Abstract
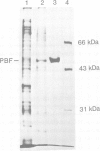
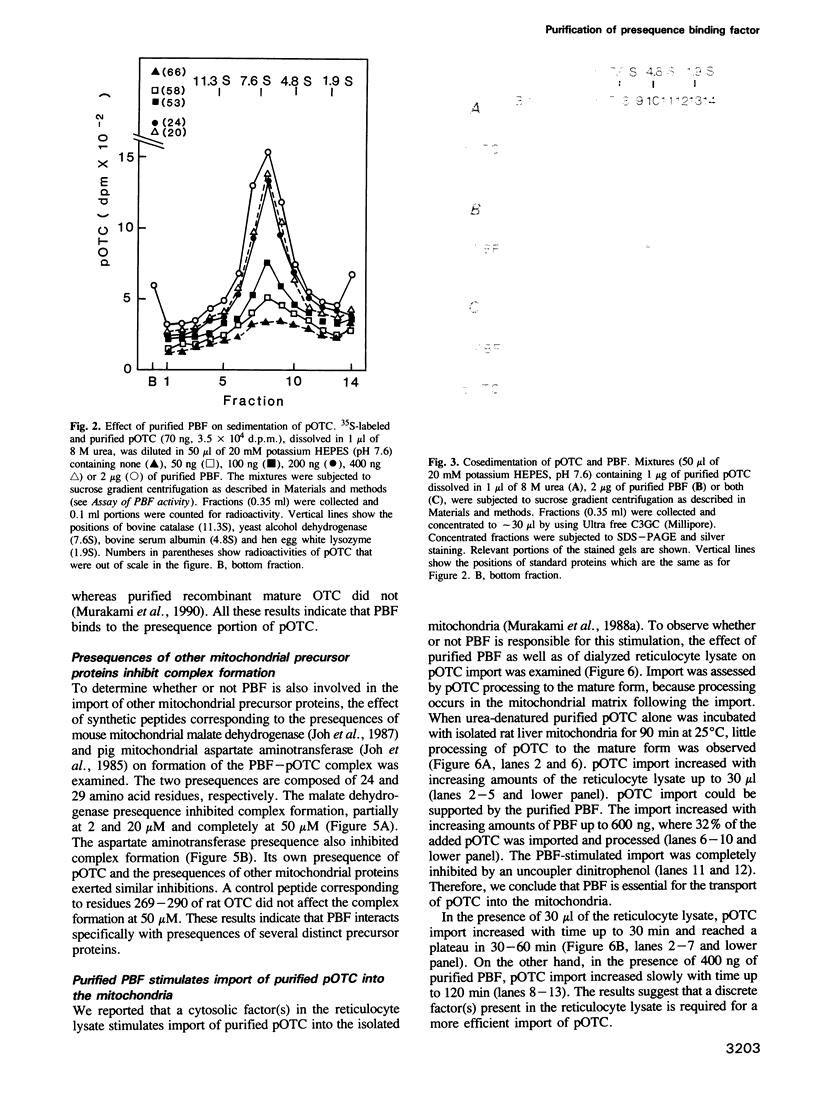
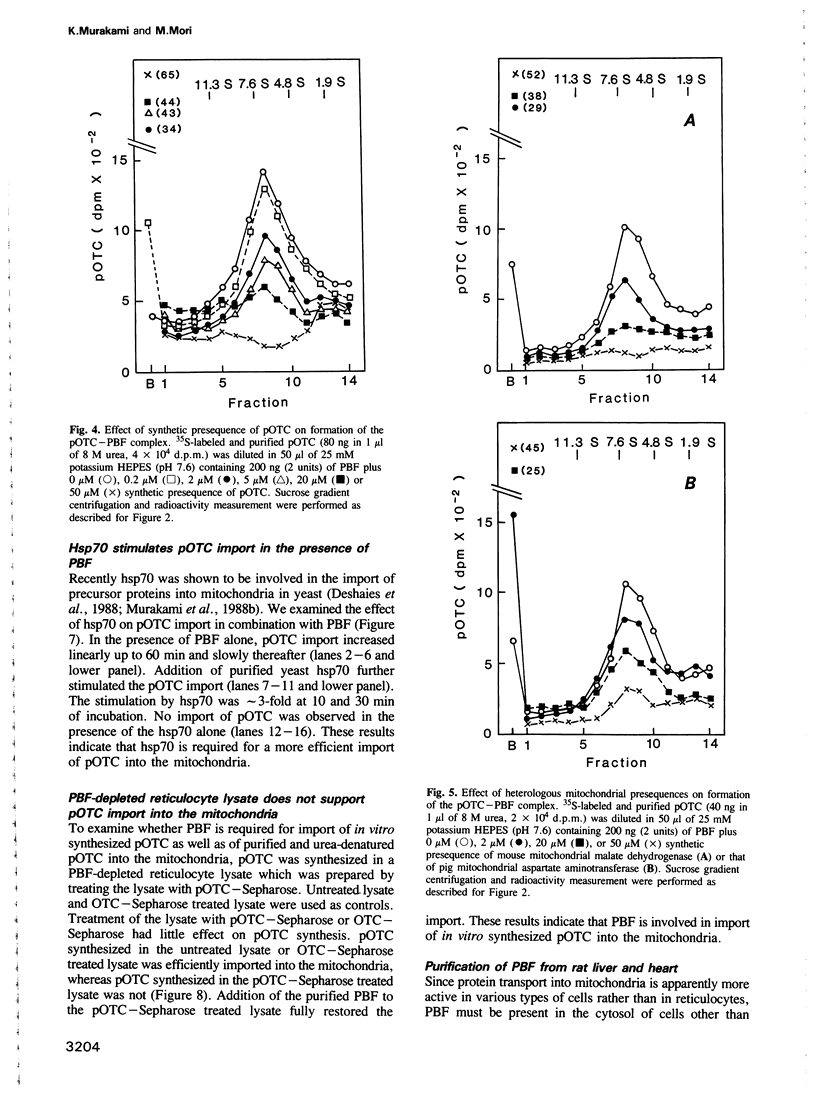
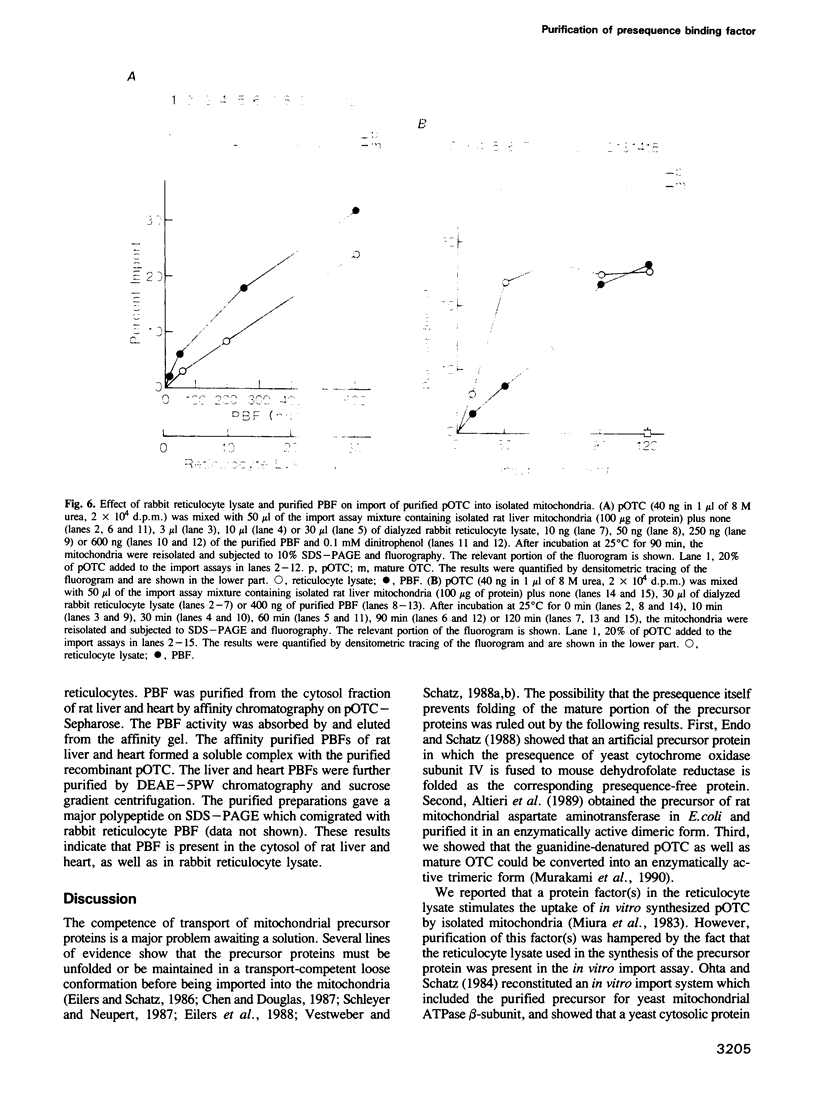
In vitro mitochondrial import of the purified precursor form (pOTC) of rat ornithine carbamoyltransferase (OTC) is stimulated by a cytosolic factor(s) contained in rabbit reticulocyte lysate. A protein factor that binds to pOTC but not to mature OTC and was named presequence binding factor or PBF, was purified 91,000-fold from the lysate by affinity chromatography using pOTC-bound Sepharose, DEAE-5PW HPLC and sucrose gradient centrifugation. The purified PBF migrated as a single polypeptide of 50,000 daltons on SDS-PAGE. On sucrose gradients, urea-denatured pOTC sedimented to the bottom, whereas PBF sedimented with an S20,w value of 5.5S. When pOTC and PBF were centrifuged together, both polypeptides sedimented as a complex of 7.1S. Formation of the pOTC-PBF complex was inhibited by micromolar concentrations of the synthetic presequence of pOTC and those of other mitochondrial precursor proteins. The purified PBF markedly stimulated the import of purified or in vitro synthesized pOTC into the mitochondria. PBF-stimulated pOTC import was further enhanced by a 70 kd heat shock protein (hsp 70) purified from yeast; the hsp70 alone had little effect. Thus, PBF binds to the presequence portion of the precursors and may hold them in a transport-competent form in cooperation with hsp70.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri F., Mattingly J. R., Jr, Rodriguez-Berrocal F. J., Youssef J., Iriarte A., Wu T. H., Martinez-Carrion M. Isolation and properties of a liver mitochondrial precursor protein to aspartate aminotransferase expressed in Escherichia coli. J Biol Chem. 1989 Mar 25;264(9):4782–4786. [PubMed] [Google Scholar]
- Chen W. J., Douglas M. G. The role of protein structure in the mitochondrial import pathway. Analysis of the soluble F1-ATPase beta-subunit precursor. J Biol Chem. 1987 Nov 15;262(32):15598–15604. [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Conboy J. G., Kalousek F., Rosenberg L. E. In vitro synthesis of a putative precursor of mitochondrial ornithine transcarbamoylase. