Key Points
Donor T cells lacking AhR demonstrate decreased aGVHD because of reduced donor T-cell proliferation early after transplant.
Absence of AhR on donor cells increased pTreg cells in the colon; in vitro blockade increased the number of human iTreg from CD4+ T cells.
Abstract
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that affects the function and development of immune cells. Here, we show that recipient mice receiving AhR−/− T cells have improved survival and decreased acute graft-versus-host disease (aGVHD) in 2 different murine allogeneic bone marrow transplant (BMT) models. We also show that CD4+ T cells lacking AhR demonstrate reduced accumulation in secondary lymphoid tissue because of low levels of proliferation 4 days after BMT. Additionally, we found a significant increase in the quantity of peripherally induced regulatory donor T (pTreg) cells in the colon of recipients transplanted with AhR−/− T cells 14 days after transplant. Blockade of AhR using a clinically available AhR antagonist greatly enhanced the in vitro generation of inducible Treg (iTreg) cells from naïve CD4+ human T cells. We have identified AhR as a novel target on donor T cells that is critical to the pathogenesis of aGVHD.
Introduction
Acute graft-versus-host disease (aGVHD) is a significant complication of allogeneic hematopoietic stem cell transplantation. Despite standard prophylactic regimens, 10% to 80% of transplant recipients develop aGVHD, depending on the degree of major histocompatibility complex antigen mismatch, the type of conditioning regimen used, the age of the donor and recipient, and other factors.1 As shown, aGVHD is characterized by the migration of naïve donor T cells, first to secondary lymphoid tissues (SLTs) where they undergo expansion followed by migration to target organs such as the colon, small bowel, liver, and skin where they cause tissue damage.2,3 The control of peripheral immune responses to host antigens is mediated by negative selection of T cells with high affinity for self-antigens and peripheral immunosuppression mediated by different populations of immune cells. Of these, the best characterized are CD4+ regulatory T (Treg) cells that express the canonical transcription factor FoxP3. Treg cells can either be selected in the thymus (tTreg cells) or induced in the periphery (pTreg cells).4-6 The transfer of Treg cells prior to or with donor T cells diminished the incidence of aGVHD and improved overall survival. The infusion of donor Treg cells diminished the incidence of aGVHD without eliminating the graft-versus-tumor (GVT) response.7-10 Additionally, clinical trials involving the infusion of Treg cells showed a reduced incidence of aGVHD.11,12 However, because of the difficulty with generating sufficient numbers of homogenous tTreg cells ex vivo, many studies have focused on the expansion of inducible Treg (iTreg) cells. Unfortunately, iTreg cells can be unstable leading to poor persistence in vivo and the acquisition of a proinflammatory phenotype mediated by the expression of interferon γ (IFN-γ).13,14 Increasing the number and stability of iTreg cells is an active area of investigation.15,16
The aryl hydrocarbon receptor (AhR) is a ligand-dependent transcription factor, expressed by immune and epithelial cells and characterized for sensing and influencing the effects of environmental toxins.17 Previous work has shown that this response is mediated through the development and function of both innate and adaptive immune cells, which alter the polarization of CD4+ T cells into T helper 17 (Th17) cells or Treg cells. For example, FICZ, an endogenous by-product of l-tryptophan metabolism, promotes the generation of Th17 cells via AhR activation.18 In contrast, kynurenine, another tryptophan metabolite, promotes pTreg generation upon AhR activation.19 AhR activation upon binding to the xenobiotic ligand 2,3,7,8-tetrachorodibenzodioxin enhances iTreg generation from CD4+ cells in vitro while inhibiting the generation of Th1 and Th17 cells.20,21 Several mechanisms by which AhR promotes Th17 differentiation have been proposed, such as the direct binding of AhR to the Il17 gene locus, or inhibition of STAT1 phosphorylation, which reduces Th1 differentiation and enhances Th17 differentiation.22,23
Given that previous investigators demonstrated that activation of AhR can lead to enhanced Th17 generation, we hypothesized that the loss of AhR would conversely enhance iTreg generation and potentially diminish the manifestations of aGVHD. Here, we demonstrate that loss of AhR from donor T cells reduced the proliferation of effector CD4+ T cells in SLT. Additionally, the absence of AhR on murine donor T cells enhances the number of pTreg cells in the colon of recipients of AhR−/− T cells. Finally, blocking AhR on human T cells using an AhR antagonist increased the number of activated iTreg cells generated in vitro. Our study suggests that antagonists of AhR could be used to diminish the occurrence of aGVHD and enhance the generation of iTreg cells.
Materials and methods
Information regarding mouse strains, transplant protocol, histopathology, messenger RNA (mRNA) sequencing, cytokine determination, lymphocyte isolation and flow cytometry analysis, and immunohistochemistry can be found in the supplemental Methods (available on the Blood Web site).
pTreg identification
Donor T cells were isolated from wild type (WT) FoxP3-IRES-mRFP (FIR) or AhR-FIR mice. Before transfer to lethally irradiated B6D2 mice, donor Treg cells were removed by sorting for multimeric red flourescent protein (mRFP)–negative cells on a FACSAria II. pTreg cells were identified by mRFP expression using a BDLSR Fortessa. For a separate set of experiments, pTreg cells were identified by differences in the expression of the proteins Neuropilin-1 (3DS304M) and Helios (22F6) from eBioscience using monoclonal antibody staining at a dilution of 3:100. All flow cytometry data were analyzed using FlowJo version 10.1 (Tree Star Inc.).
