Abstract
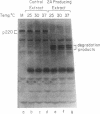
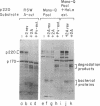
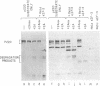
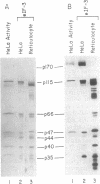
After cultured cells are infected with poliovirus, cellular mRNA fails to bind to ribosomes, and synthesis of the majority of cellular proteins ceases. The defective step has been localized to the cap-dependent activity of the eukaryotic translation initiation factor 4F. Inactivation of this factor correlates with the cleavage of its largest subunit, p220, into characteristic products observed in infected cells. This cleavage is mediated by the poliovirus protease 2Apro. Previous work suggests that 2Apro does not catalyze the reaction directly, suggesting that one or more cellular proteins is required for the degradation of p220. To identify such a protein, we have developed an assay in which cleavage of a p220 substrate in the presence of poliovirus 2Apro is dependent upon the addition of HeLa cell proteins. By using this assay, we show that another factor, eukaryotic translation initiation factor 3, is required for 2Apro-dependent cleavage of p220.
Full text
PDF


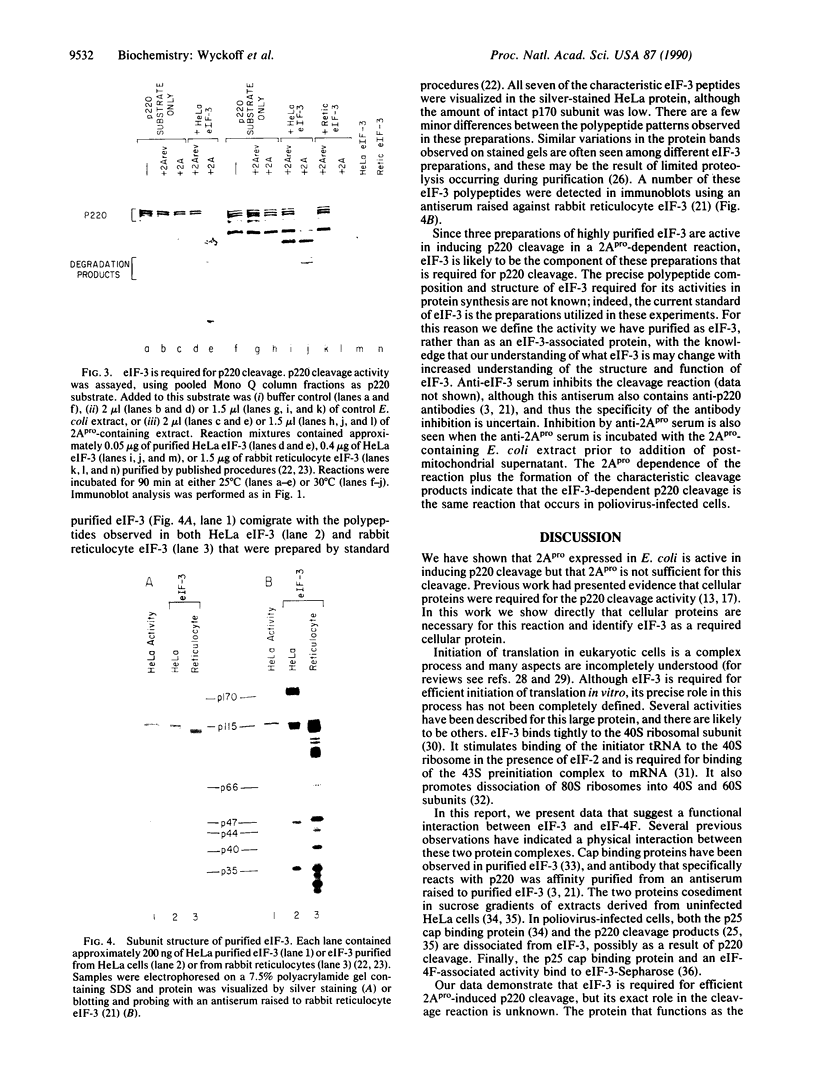

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R., Brown-Luedi M. L., Hershey J. W. Protein synthesis initiation factors from rabbit reticulocytes: purification, characterization, and radiochemical labeling. Methods Enzymol. 1979;60:15–35. doi: 10.1016/s0076-6879(79)60005-8. [DOI] [PubMed] [Google Scholar]
- Benne R., Hershey J. W. Purification and characterization of initiation factor IF-E3 from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3005–3009. doi: 10.1073/pnas.73.9.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Hershey J. W. The mechanism of action of protein synthesis initiation factors from rabbit reticulocytes. J Biol Chem. 1978 May 10;253(9):3078–3087. [PubMed] [Google Scholar]
- Bernstein H. D., Sonenberg N., Baltimore D. Poliovirus mutant that does not selectively inhibit host cell protein synthesis. Mol Cell Biol. 1985 Nov;5(11):2913–2923. doi: 10.1128/mcb.5.11.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Luedi M. L., Meyer L. J., Milburn S. C., Yau P. M., Corbett S., Hershey J. W. Protein synthesis initiation factors from human HeLa cells and rabbit reticulocytes are similar: comparison of protein structure, activities, and immunochemical properties. Biochemistry. 1982 Aug 31;21(18):4202–4206. doi: 10.1021/bi00261a002. [DOI] [PubMed] [Google Scholar]
- Brown B. A., Ehrenfeld E. Translation of poliovirus RNA in vitro: changes in cleavage pattern and initiation sites by ribosomal salt wash. Virology. 1979 Sep;97(2):396–405. doi: 10.1016/0042-6822(79)90350-7. [DOI] [PubMed] [Google Scholar]
- Dieckmann C. L., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP6, a yeast nuclear gene necessary for synthesis of cytochrome b. J Biol Chem. 1985 Feb 10;260(3):1513–1520. [PubMed] [Google Scholar]