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5724–5727. doi: 10.1073/pnas.76.11.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., McCammon M. T., Vassarotti A. Targeting proteins into mitochondria. Microbiol Rev. 1986 Jun;50(2):166–178. doi: 10.1128/mr.50.2.166-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M., Hwang S., Schatz G. Unfolding and refolding of a purified precursor protein during import into isolated mitochondria. EMBO J. 1988 Apr;7(4):1139–1145. doi: 10.1002/j.1460-2075.1988.tb02923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Endo T., Schatz G. Latent membrane perturbation activity of a mitochondrial precursor protein is exposed by unfolding. EMBO J. 1988 Apr;7(4):1153–1158. doi: 10.1002/j.1460-2075.1988.tb02925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Williams K. R., Kalousek F., Kraus J. P., Doolittle R. F., Konigsberg W., Rosenberg L. E. Structure and expression of a complementary DNA for the nuclear coded precursor of human mitochondrial ornithine transcarbamylase. Science. 1984 Jun 8;224(4653):1068–1074. doi: 10.1126/science.6372096. [DOI] [PubMed] [Google Scholar]
- Joh T., Nomiyama H., Maeda S., Shimada K., Morino Y. Cloning and sequence analysis of a cDNA encoding porcine mitochondrial aspartate aminotransferase precursor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6065–6069. doi: 10.1073/pnas.82.18.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh T., Takeshima H., Tsuzuki T., Shimada K., Tanase S., Morino Y. Cloning and sequence analysis of cDNAs encoding mammalian mitochondrial malate dehydrogenase. Biochemistry. 1987 May 5;26(9):2515–2520. doi: 10.1021/bi00383a017. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Mori M., Amaya Y., Tatibana M. A mitochondrial protease that cleaves the precursor of ornithine carbamoyltransferase. Purification and properties. Eur J Biochem. 1982 Mar 1;122(3):641–647. [PubMed] [Google Scholar]
- Miura S., Mori M., Tatibana M. Transport of ornithine carbamoyltransferase precursor into mitochondria. Stimulation by potassium ion, magnesium ion, and a reticulocyte cytosolic protein(s). J Biol Chem. 1983 Jun 10;258(11):6671–6674. [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Processing of a putative precursor of rat liver ornithine transcarbamylase, a mitochondrial matrix enzyme. J Biochem. 1980 Dec;88(6):1829–1836. doi: 10.1093/oxfordjournals.jbchem.a133158. [DOI] [PubMed] [Google Scholar]