Human iTreg in vivo generation
CD25−CD4+CD45RA+ T cells were isolated using magnetic-activated cell sorting technology from human blood.15 Purified cells were cultured with Dynabeads Human Activator CD3/CD28 beads from Life Technology, with interleukin-2 (IL-2; 300 U/mL) and transforming growth factor β1 (TGF-β1; 10 ng/mL) from Peprotech. The AhR antagonist StemRegenin-1 (SR1) was added to cultured cells at final concentration of 0.75 μM, 1.5 μM, or 2.25 μM.24 Rapamycin was added at 109 nM.15 iTreg cells were identified as CD4+FoxP3+ T cells. Human CD4 (OKT4) and FoxP3 (236A/E7) antibodies were from eBioscience.
GVT analysis
Murine mastocytoma cells’ (P815) tumor growth was measured after the injection of luciferin using the IVIS Kinetics Imaging System as described previously.25 Tumor growth was visualized weekly.
Statistics
All survival curves were constructed using the Kaplan-Meier method. Median survival times were compared using the log-rank (Mantel-Cox) test. To compare mean clinical scores, an analysis of variance was used. All other continuous variables were compared by Student t test with a correction for multiple comparisons using the Holm-Sidak method. All graphs and statistics were constructed and analyzed in GraphPad Prism. P values >.05 were considered significant. Error bars represent the standard error of the mean.
Results
Recipients of AhR−/− T cells demonstrate attenuated aGVHD
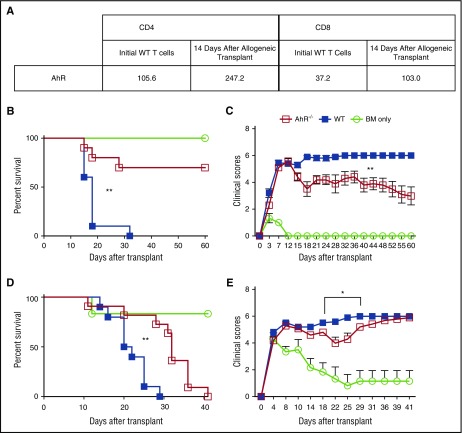
Using mRNA sequencing, we found that AhR is upregulated in both CD4+ and CD8+ donor T cells 14 days after allogeneic bone marrow transplant (allo-BMT) (Figure 1A). Given this finding and the role of AhR in Treg/Th17 polarization, we wanted to determine how the loss of AhR affected the incidence and severity of aGVHD. After allo-BMT using B6 T cells with TCD BM given to lethally irradiated B6D2 recipients, all recipients of WT T cells died as a result of aGVHD by day 32. In contrast, 70% of recipients receiving donor AhR−/− T cells and TCD BM survived until day 60 (Figure 1B). We also saw a significant decrease in clinical score for recipients receiving AhR−/− T cells compared with WT T cells (Figure 1C). We confirmed our findings in a second major histocompatibility complex–mismatch model using BALB.b recipients (Figure 1D-E). Again, we found a significant difference in median survival, and clinical score from day 18 to 29, of BALB.b recipients transplanted with AhR−/− T cells compared with BALB.b recipients receiving WT T cells. Our data indicate that the absence of expression of AhR on donor T cells decreased the incidence and severity of aGVHD.
Figure 1.
Absence of AhR leads to attenuated aGVHD. (A) Spleens from 5 B6D2 recipients were pooled sorted for CD4+ and CD8+ T cells 14 days after transplant, and mRNA sequencing was performed as described in the supplemental Methods. (B) 4.0 × 106 WT or AhR−/− T cells were transferred along with 3.0 × 106 T cell–depleted bone marrow (TCD BM) cells into lethally irradiated B6D2 recipients. Recipients were monitored for survival (B) and clinical score of GVHD (C). n = 10 for WT and AhR−/− groups; n = 3 for BM only. Representative data of 2 independent experiments. Clinical score data were found to be significant starting from day 18 after transplant. (D) 5.0 × 106 WT or AhR−/− T cells were transferred along with 3.0 × 106 TCD BM cells into lethally irradiated BALB.b recipients and monitored for survival (D) and clinical GVHD score (E). Pooled data from 2 independent experiments. n = 10 for WT, n = 11 for AhR−/−, and n = 6 for BM only. *P < .05; **P < .001 by log-rank (Mantel-Cox) test for survival curves and 2-way analysis of variance for clinical scores comparing WT and AhR−/−.
Evaluation of T cells at baseline in AhR−/− mice
One hypothesis for the decreased incidence and severity of aGVHD using AhR−/− donor T cells is differences in the number or frequency of donor T cells at baseline compared with WT mice. To evaluate this hypothesis, we examined the T-cell phenotype from AhR−/− donor T cells prior to transplantation. There was no difference in the frequency of CD4+ T cells between WT and AhR−/− donor T cells prior to infusion, but there was a reduction in CD8+ T cells (supplemental Figure 1A). We did not find a difference in the expression of T-bet, GATA3, or ROR-γ in the WT or AhR−/− T cell populations prior to infusion (supplemental Figure 1B). There was reduced frequency of AhR−/− T cells expressing CD44 compared with WT T cells (supplemental Figure 1C), but no difference in the number of naïve or central memory T cells based on the expression of CD62L. We also noted a very small but significant increase in FoxP3+ T cells in AhR−/− compared with WT mice; however, the CD4+FoxP3+ T cells expressed lower levels of Helios than those in WT T cells (supplemental Figure 1D-E) suggesting that there are fewer activated Treg cells from AhR−/− donor mice. Thus, these data would suggest that the difference in outcome using AhR−/− T cells is not correlated with decreased number of naïve T cells. As we infused CD25-depleted donor T cells, the very small difference in Treg cells at baseline in WT compared with AhR−/− mice would not mediate the significant difference in overall survival found using AhR−/− donor T cells.
