity by N-acetylglucosamine-Sepharose affinity chromatography. The NH2-terminal peptide sequence of purified GT confirmed the cleavage site of the fusion protein by bacterial signal peptidase. This expression system was utilized to produce mutant forms of GT in order to identify specific amino acids involved in substrate binding sites. Photoaffinity labeling of GT with UDP-galactose analog, 4-azido-2-nitrophenyluridylylpyrophosphate (ANUP), followed by cyanogen bromide (CNBr) cleavage revealed that ANUP bound to a fragment of GT composed of amino acid residues from Asp276 to Met328. Within this peptide segment, Tyr284, Tyr287, Tyr309, Trp310 and Trp312 were separately substituted into Gly and Tyr287 into Phe by site-directed mutagenesis. Enzymatic activity assay showed drastic reduction of the activity in all of the mutants except that Tyr287----Phe remained as active as wild-type GT. Kinetic studies of the mutated GT showed that Tyr284, Tyr309 and Trp310 are critically involved in the N-acetyglucosamine binding and Tyr309 is involved in UDP-galactose binding as well.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appert H. E., Rutherford T. J., Tarr G. E., Thomford N. R., McCorquodale D. J. Isolation of galactosyltransferase from human milk and the determination of its N-terminal amino acid sequence. Biochem Biophys Res Commun. 1986 Jul 16;138(1):224–229. doi: 10.1016/0006-291x(86)90269-x. [DOI] [PubMed] [Google Scholar]
- Appert H. E., Rutherford T. J., Tarr G. E., Wiest J. S., Thomford N. R., McCorquodale D. J. Isolation of a cDNA coding for human galactosyltransferase. Biochem Biophys Res Commun. 1986 Aug 29;139(1):163–168. doi: 10.1016/s0006-291x(86)80094-8. [DOI] [PubMed] [Google Scholar]
- Barker R., Olsen K. W., Shaper J. H., Hill R. L. Agarose derivatives of uridine diphosphate and N-acetylglucosamine for the purification of a galactosyltransferase. J Biol Chem. 1972 Nov 25;247(22):7135–7147. [PubMed] [Google Scholar]
- Bell J. E., Beyer T. A., Hill R. L. The kinetic mechansim of bovine milk galactosyltransferase. The role of alpha-lactalbumin. J Biol Chem. 1976 May 25;251(10):3003–3013. [PubMed] [Google Scholar]
- Berger E. G., Mandel T., Schilt U. Immunohistochemical localization of galactosyltransferase in human fibroblasts and HeLa cells. J Histochem Cytochem. 1981 Mar;29(3):364–370. doi: 10.1177/29.3.6787115. [DOI] [PubMed] [Google Scholar]
- Clymer D. J., Geren C. R., Ebner K. E. Ultraviolet photoinactivation of galactosyltransferase. Protection by substrates. Biochemistry. 1976 Mar 9;15(5):1093–1097. doi: 10.1021/bi00650a022. [DOI] [PubMed] [Google Scholar]
- Coleman J., Inukai M., Inouye M. Dual functions of the signal peptide in protein transfer across the membrane. Cell. 1985 Nov;43(1):351–360. doi: 10.1016/0092-8674(85)90040-6. [DOI] [PubMed] [Google Scholar]
- D'Agostaro G., Bendiak B., Tropak M. Cloning of cDNA encoding the membrane-bound form of bovine beta 1,4-galactosyltransferase. Eur J Biochem. 1989 Jul 15;183(1):211–217. doi: 10.1111/j.1432-1033.1989.tb14915.x. [DOI] [PubMed] [Google Scholar]
- Geren C. R., Magee S. C., Ebner K. E. Circular dichroism changes in galactosyltransferase upon substrate binding;. Biochemistry. 1975 Apr 8;14(7):1461–1463. doi: 10.1021/bi00678a017. [DOI] [PubMed] [Google Scholar]
- Ghrayeb J., Kimura H., Takahara M., Hsiung H., Masui Y., Inouye M. Secretion cloning vectors in Escherichia coli. EMBO J. 1984 Oct;3(10):2437–2442. doi: 10.1002/j.1460-2075.1984.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joziasse D. H., Shaper J. H., Van den Eijnden D. H., Van Tunen A. J., Shaper N. L. Bovine alpha 1----3-galactosyltransferase: isolation and characterization of a cDNA clone. Identification of homologous sequences in human genomic DNA. J Biol Chem. 1989 Aug 25;264(24):14290–14297. [PubMed] [Google Scholar]
- Khatra B. S., Herries D. G., Brew K. Some kinetic properties of human-milk galactosyl transferase. Eur J Biochem. 1974 May 15;44(2):537–560. doi: 10.1111/j.1432-1033.1974.tb03513.x. [DOI] [PubMed] [Google Scholar]
- Kobata A., Ginsburg V. Oligosaccharides of human milk. 3. Isolation and characterization of a new hexasaccharide, lacto-N-hexaose. J Biol Chem. 1972 Mar 10;247(5):1525–1529. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee T. K., Wong L. J., Wong S. S. Photoaffinity labeling of lactose synthase with a UDP-galactose analogue. J Biol Chem. 1983 Nov 10;258(21):13166–13171. [PubMed] [Google Scholar]