- Mori M., Morita T., Ikeda F., Amaya Y., Tatibana M., Cohen P. P. Synthesis, intracellular transport, and processing of the precursors for mitochondrial ornithine transcarbamylase and carbamoyl-phosphate synthetase I in isolated hepatocytes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6056–6060. doi: 10.1073/pnas.78.10.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Morita T., Miura S., Tatibana M. Uptake and processing of the precursor for rat liver ornithine transcarbamylase by isolated mitochondria. Inhibition by uncouplers. J Biol Chem. 1981 Aug 25;256(16):8263–8266. [PubMed] [Google Scholar]
- Morita T., Miura S., Mori M., Tatibana M. Transport of the precursor for rat-liver ornithine carbamoyltransferase into mitochondria in vitro. Eur J Biochem. 1982 Mar 1;122(3):501–509. doi: 10.1111/j.1432-1033.1982.tb06465.x. [DOI] [PubMed] [Google Scholar]
- Murakami H., Pain D., Blobel G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988 Dec;107(6 Pt 1):2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Amaya Y., Takiguchi M., Ebina Y., Mori M. Reconstitution of mitochondrial protein transport with purified ornithine carbamoyltransferase precursor expressed in Escherichia coli. J Biol Chem. 1988 Dec 5;263(34):18437–18442. [PubMed] [Google Scholar]
- Murakami K., Tokunaga F., Iwanaga S., Mori M. Presequence does not prevent folding of a purified mitochondrial precursor protein and is essential for association with a reticulocyte cytosolic factor(s). J Biochem. 1990 Aug;108(2):207–214. doi: 10.1093/oxfordjournals.jbchem.a123182. [DOI] [PubMed] [Google Scholar]
- Ohta S., Schatz G. A purified precursor polypeptide requires a cytosolic protein fraction for import into mitochondria. EMBO J. 1984 Mar;3(3):651–657. doi: 10.1002/j.1460-2075.1984.tb01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H., Tuboi S. The cytosolic factor required for import of precursors of mitochondrial proteins into mitochondria. J Biol Chem. 1988 Mar 5;263(7):3188–3193. [PubMed] [Google Scholar]
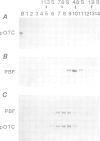