Reduced inflammation in the colon following allo-BMT
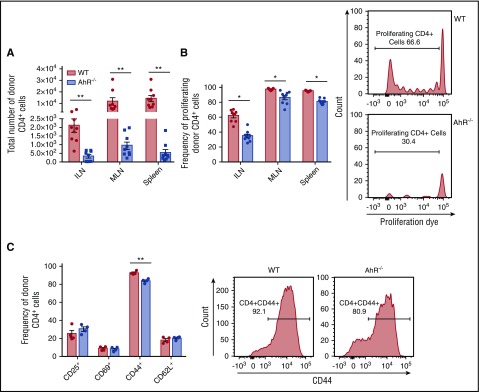
To understand the mechanism for the difference in aGVHD in the absence of AhR expression by donor T cells, we evaluated the migration of donor T cells to SLT.25-27 Interestingly, we found a significant difference in the accumulation of donor CD4+ T cells in the absence of AhR 4 days after allo-BMT in the inguinal lymph nodes (ILNs), mesenteric lymph nodes (MLNs), and spleen (Figure 2A). This correlated with decreased proliferation of AhR−/− T cells (Figure 2B) and reduced CD44 expression by AhR−/− CD4+ T Cells in the spleen (Figure 2C). We also noted a difference in accumulation of CD8+ T cells (supplemental Figure 2A), which was not accompanied by reduced proliferation but was associated with reduced expression of CD44 and increased expression of CD62L (supplemental Figure 2B-C) by donor T cells in the spleen. We found reduced migration of donor CD8+ T cells, but not CD4+ T cells into the spleen from AhR−/− T cells compared with WT T cells (supplemental Figure 3). In contrast, we did not see a reduction in the accumulation of AhR−/− T cells compared with WT T cells in BALB.b recipients and found increased proliferation of CD8+ AhR−/− donor T cells in SLT and increased proliferation of CD4+ T cells specifically in the ILN (supplemental Figure 4). Interestingly, the difference in T-cell accumulation in SLT did not mediate quantitative differences in donor CD4+ or CD8+ T cells in the lung, liver, colon, or spleen 7, 14, or 20 days after transplant (supplemental Figure 5). To further evaluate the mechanism for the decreased numbers of AhR−/− T cells on day 4, we performed in vitro assessments. We did not find a difference in the induction of apoptosis comparing WT with AhR−/− T cells suggesting that this was not the cause for the difference in T-cell accumulation (supplemental Figure 6). Thus, the absence of AhR on donor T cells affected the early accumulation of donor T cells in lymphoid tissue in B6D2 mice but did not impact their accumulation later in GVHD target organs.
Figure 2.
Reduced accumulation of AhR−/−T cells in SLT. Donor cells were stained with Cell Proliferation Dye eFluor450 prior to transplant. 4.0 × 106 WT or AhR−/− T cells were transplanted with 3.0 × 106 TCD BM cells into lethally irradiated B6D2 recipients. (A) Number of CD4+ donor T cells in SLT 4 days after transplant. (B) Frequency of proliferating cells CD4+ in SLT 4 days after transplant. Graphs represent pooled data from 2 independent experiments; n = 9 for each group. Flow cytometry plots show representative data of the ILN from 2 independent experiments. (C) Frequency of CD4+ cells expressing specific markers in the spleen 4 days after transplant; n = 4 in each group. Flow plots show representative histograms from the spleen. *P < .05; **P < .001 as indicated by Student t test.
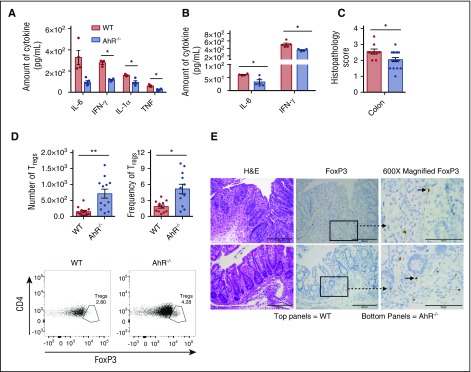
To evaluate for differences in the generation of proinflammatory cytokines by donor T cells, we evaluated the production of IL-6, IFN-γ, tumor necrosis factor (TNF), IL-1α and IL-10 by enzyme-linked immunosorbent assay on homogenates of the lung, liver, colon and spleen. At day 7 after allo-BMT, we found a nonsignificant reduction in IL-6, but significant reductions in IFN-γ, IL-1α, and TNF in the colons of B6D2 recipients transplanted with AhR−/− T cells compared with WT T cells (Figure 3A). We also saw a significant decrease in IL-6 and IFN-γ in the colon 14 days after transplant (Figure 3B). This was accompanied by a significant reduction in the histopathology score in the colon 20 days after transplant (Figure 3C), which was not seen in other organs (supplemental Figure 7). There was no difference in IL-10 expression 7 or 14 days after transplant (supplemental Figure 8A), nor did we observe a difference in the expression of TNF, IL-17, or IFN-γ in the lung, liver, or spleen or TNF and IL-17 in the colon 14 days after transplant (supplemental Figure 8B-E). Thus, the absence of AhR on donor T cells decreased the generation of proinflammatory cytokines in the colon after allo-BMT.
Figure 3.
Reduced inflammatory cytokines and increased FoxP3+Tregcells in the colons of AhR−/−T-cell recipients. Cytokine levels were determined using an enzyme-linked immunosorbent assay of colon homogenates 7 days after transplant (A) (n = 3-4 for WT; n = 4-5 for AhR−/−; representative experiment of 2 independent experiments) and 14 days after transplant (B) (n = 4-5 for WT; n = 4 for AhR−/−). Representative experiments of 2 independent experiments. (C) Colon histopathology score 20 days after transplant; n = 9 for WT, and n = 18 for AhR−/−. Pooled results from 2 independent experiments. (D) Colons were isolated and digested as described in “Materials and methods.” Lymphocytes from the colon underwent intranuclear FoxP3-staining as described in “Materials and methods” prior to analysis. N = 12 for each group; pooled results from 3 independent experiments. (E) Representative images of immunohistochemistry staining done on colon samples used for colon GVHD score evaluation. *P < .05; **P < .001 as indicated by Student t test. H&E, hematoxylin and eosin.
