Abstract
Morphine is the prototypic mu opioid, producing its analgesic actions through traditional 7 transmembrane domain (7TM) G-protein coupled receptors generated by the mu opioid receptor gene (Oprm1). However, the Oprm1 gene undergoes extensive alternative splicing to yield three structurally distinct sets of splice variants. In addition to the full length 7TM receptors, it produces a set of truncated variants comprised of only 6 transmembrane domains (6TM). This study explored the relative contributions of 7TM and 6TM variants in a range of other morphine actions. Loss of the 6TM variants in an exon 11 knockout (E11 KO) mouse did not affect morphine analgesia, reward or respiratory depression. However, E11 KO mice lacking 6TM variants failed to show morphine-induced hyperalgesia, developed tolerance more slowly than wildtype mice and did not display hyperlocomotion. Together, our findings confirm the established role of 7TM mu receptor variants in morphine analgesia, reward and respiratory depression, but reveal an unexpected obligatory role for 6TM variants in morphine-induced hyperalgesia and a modulatory role in morphine tolerance and dependence.
Introduction
Morphine acts through the mu opioid receptor, a G-protein coupled receptor. Originally proposed based upon rigid structure-activity relationships (Beckett 1959; Portoghese 1966), it was first demonstrated in binding assays in 1973 (Pert and Snyder 1973; Simon et al. 1973; Terenius 1973) and finally cloned in 1993 (Chen et al. 1993; Pasternak and Pan 2013; Thompson et al. 1993; Wang et al. 1993). The importance of the cloned mu receptors in morphine analgesia was first demonstrated using an antisense approach (Rossi et al. 1994) and subsequently in a number of knockout mouse models (Charbogne et al. 2014; Kitanaka et al. 1998; Loh et al. 1998; Matthes et al. 1996; Schuller et al. 1999). While only a single mu opioid receptor gene, Oprm1, has been identified, the gene undergoes extensive splicing to generate dozens of splice variants (see review (Pasternak and Pan 2013), revealing a complexity exceeding the original proposal of mu opioid receptor subtypes (Wolozin and Pasternak 1981).
Oprm1 gene splice variants can be divided into three general groups based upon their structures (Supplemental Figure 1). Most of the variants are traditional 7 transmembrane domain (7TM) G-protein coupled receptors (GPCR) associated with the exon 1 promoter. A second set containing only a single transmembrane domain (1TM) potentiates morphine analgesia through a chaperone function that stabilizes the 7TM variants (Xu et al. 2013). The last group contains only 6 transmembrane (6TM) domains and is produced through the exon 11 promoter, which is distinct from the exon 1 promoter responsible for the 7TM and 1TM variants. All three groups undergo 3′ splicing to yield additional variants with alternative C-terminals.
Understanding the contributions of these individual splice variants in selected opioid actions has been difficult since many exons of the Oprm1 gene are shared among multiple splice variants. However, several knockout models have given some insights. In one model with a disruption of exon 1 (E1 KO), the traditional 7TM receptors and 1TM variants are lost while the 6TM variants, which do not contain exon 1, continue to be expressed (Schuller et al. 1999). The 7TM variants bind mu drugs with high affinity, but the 1TM do not. The 1TM produce their actions by stabilizing and thereby increasing the expression of the 7TM variants (Xu et al. 2013). Another knockout model has a disruption of exon 11 (E11 KO), eliminating the 6TM variants while maintaining 7TM expression (Pan et al. 2009). Morphine analgesia is lost in the E1 KO mouse and in other Oprm1 knockout models lacking 7TM variants (Kieffer 1999; Loh et al. 1998; Matthes et al. 1996; Sora et al. 1997). In contrast, morphine analgesia is unaffected by the selective elimination of 6TM variants in the E11 KO mouse (Pan et al. 2009). However, the activity of other analgesics is dependent upon 6TM variants, such as buprenorphine (Grinnell et al. 2016). Morphine also produces hyperalgesia that can become manifest after its analgesic actions wane. It was suggested that this might be associated with opioid receptor mediated excitatory mechanisms (Crain and Shen 1990) acting through the μ3 receptor (Stefano et al. 1995), a truncated 6TM mu receptor variant (Cadet et al. 2003), and more recently through another 6TM receptor, MOR-1K (Gris et al. 2010; Oladosu et al. 2015a). In the current study, we assessed the contributions of 6TM variants in a range of morphine actions in mice, including hyperalgesia.