- Pfaller R., Neupert W. High-affinity binding sites involved in the import of porin into mitochondria. EMBO J. 1987 Sep;6(9):2635–2642. doi: 10.1002/j.1460-2075.1987.tb02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Hartl F. U., Neupert W. Import of proteins into mitochondria: a multi-step process. Eur J Biochem. 1988 Aug 1;175(2):205–212. doi: 10.1111/j.1432-1033.1988.tb14185.x. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Müller H. K., Harmey M. A., Neupert W. Mitochondrial protein import: involvement of the mature part of a cleavable precursor protein in the binding to receptor sites. EMBO J. 1987 Nov;6(11):3449–3454. doi: 10.1002/j.1460-2075.1987.tb02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Tropschug M., Neupert W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell. 1987 Jun 19;49(6):815–823. doi: 10.1016/0092-8674(87)90619-2. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Rhoads R. E., McKnight G. S. Assay of ovalbumin mRNA in reticulocyte lysate. Methods Enzymol. 1974;30:694–701. doi: 10.1016/0076-6879(74)30066-3. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi M., Miura S., Mori M., Tatibana M., Nagata S., Kaziro Y. Molecular cloning and nucleotide sequence of cDNA for rat ornithine carbamoyltransferase precursor. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7412–7416. doi: 10.1073/pnas.81.23.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner K., Schatz G. Import of an incompletely folded precursor protein into isolated mitochondria requires an energized inner membrane, but no added ATP. EMBO J. 1987 Aug;6(8):2449–2456. doi: 10.1002/j.1460-2075.1987.tb02524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. A chimeric mitochondrial precursor protein with internal disulfide bridges blocks import of authentic precursors into mitochondria and allows quantitation of import sites. J Cell Biol. 1988 Dec;107(6 Pt 1):2037–2043. doi: 10.1083/jcb.107.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. Point mutations destabilizing a precursor protein enhance its post-translational import into mitochondria. EMBO J. 1988 Apr;7(4):1147–1151. doi: 10.1002/j.1460-2075.1988.tb02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]