Increased number of Treg cells in the colon of AhR−/− T-cell recipients
To characterize the mechanism(s) for the reduced proinflammatory cytokines in AhR−/− T-cell recipients, we immunophenotyped the donor immune cells found in the gastrointestinal (GI) tract after transplantation. Using intracellular staining and flow cytometry, we found a twofold increase in the number and frequency of CD4+FoxP3+ Treg cells in the colons of AhR−/− T-cell recipients (Figure 3D). This finding was confirmed using immunohistochemistry staining for FoxP3 in the colon 20 days after transplant (Figure 3E). This finding was specific to CD4+ Treg cells in the colon as we did not see increased CD8+ Treg cells or increased CD4+ Treg cells in other organs (supplemental Figure 9).
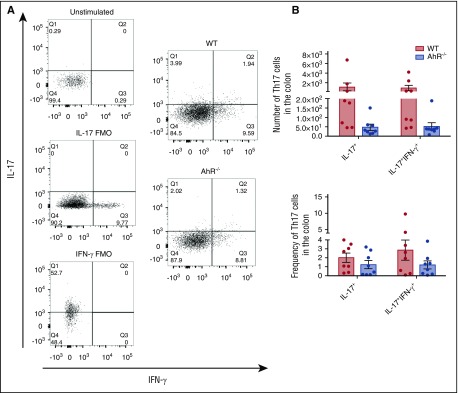
We observed a nonsignificant reduction in IL-17A (P = .19) and dual IL-17A/IFN-γ–producing donor T cells (P = .12) in the colon 14 days after transplant (Figure 4). We did not see a difference in Th1 cells in the colon 14 days after transplant (supplemental Figure 10A), nor differences in Th1, Th17, or Th17/IFN-γ T cells in the liver or spleen on days 7 (supplemental Figure 11) or 14 (supplemental Figure 10B-C) posttransplant. We also saw no difference in Tc1, Tc17, or Tc17/IFN-γ cells in the colon, liver, or spleen 14 days after transplant (supplemental Figure 12). Thus, the absence of AhR on donor T cells led to a significant increase in the accumulation of donor Treg cells in the colon concurrent with a nonsignificant decrease in the accumulation of IL-17A and dual IL-17A/IFN-γ–producing T cells in the GI tract.
Figure 4.
Assessment of Th17 cells in the colons of AhR−/−T-cell recipients 14 days after transplant. (A) Schema for the analysis of T cells isolated from the colon and a representative histogram characterizing donor B6 or AhR T cells from the colon of B6D2 recipient mice 14 days posttransplant. (B) Total number of donor T cells from WT or AhR mice and the frequency of donor T cells on day 14 posttransplant. N = 8 recipient B6D2 mice per group receiving either WT or AhR−/− T cells with WT TCD BM. Data were pooled from 2 separate experiments. FMO, fluorescence minus one controls.
Loss of AhR leads to increased number and frequency of pTreg cells in the colon
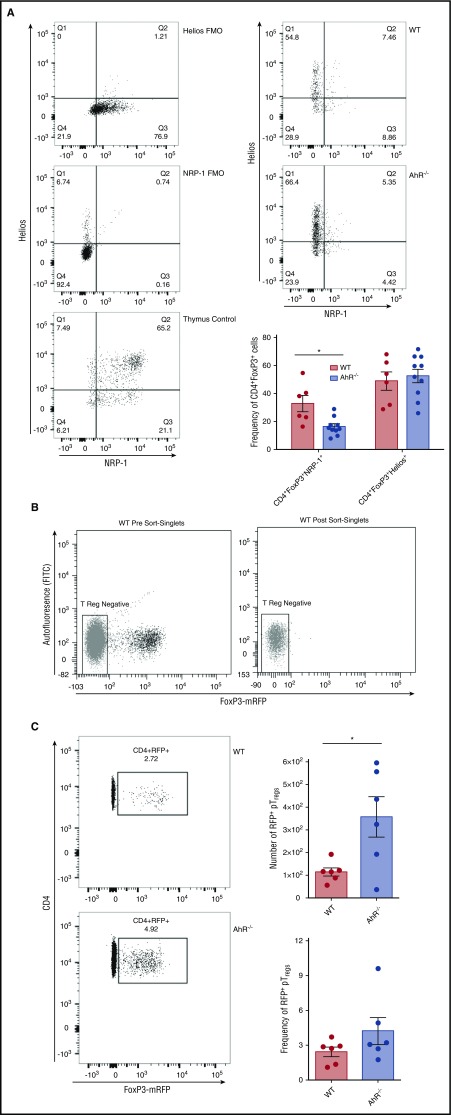
Treg cells in the colon can be either tTreg cells or pTreg cells, and these cells can be differentiated to some extent by higher expression of the type 1 transmembrane glycoprotein Neuropilin-1 on tTreg cells. A smaller proportion of the Treg cells present in the colon of AhR−/− T-cell recipients expressed Neuropilin-1 than those from WT T-cell recipients, indicating that a larger portion of these cells are consistent with a pTreg phenotype (Figure 5A).28 Additionally, we found that both WT and AhR−/− colonic Treg cells were activated as demonstrated by the equivalent expression of Helios (Figure 5A).29 To confirm the enhanced accumulation of pTreg cells in the colons of recipient mice receiving AhR−/− donor T cells, we used a genetic approach. We crossed AhR−/− mice with FIR mice to identify Treg cells by mRFP expression, which correlates with FoxP3 expression.30 To ensure that we did not transfer donor tTreg cells, we transplanted mRFP-negative T cells into lethally irradiated recipient mice (Figure 5B). After allo-BMT using mRFP-negative T cells, we isolated T cells from the colons of B6D2 recipient mice transplanted with WT-FIR or AhR−/−-FIR (AhR-FIR) mRFP-negative T cells. On day 14, we saw a significant increase in the number of mRFP+ pTreg cells in the colons of B6D2 mice receiving AhR-FIR T cells compared with WT-FIR T cells (Figure 5C). We also observed a nonsignificant increase in the number of pTreg cells in the colons of BALB.b mice receiving AhR-FIR T cells (supplemental Figure 13). Thus, the absence of AhR on donor T cells leads to an enhanced accumulation of pTreg cells in the colon.