- Libby R. T., Braedt G., Kronheim S. R., March C. J., Urdal D. L., Chiaverotti T. A., Tushinski R. J., Mochizuki D. Y., Hopp T. P., Cosman D. Expression and purification of native human granulocyte-macrophage colony-stimulating factor from an Escherichia coli secretion vector. DNA. 1987 Jun;6(3):221–229. doi: 10.1089/dna.1987.6.221. [DOI] [PubMed] [Google Scholar]
- Magee S. C., Ebner K. E. Inactivation of soluble bovine milk galactosyltransferase (lactose synthetase) by sulfhydryl reagents and trypsin. Protection by substrates and products. J Biol Chem. 1974 Nov 10;249(21):6992–6998. [PubMed] [Google Scholar]
- Masri K. A., Appert H. E., Fukuda M. N. Identification of the full-length coding sequence for human galactosyltransferase (beta-N-acetylglucosaminide: beta 1,4-galactosyltransferase). Biochem Biophys Res Commun. 1988 Dec 15;157(2):657–663. doi: 10.1016/s0006-291x(88)80300-0. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Ando T., Kimura T., Narimatsu H. Cloning and sequencing of a full-length cDNA of mouse N-acetylglucosamine (beta 1-4)galactosyltransferase. J Biochem. 1988 Aug;104(2):165–168. doi: 10.1093/oxfordjournals.jbchem.a122434. [DOI] [PubMed] [Google Scholar]
- Narimatsu H., Sinha S., Brew K., Okayama H., Qasba P. K. Cloning and sequencing of cDNA of bovine N-acetylglucosamine (beta 1-4)galactosyltransferase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4720–4724. doi: 10.1073/pnas.83.13.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Ram B. P., Munjal D. D. Galactosyltransferases: physical, chemical, and biological aspects. CRC Crit Rev Biochem. 1985;17(3):257–311. doi: 10.3109/10409238509113606. [DOI] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper N. L., Hollis G. F., Douglas J. G., Kirsch I. R., Shaper J. H. Characterization of the full length cDNA for murine beta-1,4-galactosyltransferase. Novel features at the 5'-end predict two translational start sites at two in-frame AUGs. J Biol Chem. 1988 Jul 25;263(21):10420–10428. [PubMed] [Google Scholar]
- Silvia J. S., Ebner K. E. Protection by substrates and alpha-lactalbumin against inactivation of galactosyltransferase by iodine monochloride. J Biol Chem. 1980 Dec 10;255(23):11262–11267. [PubMed] [Google Scholar]
- Smith C. A., Brew K. Isolation and characteristics of galactosyltransferase from Golgi membranes of lactating sheep mammary glands. J Biol Chem. 1977 Oct 25;252(20):7294–7299. [PubMed] [Google Scholar]
- Strous G. J., Van Kerkhof P., Willemsen R., Geuze H. J., Berger E. G. Transport and topology of galactosyltransferase in endomembranes of HeLa cells. J Cell Biol. 1983 Sep;97(3):723–727. doi: 10.1083/jcb.97.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase K., Ebner K. E. Interaction of galactosyltransferase with alpha-lactalbumin and substrates. Curr Top Cell Regul. 1984;24:51–62. doi: 10.1016/b978-0-12-152824-9.50013-7. [DOI] [PubMed] [Google Scholar]
- Takase K., Ebner K. E. Interactions of substrates and alpha-lactalbumin with galactosyltransferase as measured by difference spectroscopy. J Biol Chem. 1981 Jul 25;256(14):7269–7276. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer I. P., Hill R. L. The purification and properties of the A protein of lactose synthetase. J Biol Chem. 1971 Nov;246(21):6666–6675. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weinstein J., Lee E. U., McEntee K., Lai P. H., Paulson J. C. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987 Dec 25;262(36):17735–17743. [PubMed] [Google Scholar]
- Wong L. J., Wong S. S. The sulfhydryl group microenvironment of lactose synthase from bovine milk. Int J Biochem. 1984;16(8):913–917. doi: 10.1016/0020-711x(84)90152-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto F., Marken J., Tsuji T., White T., Clausen H., Hakomori S. Cloning and characterization of DNA complementary to human UDP-GalNAc: Fuc alpha 1----2Gal alpha 1----3GalNAc transferase (histo-blood group A transferase) mRNA. J Biol Chem. 1990 Jan 15;265(2):1146–1151. [PubMed] [Google Scholar]
- Zsebo K. M., Lu H. S., Fieschko J. C., Goldstein L., Davis J., Duker K., Suggs S. V., Lai P. H., Bitter G. A. Protein secretion from Saccharomyces cerevisiae directed by the prepro-alpha-factor leader region. J Biol Chem. 1986 May 5;261(13):5858–5865. [PubMed] [Google Scholar]