- Etchison D., Etchison J. R. Monoclonal antibody-aided characterization of cellular p220 in uninfected and poliovirus-infected HeLa cells: subcellular distribution and identification of conformers. J Virol. 1987 Sep;61(9):2702–2710. doi: 10.1128/jvi.61.9.2702-2710.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Hansen J., Ehrenfeld E., Edery I., Sonenberg N., Milburn S., Hershey J. W. Demonstration in vitro that eucaryotic initiation factor 3 is active but that a cap-binding protein complex is inactive in poliovirus-infected HeLa cells. J Virol. 1984 Sep;51(3):832–837. doi: 10.1128/jvi.51.3.832-837.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Milburn S. C., Edery I., Sonenberg N., Hershey J. W. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982 Dec 25;257(24):14806–14810. [PubMed] [Google Scholar]
- Etchison D., Smith K. Variations in cap-binding complexes from uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1990 May 5;265(13):7492–7500. [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Hansen J., Etchison D., Hershey J. W., Ehrenfeld E. Association of cap-binding protein with eucaryotic initiation factor 3 in initiation factor preparations from uninfected and poliovirus-infected HeLa cells. J Virol. 1982 Apr;42(1):200–207. doi: 10.1128/jvi.42.1.200-207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett M. J., Rose J. K., Baltimore D. 5'-terminal structure of poliovirus polyribosomal RNA is pUp. Proc Natl Acad Sci U S A. 1976 Feb;73(2):327–330. doi: 10.1073/pnas.73.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Nicklin M. J., Toyoda H., Etchison D., Wimmer E. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J Virol. 1987 Sep;61(9):2711–2718. doi: 10.1128/jvi.61.9.2711-2718.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Edery I., Hanecak R., Wimmer E., Sonenberg N. Poliovirus protease 3C (P3-7c) does not cleave P220 of the eucaryotic mRNA cap-binding protein complex. J Virol. 1985 Aug;55(2):489–493. doi: 10.1128/jvi.55.2.489-493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. E., Etchison D., Ehrenfeld E. Poliovirus protease does not mediate cleavage of the 220,000-Da component of the cap binding protein complex. Proc Natl Acad Sci U S A. 1985 May;82(9):2723–2727. doi: 10.1073/pnas.82.9.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. E., Grubman M. J., Ehrenfeld E. Relationship of p220 cleavage during picornavirus infection to 2A proteinase sequencing. J Virol. 1988 Nov;62(11):4216–4223. doi: 10.1128/jvi.62.11.4216-4223.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. E., Jense H. G., Ehrenfeld E. Restriction of translation of capped mRNA in vitro as a model for poliovirus-induced inhibition of host cell protein synthesis: relationship to p220 cleavage. J Virol. 1987 Aug;61(8):2480–2488. doi: 10.1128/jvi.61.8.2480-2488.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. E., Toyoda H., Etchison D., Wimmer E., Ehrenfeld E. Cleavage of the cap binding protein complex polypeptide p220 is not effected by the second poliovirus protease 2A. Virology. 1986 Apr 15;150(1):299–303. doi: 10.1016/0042-6822(86)90291-6. [DOI] [PubMed] [Google Scholar]
- Meyer L. J., Brown-Luedi M. L., Corbett S., Tolan D. R., Hershey J. W. The purification and characterization of multiple forms of protein synthesis eukaryotic initiation factors 2, 3, and 5 from rabbit reticulocytes. J Biol Chem. 1981 Jan 10;256(1):351–356. [PubMed] [Google Scholar]