Figure 5.
Recipients of AhR−/−T cells have increased numbers of pTregcells in the colon. (A) Colons were isolated 14 days after transplant. Cells were gated on donor CD4+FoxP3+ T cells. These cells were analyzed for Neuropilin-1 (NRP-1) and Helios expression. Graph depicts pooled results from 2 independent experiments; n = 6 for WT, and n = 10 for AhR−/− for each group. (B) Representative flow cytometry histograms of T-cell sorting for mRFP-negative cells. (C) 14 days after transplant, colons were isolated from recipients of either WT-FIR or AhR−/−-FIR T cells and analyzed for mRFP expression. Flow cytometry panels are representative images of 3 independent experiments. Graphs represent pooled data of 3 individual experiments; n = 6 in each group. *P < .05 by Student t test. FITC, fluorescein isothiocyanate.
GVT response of AhR−/− T cells
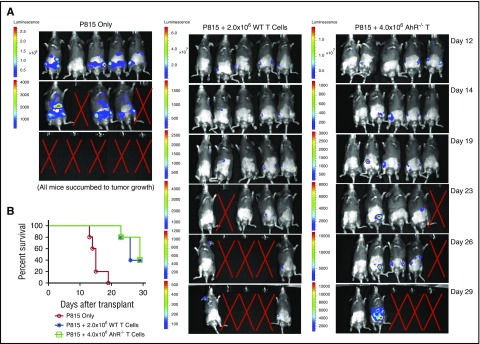
The same populations of donor T cells that mediate GVHD are critical to the GVT response that is critical to the success of allo-BMT. To determine whether AhR−/− T cells still mediate GVT, we transplanted P815-luciferase cells along with AhR−/− T cells (or WT T cells for comparison) and TCD BM cells. Recipient mice that received 4.0 × 106 WT donor T cells did not develop tumors with all mice dying from GVHD (supplemental Figure 14). To better evaluate the antitumor activity of WT T cells in the absence of lethal GVHD, we infused 2.0 × 106 WT T cells compared with 4.0 × 106 AhR−/− T cells. There was similar tumor growth in B6D2 mice receiving 2.0 × 106 WT T cells compared with recipients of 4.0 × 106 AhR−/− T cells (Figure 6A). However, unlike the use of WT T cells, the infusion of 4.0 × 106 AhR−/− T cells did not induce GVHD. Thus, elimination of AhR diminished GVHD with 4.0 × 106 AhR−/− T cells having the same GVT activity as 2.0 × 106 WT T cells (Figure 6B).
Figure 6.
Antitumor response using AhR−/−T cells. (A) 2.5 × 104 luciferase-expressing P815 cells were transferred along with 2.0 × 106 WT or 4.0 × 106 AhR−/− T cells and 3.0 × 106 TCD BM cells. Mice were monitored for tumor growth as indicated using an IVIS Kinetic System following luciferin injection. The “X” indicates death of mouse because of tumor growth; n = 5 for each group. (B) Kaplan-Meier survival for mice described in panel A.
Blocking AhR enhances the generation of human iTreg cells
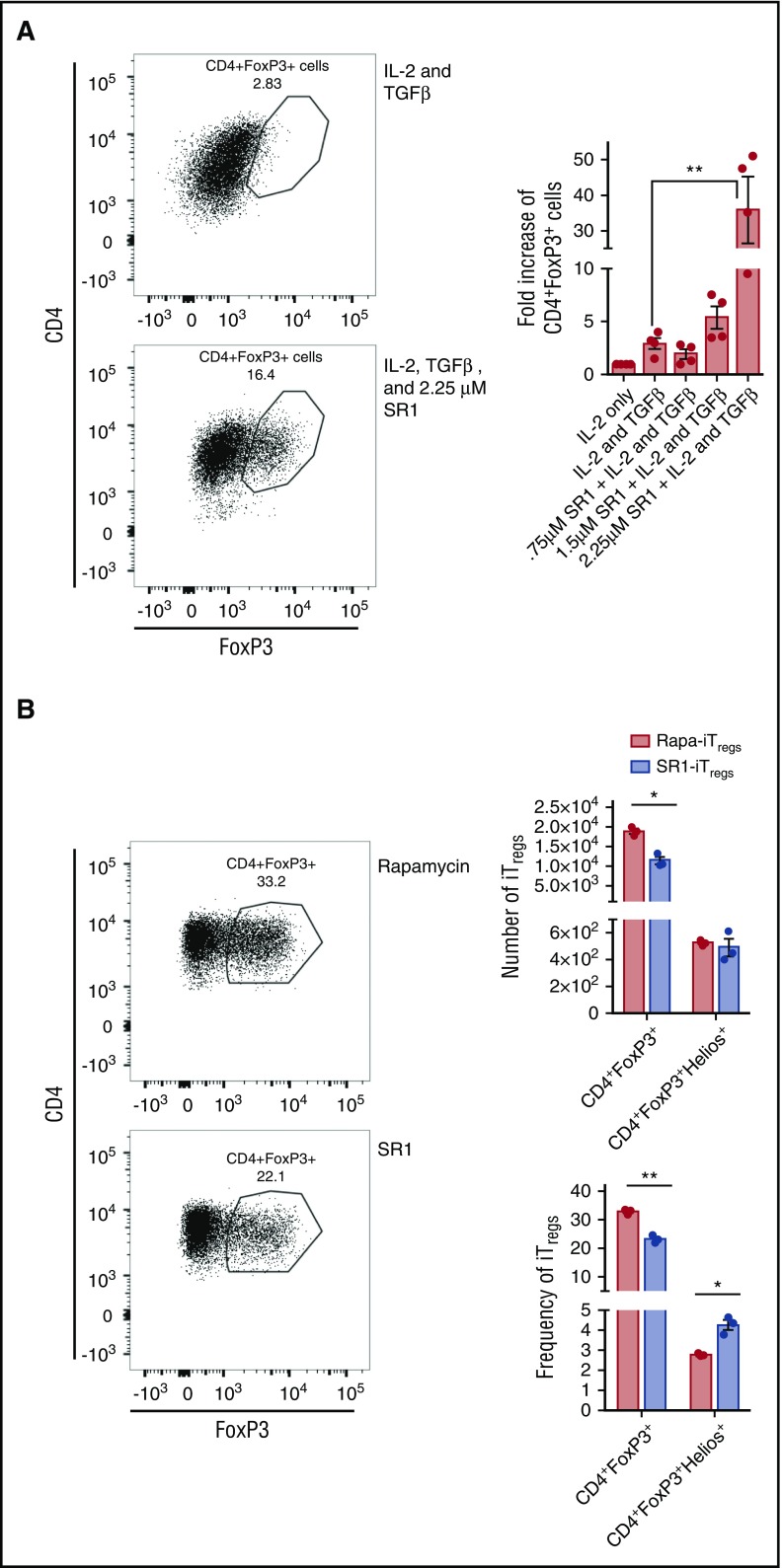
We next wanted to extrapolate our findings to human T cells, as investigators are currently trying to develop novel methods of iTreg induction that preserve iTreg stability in vivo.15,16,31 We hypothesized that inhibition of AhR on human T cells would enhance the generation of iTreg cells ex vivo. To test our hypothesis, we used SR1, a human antagonist of AhR, which specifically binds and inhibits AhR.24 We isolated naïve human CD4+ T cells from human blood and cultured them for 14 days in the presence of IL-2, TGF-β, human activator CD3/CD28 dynabeads, and with or without SR1. On day 14, T cells were isolated and analyzed for FoxP3 expression by flow cytometry. We found that naïve CD4+ cells cultured with 2.25 μM of SR1 generated eightfold more iTreg cells than those cultured with IL-2 and TGF-β alone (Figure 7A). This demonstrated that inhibiting AhR is a novel method to enhance iTreg generation.
Figure 7.