Methods
Mice
C57/Bl6 wild type (C57; WT) (Jackson Laboratories) and exon 11 KO (E11 KO) (Pan et al. 2009) mice were bred in our laboratory. E11 KO animals were derived as previously described (Pan et al. 2009) and backcrossed more than 10 generations on a C57/Bl6 background. Since no obvious sex differences were noted, both male and female mice were included. WT and E11 KO were matched for age and sex. All mice were maintained on a 12-h light/dark cycle with food and water available ad libitum and housed in groups of five until testing. All animal studies were approved by the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals in an AAALAC accredited facility.
Drugs
Morphine sulfate and naloxone were obtained from the Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD). Naloxonazine (Hahn et al. 1982)and IBNtxA (3-iodobenzoyl naltrexamine)(Majumdar et al. 2011) were synthesized in our laboratory.
Analgesia and hyperalgesia
Analgesia was assessed with the radiant heat tail flick assay using a Ugo Basile radiant heat tail flick machine (Varese, Italy) with baseline values between 2–3 sec and a maximum latency of 10 sec. Data were analyzed both as percent maximal possible effect (%MPE) and quantally, defined as a doubling or greater of baseline latency. %MPE was calculated with the formula: [(response - baseline)/(10 - baseline)] x 100. Both methods of analysis yielded similar results. Tail flick analgesia was tested at peak effect at 30 min after morphine administration. ED50 values were calculated by nonlinear regression analysis (GraphPad Prism, Carlsbad, CA).
Hyperalgesia was assessed as previously described (Elhabazi et al. 2014). Mice were acclimated by handling them for 2–3 days prior to testing. At least 2 days prior to drug administration, baseline tail flick latencies were obtained using a tail immersion assay (Neslab, GP-100 Water Bath, 47°C). Baselines are reported as the average of 3 tail flick latencies. Then, mice were administered saline or equianalgesic doses of morphine (10 mg/kg, s.c.) or IBNtxA (1.6 mg/kg, s.c.) and tested for tail flick latency 24 hours later. This was repeated daily for a total of 4 times.
Tolerance and dependence
Mice were injected daily with morphine (10 mg/kg, s.c.) and analgesia assessed on the indicated days using the radiant tail flick assay. On day 21, a dose response curve was generated using cumulative dosing. The ED50 values (95% confidence intervals) were calculated by nonlinear regression analysis (GraphPad Prism).
On day 21 of chronic daily morphine dosing (10 mg/kg, s.c.) mice were injected with naloxone (1 mg/kg, s.c.), and jumping was quantified for the next15 minutes.
Locomotor Activity
Open field locomotor activity was obtained in a MedAssociates ENV-510 activity chamber (St Albans, VT) using MedAssociates Activity Monitor software. Mice were injected with saline or morphine (10 mg/kg, s.c.) and immediately placed in an open field box for 60 minutes. Total distance traveled and distance traveled in 2 minutes bins were compared using a one-way or repeated measures ANOVA followed by Bonferroni multiple comparisons test (GraphPad Prism).
Respiratory Depression
Respiratory rate was assessed in freely moving adult mice with the Mouse Ox pulse oximeter system (Starr Life Sciences)(Majumdar et al. 2011). Mice were shaved around the neck 24 hours prior to testing. Mice were habituated to the device for at least 1 hour prior to testing. A 5 second average breath rate was assessed at 5 minute intervals. A baseline was obtained over a 25 minute period before drug injection. Then, mice (n=5–7 per group) received saline or morphine (1, 2.5, 5, or 10 mg/kg, s.c.). Testing began 15 minutes post injection and continued for 35 minutes. Data are reported as % of baseline readings.
Conditioned Place Preference
A 3 chamber conditioned place preference paradigm (Med Associates) was used to assess morphine reward behavior in WT and E11 KO mice. First, mice underwent a 20 min pre-conditioning session during which they had free access to all chambers to determine their innate preference for a black chamber with a bar floor, a gray chamber with a solid floor, or a white chamber with a grid floor. Then, mice underwent conditioning for 4 days. On each conditioning day, mice received saline i.p. and were immediately placed in the chamber they showed innate preference for (either white or black) during pre-conditioning for 20 min. Four hours later, mice were injected with morphine (10 mg/kg, i.p.) and immediately placed in the chamber they did not show preference for (either white or black) during the pre-conditioning phase. On the day following conditioning day 4, mice were tested for place preference. They were given free access to all chambers, and time spent in each chamber was quantified during a 20 min period. Preference was determined with a difference score of time spent in the drug paired chamber post conditioning minus time spent in chamber pre-conditioning.