- Meyer L. J., Milburn S. C., Hershey J. W. Immunochemical characterization of mammalian protein synthesis initiation factors. Biochemistry. 1982 Aug 31;21(18):4206–4212. doi: 10.1021/bi00261a003. [DOI] [PubMed] [Google Scholar]
- Milburn S. C., Duncan R. F., Hershey J. W. Immunoblot analysis of the structure of protein synthesis initiation factor eIF3 from HeLa cells. Arch Biochem Biophys. 1990 Jan;276(1):6–11. doi: 10.1016/0003-9861(90)90002-g. [DOI] [PubMed] [Google Scholar]
- Moldave K. Eukaryotic protein synthesis. Annu Rev Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- Nicklin M. J., Kräusslich H. G., Toyoda H., Dunn J. J., Wimmer E. Poliovirus polypeptide precursors: expression in vitro and processing by exogenous 3C and 2A proteinases. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4002–4006. doi: 10.1073/pnas.84.12.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto A., Lee Y. F., Wimmer E. The 5' end of poliovirus mRNA is not capped with m7G(5')ppp(5')Np. Proc Natl Acad Sci U S A. 1976 Feb;73(2):375–380. doi: 10.1073/pnas.73.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. E., Racaniello V. R. Inhibition of translation in cells infected with a poliovirus 2Apro mutant correlates with phosphorylation of the alpha subunit of eucaryotic initiation factor 2. J Virol. 1989 Dec;63(12):5069–5075. doi: 10.1128/jvi.63.12.5069-5075.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain V. M. Initiation of protein synthesis in mammalian cells. Biochem J. 1986 May 1;235(3):625–637. doi: 10.1042/bj2350625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Shenk T. Impact of virus infection on host cell protein synthesis. Annu Rev Biochem. 1987;56:317–332. doi: 10.1146/annurev.bi.56.070187.001533. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Pelletier J. Poliovirus translation: a paradigm for a novel initiation mechanism. Bioessays. 1989 Nov;11(5):128–132. doi: 10.1002/bies.950110504. [DOI] [PubMed] [Google Scholar]
- Sun X. H., Baltimore D. Human immunodeficiency virus tat-activated expression of poliovirus protein 2A inhibits mRNA translation. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2143–2146. doi: 10.1073/pnas.86.7.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara S. M., Morgan M. A., Shatkin A. J. Two forms of purified m7G-cap binding protein with different effects on capped mRNA translation in extracts of uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1981 Aug 10;256(15):7691–7694. [PubMed] [Google Scholar]
- Thompson H. A., Sadnik I., Scheinbuks J., Moldave K. Studies on native ribosomal subunits from rat liver. Purification and characterization of a ribosome dissociation factor. Biochemistry. 1977 May 17;16(10):2221–2230. doi: 10.1021/bi00629a028. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Nicklin M. J., Murray M. G., Anderson C. W., Dunn J. J., Studier F. W., Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986 Jun 6;45(5):761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Sonenberg N., Shatkin A. J., Rose J. K., Leong K., Bergmann J. E., Gordon J., Baltimore D. Purification of a factor that restores translation of vesicular stomatitis virus mRNA in extracts from poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):770–774. doi: 10.1073/pnas.77.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]