SR1 enhances iTreggeneration in vitro. Human CD4+CD45RA+ were cultured with IL-2 alone, IL-2 and TGF-β, or IL-2 and TGF-β along with 0.75 μM, 1.5 μM, or 2.25 μM of SR1 (A) or with 2.25 μM or 109 nM of rapamycin (B). After 14 days of culture, cells were isolated and stained for FoxP3 expression. Flow cytometry histograms are representative images of 2 independent experiments. Graph represents pooled data from the 2 experiments; n = 4 in each group. *P < .05; **P < .001 by Student t test.
As SR1 blocks human and not mouse AhR, we were not able to evaluate if blocking AhR led to enhanced number of pTreg cells in vivo. Thus, we evaluated the stability of iTreg cells in vivo comparing blocking AhR using SR1 with TGF-β (SR1-iTreg cells) with iTreg cells generated using TGF-β and rapamycin (Rapa-iTreg cells), as rapamycin has been shown to also induce iTreg cells.15 There was a significant increase in the frequency of activated SR1-iTreg cells compared with Rapa-iTreg cells as measured by Helios expression (Figure 7B). However, the overall frequency and number of iTreg cells from SR1-stimulated cultures was significantly less than those generated by stimulating with rapamycin (Figure 7B). Thus, inhibiting SR1 leads to the generation of more activated iTreg cells compared with iTreg cells generated with rapamycin at the expense of fewer overall cells generated in the culture.
Discussion
Our study demonstrated that loss of AhR in donor T cells attenuated aGVHD by reducing donor CD4+ T-cell proliferation in the first week posttransplant and later by increasing the number of pTreg cells present in the colon of allo-BMT recipients. We also showed that inhibition of AhR using a specific human AhR antagonist, SR1, increased the number of iTreg cells generated from human naïve CD4+ T cells. These data indicated that T-cell expression of AhR plays a critical role in maintaining proinflammatory T cells in the GI tract and blocking the development of pTreg cells post-BMT. Finally, we showed that blocking AhR might be an effective clinical method for ex vivo generation of iTreg cells for the prevention of aGVHD.
The absence of AhR on donor T cells had a profound impact on the induction of aGVHD through at least 2 different mechanisms. First, we found a substantial decrease in donor CD4+ T cells in SLT 4 days after BMT. Our group and others have shown that donor T cells are activated in SLT and that the absence of donor T-cell accumulation in the SLT and spleen can significantly diminish the incidence of aGVHD.25,27 Initially, we hypothesized that this difference could be because of alterations in T-cell trafficking, but instead we found that donor CD4+ T-cell proliferation was markedly less in the absence of AhR on day 4. The reduced proliferation may partially be because of reduced activation as indicated by the reduced CD44+ expression on AhR−/− CD4+ T cells. Decreased proliferation could also be mediated by changes in the expression of vascular endothelial growth factor and platelet-derived growth factor BB, which affect T-cell proliferation and are controlled in part by AhR.32-34 We observed a reduction in CD8+ T cells in SLT 4 days after BMT, but surprisingly, this affect was not caused by reduced proliferation but possibly mediated by reduced activation. Consistent with the diminished beneficial survival in BALB.b recipient mice, we did not observe reduced accumulation or proliferation of AhR−/− T cells in SLT. Thus, part of the improved outcome of B6D2 compared with BALB.b recipients may relate to differences in the trafficking of AhR−/− T cells in each model.