Results
Analgesia
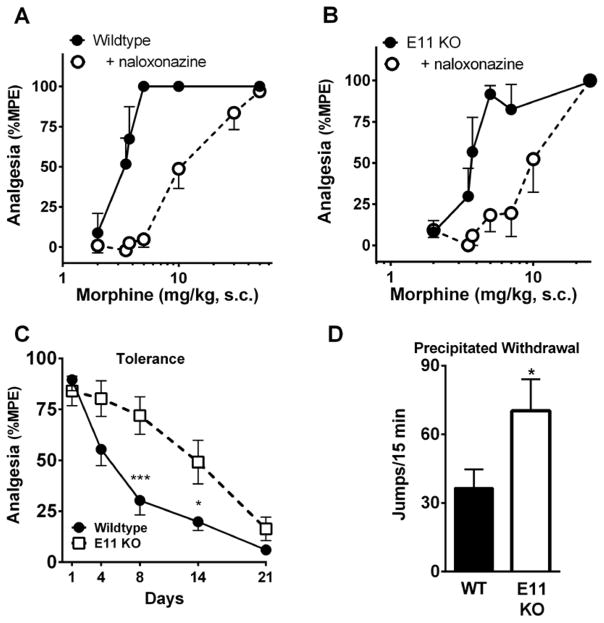
Prior studies from our laboratory found normal morphine responses in E11 KO mice in a mixed background (Pan et al. 2009). In the current study, morphine analgesia was similar in both wildtype and E11 KO mice in the C57/Bl6 background with ED50 values of 3.5 and 3.6 mg/kg, s.c., respectively (Table 1), consistent with earlier studies examining morphine analgesia in a mixed background (Pan et al. 2009). The antagonists naloxazone and naloxonazine differentially antagonize morphine analgesia without blocking respiratory depression, physical dependence and a number of other morphine actions (Hahn et al. 1982; Ling et al. 1984; Ling et al. 1985; Pasternak 2001; Pasternak and Pan 2013; Spiegel et al. 1982), which led to the original suggestion of mu receptor subtypes (Wolozin and Pasternak 1981). Naloxonazine shifted the morphine ED50 value in both wildtype and E11 KO mice by approximately 3-fold (Figure 1a, b; Table 1), consistent with a similar receptor mechanism of morphine analgesia in both groups of mice. It also implied that the naloxonazine-sensitive analgesic target was independent of 6TM variants.
Table 1.
ED50s of WT and E11 KO mice following naloxonazine and morphine tolerance.
| Pretreatment | WT Morphine ED50 | E11 KO Morphine ED50 |
|---|---|---|
| Control | 3.5a (3.2, 3.8) | 3.6a (3.5, 3.8) |
| Naloxonazine | 9.7a **** (8.3, 11.4) | 9.2a #### (7.0, 12.1) |
| 21 days morphine | 25.3a **** (18.6, 34.5) | 13.0a ####,† (11.2, 15.2) |
Groups of mice (n=11–20) received morphine (s.c.) and were tested for analgesia with the tail flick assay. Cumulative dosing was used to generate analgesic dose response curves. The ED50s (95% confidence intervals) were calculated by nonlinear regression analysis (GraphPad Prism). Significance between groups was determined with an F-test. Data are reported with 2 experiments pooled to generate one dose response curve. The ED50 values for morphine in wildtype and E11 KO mice were not significantly different in the control or naloxonazine groups. Naloxonazine shifted the ED50 of morphine by approximately 3-fold in both WT (F1,82= 56.67, p<0.0001) and E11 KO (F1,86= 36.99, p<0.0001) mice compared to controls. Following 21 days of morphine administration, the analgesic ED50 of morphine was significantly lower in E11 KO than WT mice (F1,94= 18.27, p<0.0001). Both morphine treated groups were significantly different than their respective control groups (WT: F1,73=35.1, p<0.0001; E11 KO: F1,65=25.94; p<0.0001).
Figure 1. Role of exon 11 variants in morphine analgesia, tolerance and dependence.
A and B) Groups of mice (n=6–11) received either saline or naloxonazine (35 mg/kg, s.c.) 24 hr prior to testing with the indicated dose of morphine in the radiant heat tail flick assay. For ED50 values and statistics, see Table 1. C) Time course of morphine tolerance. Groups of mice (n=17–20) received morphine (10 mg/kg, s.c. daily), and analgesia was assessed using the radiant tail flick assay on the indicated days. Experiments were performed twice with similar results observed with each replicate. The response in wildtype (WT) mice was significantly attenuated on Day 4 (repeated measures ANOVA with Bonferroni multiple comparisons test, p=0.0003 vs. WT Day 1), whereas E11 KO mice did not show a significant reduction in analgesic response until Day 14 (repeated measures ANOVA with Bonferroni multiple comparisons test, p=0.0008 vs. E11 KO Day 1). E11 KO mice were slower to develop morphine tolerance compared to WT mice (repeated measures ANOVA with Bonferroni post hoc comparisons test: time F4,140=50.2 p<0.0001, genotype: F1,35=8.76 p=0.0055, interaction: F4,140=4.86 p=0.0011), *p<0.05, ***p<0.001 compared to E11 KO. D) Withdrawal following 21 days of morphine. Groups of mice (n=12) received morphine for 21 days (10 mg/kg, s.c. 1 x daily). Withdrawal was precipitated with naloxone (1 mg/kg s.c.) and jumping was quantified for 15 minutes. Experiments were performed twice with similar results obtained following each determination. Data in figure are pooled between experiments. Significance was determined with a student’s t-test (t22=2.11, p=0.047) *p<0.05, **p<0.01.
Tolerance and dependence
The 6TM variants contribute to the development of morphine tolerance. When dosed daily (10 mg/kg, s.c.), morphine responses in wildtype mice gradually diminished over time (Figure 1c), with more than a 7-fold shift to the right of the ED50 after 21 days (Table 1). E11 KO mice also developed tolerance to morphine, but at a significantly slower rate. The response was significantly attenuated on Day 4 in wildtype animals, whereas E11 KO mice showed no significant reduction in their response until Day 14. Area under the curve analysis over the 21 days revealed a significantly lower response in the WT compared to the E11 KO group (Supplemental Figure 2). By Day 21, the responses in both WT and E11 KO mice returned to near baseline levels. However, dose-response curves on Day 21, revealed that E11 KO mice were still significantly more sensitive to morphine (ED5013 mg/kg) than wildtype mice (ED50 25.3 mg/kg) (Table 1).
The antagonist naloxone precipitates withdrawal in mice chronically administered morphine. After 21 days of daily morphine treatment, naloxone (1 mg/kg, s.c.) precipitated withdrawal in wildtype mice, quantified by jumping (Figure 1d). Despite a diminished level of tolerance, the E11 KO mice jumped significantly more frequently than WT mice, suggesting a possible increased level of physical dependence and a dissociation of tolerance and physical dependence mechanisms. Together, the loss of E11-associated variants enhanced physical dependence and decreased tolerance.
Respiratory Depression
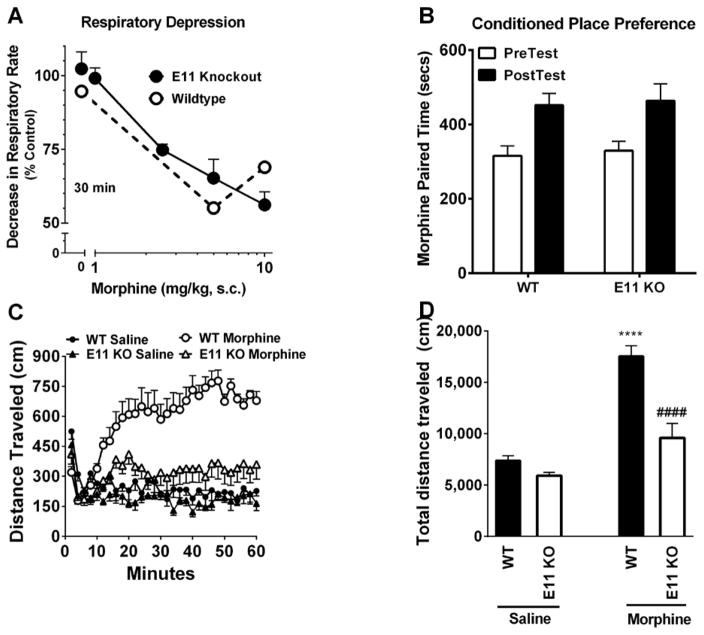
Opioid induced respiratory depression is a potentially lethal side-effect of opioid therapy. In both wildtype and E11 KO mice, morphine produced similar drops in respiratory rate 30 min after morphine administration (Figure 2a). Time action curves with the different morphine doses confirmed the similar responses over the full 50 minutes of observation (Supplemental Figure 3). Thus, respiratory depression was independent of 6TM Oprm1 variants.
Figure 2. Role of exon 11 variants in respiratory depression, CPP and locomotion.