The second mechanism by which we found loss of AhR to impact aGVHD severity was through significantly increased numbers of donor pTreg cells in the colon posttransplant. This correlated with a nonsignificant decrease in the generation of Th17 and Th17/IFN-γ cells in the colon of AhR−/− T cell recipients compared with those receiving WT T cells after allo-BMT. We saw a nonsignificant increase in pTreg cells in the colons of BALB.b recipients, which additionally may have impacted the more modest decrease in aGVHD in this model. The difference in the induction of pTreg cells was specific to the colon, a critical site for the activity of pTreg cells.35,36 The generation of pTreg cells in the GI tract is a complex process mediated by innate immune cells and the host microbiome. CD103+ dendritic cells in the MLN can induce the development of pTreg cells in a retinoic acid and TGF-β–dependent manner.37 Additionally, commensal bacterial antigens, which are more abundant in the colon than the lung, liver, or spleen, induce the generation of pTreg cells.38 The function of different populations of innate cells and the microbiome in generating pTreg cells in the colon may be 1 hypothesis why the absence of AhR only affected the maintenance of pTreg cells in the GI tract.
Our data indicate that AhR expression in donor T cells enhanced the proinflammatory response found after allo-BMT. This is contrary to previous findings using exogenous ligands of AhR. The exogenous ligand 2,3,7,8-tetrachorodibenzodioxin generates Tr1-like (CD4+CD25+FoxP3−) Treg cells found in the spleen 2 to 3 days after transplant that are capable of attenuating GVHD.21,39 Benzimidazoisoquinolines, a novel class of AhR agonists, decreased acute GVHD by generating Tr1 cells early after transplant.40 However, the function of ligands of AhR is quite complex as different ligands have been shown to both induce immunosuppressive Treg cells and proinflammatory Th17 cells. Thus, although the absence of AhR on donor T cells diminished the incidence and severity of acute GVHD, it is less clear if exogenous AhR ligands would enhance or diminish acute GVHD of the colon. Future experiments could use mice with constitutive Cyp1a1 expression that have been shown to deplete endogenous AhR ligands41 to better understand the in vivo function of AhR ligands posttransplant. Additionally, it would be clinically relevant to address the effect of dietary indoles, such as indole-3-carbinol, a ligand for AhR that is found in cruciferous vegetables, on GVHD pathogenesis.42 As indole-3-carbinol has been shown to reduce Th17 cells and increase Treg cells in the setting of delayed-type hypersensitivity, it is possible that the absence of certain dietary ligands posttransplant could enhance GVHD.18
Restoring immune regulation through the transfer of donor Treg cells to prevent aGVHD has received significant enthusiasm. Unfortunately, a significant barrier to the clinical application of this therapy has been the difficulty with obtaining sufficient quantities of donor Treg cells. To circumvent this concern, previous groups have generated iTreg cells by culturing CD4+ T cells in the presence of immunosuppressive cytokines such as TGF-β or expansion in the presence of the mechanistic target of rapamycin.15 However, the in vivo function of these cells is not clear, as iTreg cells expanded with either allogeneic dendritic cells or anti-CD3/anti-CD28 monoclonal antibodies did not protect mice from lethal aGVHD,13,14 whereas human iTreg cells expanded with rapamycin suppress GVHD in a xenogeneic model.15 In the current manuscript, we have characterized another novel approach for enhancing iTreg numbers: the blockade of AhR. Blocking AhR in vitro using SR1 substantially increased the number of human iTreg cells after 2 weeks in culture. We found that iTreg cells induced by SR1 were more activated as demonstrated by increased expression of Helios compared with iTreg cells induced by rapamycin. Whether the enhanced activation of iTreg cells in vitro with SR1 at the expense of the overall number of iTreg cells generated will mediate greater reduction of acute GVHD in vivo could not be determined as SR1 is not an antagonist for mouse AhR. As SR1 is being evaluated in clinical trials for hematopoietic stem cell expansion (Cancer Therapy Evaluation Program; www.clinicaltrials.gov, #NCT02765997), this approach could be rapidly translated into the clinic.
In conclusion, we demonstrate a critical role for AhR in blocking the generation of pTreg cells in the lower GI tract post-BMT. AhR inhibitors, which are being evaluated to treat autoimmunity, should be evaluated clinically for the treatment/prevention of GVHD.
Acknowledgments
The authors would also like to thank the University of North Carolina Flow Cytometry Core for performing fluorescence-activated cell sorting. Animal histopathology was performed in the Lineberger Comprehensive Cancer Center (LCCC) Animal Histopathology Core Facility at the University of North Carolina at Chapel Hill with special assistance from Dawud Hilliard.
This work was supported by grants from the National Cancer Institute (R01 CA166794 [J.S.S.] and P30 CA016086 [Flow Cytometry Core]) and National Heart, Lung, and Blood Institute (R01 HL115761 [J.S.S.] and R01 HL11879 [B.R.B.]), National Institutes of Health. Research reported in this publication was supported in part by the North Carolina Biotech Center Institutional Support Grant 2012-IDG-1006. The LCCC Animal Histopathology Core is supported in part by a National Cancer Institute Center Core Support Grant (2P30CA016086-40) (University of North Carolina LCCC).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.A.D. performed experiments and wrote the manuscript; K.L.L. performed experiments; D.W.B. performed experiments and edited the manuscript; S.A.M. evaluated immunohistochemistry staining; O.V.K. and H.B. performed experiments and edited the manuscript; L.M.B. aided in flow cytometry analysis techniques; J.T.W. analyzed histopathology samples; K.P.M. edited the manuscript; F.J.G. provided the AhR−/− murine line and edited the manuscript; B.R.B. provided the SR1 molecule and reviewed and edited the manuscript; B.G.V. performed the RNA-sequencing analysis and reviewed and edited the manuscript; J.M.C. reviewed and edited the manuscript; and J.S.S. conceived of and supervised completion of this project and reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan S. Serody, University of North Carolina at Chapel Hill, 5012 Marsico Hall, 125 Mason Farm Rd, Chapel Hill, NC 27599-7295; e-mail: jonathan_serody@med.unc.edu.