A) Respiratory depression: Groups of mice (n=5–8) received the indicated dose of morphine after acclimating them and the respiratory rate was measured over 50 min. The values at 30 min were then used to evaluate the dose-response relationships. (Full time action curves are in Supplemental Figure 3). Nonlinear regression analysis revealed similar ED50 values (95% confidence limits) in the wildtype (0.9 mg/kg (0.25, 3.4)) and E11 KO mice (2.5 mg/kg (1.8, 3.4)). B) Morphine-Induced Conditioned Place Preference in E11 KO mice. E11 KO animals (n=10) demonstrated similar morphine CPP as WT controls (n=10; Two-way ANOVA, main effect of day (pre-and post-conditioning) F1,36= 31.55; p = 0.0002 but not of genotype (WT, mutant) F1,36= 0.28; p = 0.7048)). Both WT and E11 KO mice acquired a similar place preference to morphine (10 mg/kg i.p.) as demonstrated by a significant increase in time spent in the morphine paired chamber on post-conditioning versus pre-conditioning test day (Bonferonni posthoc, *p < 0.05). C) Time course of locomotor activity. Groups of mice (n=7–8) received saline or morphine (10 mg/kg, s.c.), and locomotor activity was quantified for 60 min. A repeated measures ANOVA (F87,754=6.32, p<0.0001) with Bonferroni multiple comparisons tests indicated that morphine in WT animals was significantly different from saline treated WT and E11 KO treated morphine at all time points following 5 min after injection. E11 KO morphine did not differ from E11 KO saline at any time point except 20 min post injection (p<0.01). D) Open field locomotor activity. Groups of mice (n=7–8) received morphine (10 mg/kg, s.c.) and distance traveled was quantified for 60 min. A one-way ANOVA (F3,26=29.15, p<0.0001) with Bonferroni post hoc comparisons test indicated that WT response to morphine was significantly different from WT response to saline. E11 KO response to morphine did not differ from E11 KO response to saline. **** significantly different from WT saline p<0.0001, #### significantly different from WT morphine p<0.0001
Conditioned Place Preference
To determine the role of 6TM variants in morphine reward, wildtype and E11 KO mice were tested in a 3-chamber conditioned place preference paradigm (Figure 2b). In this study both genotypes demonstrated equivalent preference for morphine-paired contexts, implying that 6TM variants are not involved with morphine reward behavior.
Locomotor Activity
Morphine (10 mg/kg, s.c.) produced a robust increase in locomotor activity in wildtype mice compared to saline controls which persisted for more than 60 minutes (Figure 2c). In contrast, E11 KO mice failed to show a significant increase in locomotor activity following morphine either in time-action or cumulative distance measures. Integrating the time-action curves confirmed these differences (Figure 2d).
Hyperalgesia
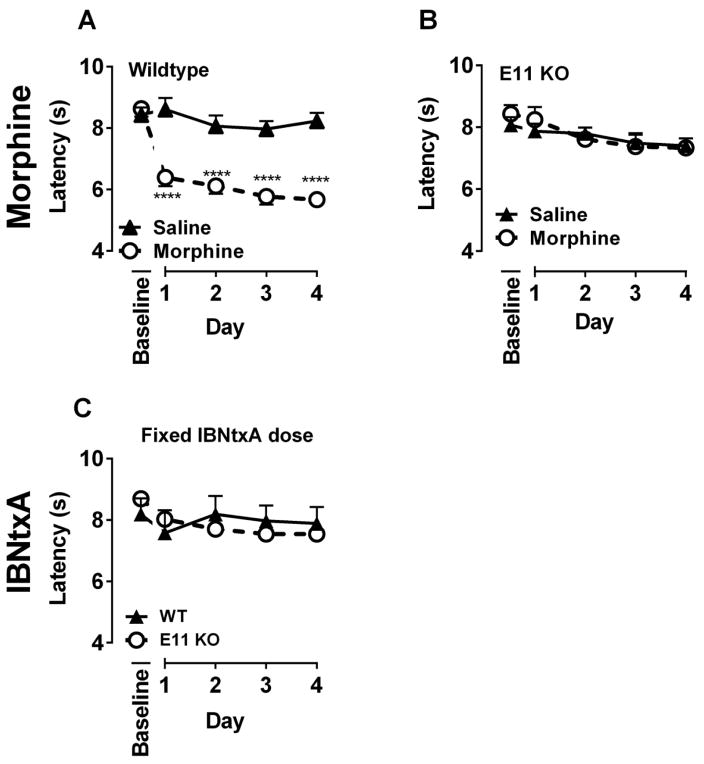
Opioid induced hyperalgesia is defined by an enhanced sensitivity to a painful stimulus following opioid exposure (see reviews (Oladosu et al. 2015b; Roeckel et al. 2016). Wildtype mice and E11 KO mice both displayed maximal analgesia 30 min following morphine (10 mg/kg, s.c.) administration (Supplemental Figure 4). The wildtype mice exhibited hyperalgesia with significant decreases in tail withdrawal latencies in the warm water withdrawal assay 24 hr after morphine (Figure 3a). This effect continued with repeated morphine dosing for 4 days. In contrast, E11 KO mice failed to show a change in withdrawal latency during this 4 day period despite the potent analgesia seen at 30 min (Figure 3b). Thus, 6TM variants are important in the expression of morphine hyperalgesia but not analgesia.
Figure 3. Role of exon 11 variants in morphine induced hyperalgesia.
A and B) Groups of WT and E11 KO mice (n=10–20 per group) were tested in the warm water tail immersion assay 3 times to obtain a baseline. Then, mice were administered saline or morphine (10 mg/kg, s.c.) and latencies were determined again 24 later. Following testing, drug was administered again, and tail withdrawal latency was assessed again 24 hours later. This was repeated two more times. Data are reported as average latency. A repeated measures ANOVA indicated that morphine significantly decreased tail flick latency in WT (F4,148=14.75, p<0.0001) but not E11 KO mice (F4,148=0.7, p=0.6). Bonferroni post comparisons test **** indicates p<0.0001. C) Groups of WT and E11 KO mice (n=10–20 per group) were tested in the warm water tail immersion assay 3 times to obtain a baseline. Then, mice were given saline or IBNtxA (1.6 mg/kg, s.c.)and tested 24 hours later. Following testing, drug was administered again, and tail withdrawal latency was assessed again 24 hours later. This was repeated two more times. Data are reported as mean ± s.e.m.. A repeated measures ANOVA indicated that IBNtxA did not produce a significant change in tail flick latency in WT or E11 KO mice.
To determine if the direct stimulation of a 6TM target also induced hyperalgesia, we examined IBNtxA. IBNtxA analgesia requires 6TM variants, but not the classical mu, delta or kappa receptors and IBNtxA labels a 6TM-dependent binding site in brain unrelated to any traditional opioid binding sites (Majumdar et al. 2011). In the tail immersion assay, IBNtxA at a dose approximately 5-fold higher than its ED50 revealed near maximal analgesia 30 min after administration as anticipated (Supplemental Figure 4), which did not decline with repeated testing over 3 days. This response was markedly decreased in the E11 KO mice, dropping to nearly baseline levels. The slight, but significant, increased latency remaining in the E11 KO mice presumably reflected interactions with an alternative non-E11 site.
Despite its analgesic activity, this dose of IBNtxA (1.6 mg/kg, s.c.) failed to produce hyperalgesia after 24 h in either wildtype or in E11 KO mice over the 4 days of testing (Figure 3c). A prior study reported IBNtxA hyperalgesia utilizing an escalating paradigm with IBNtxA doses higher than our own (Samoshkin et al. 2015). Utilizing their dosing regimen, we observed similar decreased tail withdrawal latencies (Supplemental Figure 5). However, these decreases seen using higher IBNtxA doses represented ‘off target’ effects and were not mediated through E11 sites since similar effects were present in both wildtype and E11 KO mice.
Discussion
Morphine produces a range of activities, including, paradoxically, hyperalgesia. Early studies suggested different receptor mechanisms for various morphine actions based upon the ability of antagonists such as naloxonazine to selectively block some morphine actions and not others, leading to the concept of mu opioid receptor subtypes (Wolozin and Pasternak 1981). The cloning of dozens of Oprm1 splice variants confirms this concept (see review: Pasternak and Pan 2013). Both antisense (Rossi et al. 1994; Uhl et al. 1994)and knockout approaches have established the importance of the 7TM variants in morphine analgesia (Charbogne et al. 2014; Kitanaka et al. 1998; Loh et al. 1998; Matthes et al. 1996; Schuller et al. 1999). 6TM variants are not involved, as illustrated by the maintenance of morphine analgesia in the E11 KO mice. The continued sensitivity of morphine analgesia in the E11 KO mice to naloxonazine was consistent with the same receptor mechanism of action in both groups of mice.