References
- 1.Dignan FL, Clark A, Amrolia P, et al. ; Haemato-oncology Task Force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158(1):30-45. [DOI] [PubMed] [Google Scholar]
- 2.Beilhack A, Schulz S, Baker J, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106(3):1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105(11):4191-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167(8):4245-4253. [DOI] [PubMed] [Google Scholar]
- 6.Trenado A, Charlotte F, Fisson S, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112(11):1688-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144-1150. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trzonkowski P, Bieniaszewska M, Juścińska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133(1):22-26. [DOI] [PubMed] [Google Scholar]
- 10.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921-3928. [DOI] [PubMed] [Google Scholar]
- 11.Brunstein CG, Miller JS, McKenna DH, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127(8):1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beres A, Komorowski R, Mihara M, Drobyski WR. Instability of Foxp3 expression limits the ability of induced regulatory T cells to mitigate graft versus host disease. Clin Cancer Res. 2011;17(12):3969-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenecke C, Czeloth N, Bubke A, et al. Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur J Immunol. 2009;39(11):3091-3096. [DOI] [PubMed] [Google Scholar]
- 15.Hippen KL, Merkel SC, Schirm DK, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11(6):1148-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, Tey SK, Koyama M, et al. Induced regulatory T cells promote tolerance when stabilized by rapamycin and IL-2 in vivo. J Immunol. 2013;191(10):5291-5303. [DOI] [PubMed] [Google Scholar]
- 17.Kerkvliet NI, Shepherd DM, Baecher-Steppan L. T lymphocytes are direct, aryl hydrocarbon receptor (AhR)-dependent targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): AhR expression in both CD4+ and CD8+ T cells is necessary for full suppression of a cytotoxic T lymphocyte response by TCDD. Toxicol Appl Pharmacol. 2002;185(2):146-152. [DOI] [PubMed] [Google Scholar]
- 18.Singh NP, Singh UP, Rouse M, et al. Dietary indoles suppress delayed-type hypersensitivity by inducing a switch from proinflammatory Th17 cells to anti-inflammatory regulatory T cells through regulation of microRNA. J Immunol. 2016;196(3):1108-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veiga-Parga T, Suryawanshi A, Rouse BT. Controlling viral immuno-inflammatory lesions by modulating aryl hydrocarbon receptor signaling. PLoS Pathog. 2011;7(12):e1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol. 2005;175(7):4184-4188. [DOI] [PubMed] [Google Scholar]
- 22.Cui G, Qin X, Wu L, et al. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Invest. 2011;121(2):658-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105(28):9721-9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coghill JM, Carlson MJ, Panoskaltsis-Mortari A, et al. Separation of graft-versus-host disease from graft-versus-leukemia responses by targeting CC-chemokine receptor 7 on donor T cells. Blood. 2010;115(23):4914-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fulton LM, Carlson MJ, Coghill JM, et al. Attenuation of acute graft-versus-host disease in the absence of the transcription factor RORγt. J Immunol. 2012;189(4):1765-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beilhack A, Schulz S, Baker J, et al. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111(5):2919-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav M, Louvet C, Davini D, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209(10):1713-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkord E, Abd Al Samid M, Chaudhary B. Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP. Oncotarget. 2015;6(24):20026-20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102(14):5126-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierini A, Strober W, Moffett C, et al. TNF-α priming enhances CD4+FoxP3+ regulatory T-cell suppressive function in murine GVHD prevention and treatment. Blood. 2016;128(6):866-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terashima J, Tachikawa C, Kudo K, Habano W, Ozawa S. An aryl hydrocarbon receptor induces VEGF expression through ATF4 under glucose deprivation in HepG2. BMC Mol Biol. 2013;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaguin M, Fardel O, Lecureur V. AhR-dependent secretion of PDGF-BB by human classically activated macrophages exposed to DEP extracts stimulates lung fibroblast proliferation. Toxicol Appl Pharmacol. 2015;285(3):170-178. [DOI] [PubMed] [Google Scholar]
- 34.Chen CF, Feng X, Liao HY, et al. Regulation of T cell proliferation by JMJD6 and PDGF-BB during chronic hepatitis B infection. Sci Rep. 2014;4:6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe N, Kaminuma O, Kitamura N, Hiroi T. Induced Treg cells augment the Th17-mediated intestinal inflammatory response in a CTLA4-dependent manner. PLoS One. 2016;11(3):e0150244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232-236. [DOI] [PubMed] [Google Scholar]
- 37.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lathrop SK, Bloom SM, Rao SM, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohlman D, Punj S, Pennington J, Bradford S, Kerkvliet NI. Suppression of acute graft-versus-host response by TCDD is independent of the CTLA-4-IFN-γ-IDO pathway. Toxicol Sci. 2013;135(1):81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Punj S, Kopparapu P, Jang HS, et al. Benzimidazoisoquinolines: a new class of rapidly metabolized aryl hydrocarbon receptor (AhR) ligands that induce AhR-dependent Tregs and prevent murine graft-versus-host disease. PLoS One. 2014;9(2):e88726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiering C, Wincent E, Metidji A, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542(7640):242-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujioka N, Ransom BW, Carmella SG, et al. Harnessing the power of cruciferous vegetables: developing a biomarker for Brassica vegetable consumption using urinary 3,3′-diindolylmethane. Cancer Prev Res (Phila). 2016;9(10):788-793. [DOI] [PMC free article] [PubMed] [Google Scholar]