Traditional 7TM variants also mediated other morphine actions (Table 2). The reduction of respiratory rate to morphine was similar in both wildtype and E11 KO mice. The persistence of respiratory depression in the E11 KO mice is interesting since naloxonazine does not antagonize respiratory depression (Ling et al. 1985; Ling et al. 1983; Pasternak and Pan 2013; Wolozin and Pasternak 1981). This implies that 7TM variants elicit both naloxonazine-sensitive and insensitive morphine actions. Similarly, morphine conditioned place preference was maintained in the E11 KO mice, indicating the need for only 7TM variants. The lack of association of 6TM variants with respiratory depression and reward fits with earlier work examining IBNtxA. IBNtxA produces a potent analgesia totally dependent upon 6TM variants without a contribution from 7TM ones and without respiratory depression or reward activity in the place preference assay (Lu et al. 2015; Majumdar et al. 2011). Thus, targeting 6TM variants may offer an opportunity to generate potent analgesics lacking these side-effects.
Exon 11-associated 6TM variants are involved in several alternative morphine actions despite the fact that morphine has no appreciable affinity for the 6TM-dependent IBNtxA site in brain binding assays. Morphine-induced hyperlocomotion was lost in the E11 KO mice and removal of 6TM variants slowed, but did not eliminate, the development of tolerance. Conversely, the jumping in the morphine-treated E11 KO mice increased following a naloxone challenge.
Our findings indicate that different sets of Oprm1 variants mediate morphine analgesia and hyperalgesia. While 7TM receptors are essential for morphine analgesia, morphine hyperalgesia is restricted to 6TM mechanisms. ‘Triple knockout mice’ derived by crossing delta and kappa receptor knockout mice with an exon 1 knockout mu receptor mouse that lacks all 7TM variants, but still expresses 6TM variants (Schuller et al. 1999), fail to show morphine analgesia, but still demonstrate morphine hyperalgesia (Juni et al. 2007). While the earlier study rules out a role for the 7TM receptors, our observed loss of morphine-induced hyperalgesia in the E11 KO mice directly implicate the 6TM variants in morphine hyperalgesia.
Crain and colleagues reported that morphine can in duce excitatory actions (Crain and Shen 1990), followed by studies suggesting a mu3 receptor mechanism (Stefano et al. 1995). The mu3 receptor was subsequently found to correspond to a truncated 6TM mu receptor variant (Cadet et al. 2003) that shares the same predicted protein structure as MOR-1K and MOR-1L (Pasternak and Pan 2013; Shabalina et al. 2009). MOR-1K stimulation reportedly leads to excitatory effects mediated through Gs (Gris et al. 2010; Oladosu et al. 2015a), as predicted by Crain (Crain and Shen 1996; Shen and Crain 1990). While MOR-1K remains a candidate variant for hyperalgesia, our results cannot distinguish between MOR-1K and the four other 6TM variants produced by the exon 11 promoter in the mouse. Three of them translate exon 11, skip exon 1 and then read through exons 2 and 3 followed by the alternatively spliced downstream exons. MOR-1K and MOR-1L, on the other hand, do not translate exon 11, generating identical proteins whose sequence is defined by translation of exons 2, 3 and 4. Since the E11 KO eliminates all of these variants, it is not possible to distinguish among them.
While the role of 6TM, but not 7TM variants in morphine hyperalgesia is established, many questions remain. The loss of morphine hyperalgesia in the E11 KO mice implicated 6TM variants. Analgesia, mediated through 7TM variants, was fully maintained. Morphine concentrations in brain associated with analgesia (~0.2 μM) (Patrick et al. 1975) are far lower than its binding affinity for the IBNtxA 6TM binding site identified in brain (Ki > 1 μM) (Majumdar et al. 2011). In addition, analgesic doses of IBNtxA acting through 6TM targets failed to produce hyperalgesia. Together, these observations suggest that morphine was acting through an alternative 6TM-associated target. 6TM variants are involved in a broad range of actions mediated through variety of other receptor systems. Delta and kappa opioid and α2 adrenergic analgesia is dependent upon 6TM variants (Marrone et al. 2016)and MOR-1K interacts with β2 adrenergic receptors, a possible mechanism for hyperalgesia (Samoshkin et al. 2015).
The role of 6TM variants in other morphine actions was mixed. Both conditioned place preference and respiratory depression depended upon 7TM mechanisms while locomotor activity and tolerance utilized 6TM ones. The expression of E11 variants in a number of motor regions (i.e. striatum, globus pallidus and substantia nigra) is consistent with their possible involvement with motor activity (Abbadie et al. 2004). Loss of 7TM variants did not abolish morphine tolerance, but markedly slowed its appearance. The question of the relationship of morphine hyperalgesia to tolerance has not been resolved, but the involvement of 6TM variants in both raises the possibility they may be interrelated. A contribution of 6TM mechanisms in tolerance is consistent with the upregulation of 6TM mRNA transcripts following long term morphine exposure (Xu et al. 2015). Morphine administered twice daily for 6 weeks increased mMOR-1G mRNA expression by nearly 40-fold in the brainstem and 90-fold in the hypothalamus.
These findings expand our understanding of the role of 6TM variants. This study focused upon the role of 6TM variants in morphine actions. However, 6TM variants also contribute to other actions. Analgesia produced by other compounds is significantly attenuated or completely absent in E11 KO mice (Majumdar et al. 2011; Marrone et al. 2016; Pan et al. 2009). For example, buprenorphine analgesia was dependent upon both 6TM and 7TM variants (Grinnell et al. 2016), as were morphine-6β-glucuronide and fentanyl, while IBNtxA analgesia was mediated only by 6TM ones (Majumdar et al. 2011). 6TM variants also contribute to non-mu opioid analgesia (Marrone et al. 2016). Delta and kappa opioid, and α2 adrenergic analgesia required 6TM variants, although many of their non-analgesic actions remained intact in the E11 KO animals. Together, the current findings provide further evidence for the diverse pharmacology of different mu opioid receptor splice variants.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the Peter F. McManus Charitable Trust, Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, The Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center, and the National Institutes on Drug Abuse of the National Institutes of Health (DA06241 and DA07242) to GWP, a core grant from the National Cancer Institute of the National Institutes of Health (CA08748) to MSKCC and a National Science Foundation Graduate Research Fellowship Grant (DGE-1257284) to GFM. There are no competing financial interests.
References
- Abbadie C, Pan Y-X, Pasternak GW. Immunohistochemical study of the expression of exon11-containing mu opioid receptor variants in the mouse brain. Neuroscience. 2004;i:419–430. doi: 10.1016/j.neuroscience.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Beckett AH. Stereochemical factors in biological activity. Fortschr Arzneimittelforsch. 1959;1:455–530. doi: 10.1007/978-3-0348-7035-1_6. [DOI] [PubMed] [Google Scholar]
- Cadet P, Mantione KJ, Stefano GB. Molecular identification and functional expression of mu 3, a novel alternatively spliced variant of the human mu opiate receptor gene. J Immunol. 2003;170:5118–5123. doi: 10.4049/jimmunol.170.10.5118. [DOI] [PubMed] [Google Scholar]
- Charbogne P, Kieffer BL, Befort K. 15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology. 2014;76(Pt B):204–17. doi: 10.1016/j.neuropharm.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a μ-opioid receptor from rat brain. Mol Pharmacol. 1993;44:8–12. [PubMed] [Google Scholar]
- Crain SM, Shen K-F. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. Trends in Pharmacological Sciences. 1990;11:77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Modulatory effects of Gs-coupled excitatory opioid receptor functions on opioid analgesia, tolerance, and dependence. Neurochem Res. 1996;21:1347–1351. doi: 10.1007/BF02532375. [DOI] [PubMed] [Google Scholar]
- Elhabazi K, Ayachi S, Ilien B, Simonin F. Assessment of morphine-induced hyperalgesia and analgesic tolerance in mice using thermal and mechanical nociceptive modalities. J Vis Exp. 2014:e51264. doi: 10.3791/51264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell SG, Ansonoff M, Marrone GF, Lu Z, Narayan A, Xu J, Rossi G, Majumdar S, Pan XY, Bassoni DL, Pintar J, Pasternak GW. Mediation of buprenorphine analgesia by a combination of traditional and truncated mu opioid receptor splice variants. Synapse. 2016;70:395–407. doi: 10.1002/syn.21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gris P, Gauthier J, Cheng P, Gibson DG, Gris D, Laur O, Pierson J, Wentworth S, Nackley AG, Maixner W, Diatchenko L. A Novel alternatively spliced isoform of the mu-opioid receptor: functional antagonism. Mol Pain. 2010;6:33. doi: 10.1186/1744-8069-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EF, Carroll-Buatti M, Pasternak GW. Irreversible opiate agonists and antagonists: the 14-hydroxydihydromorphinone azines. J Neurosci. 1982;2:572–576. doi: 10.1523/JNEUROSCI.02-05-00572.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni A, Klein G, Pintar JE, Kest B. Nociception increases during opioid infusion in opioid receptor triple knock-out mice. Neuroscience. 2007;147:439–444. doi: 10.1016/j.neuroscience.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Kieffer BL. Opioids: first lessons from knockout mice. Trends in Pharmacological Sciences. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- Kitanaka N, Sora I, Kinsey S, Zeng ZZ, Uhl GR. No heroin or morphine 6β-glucuronide analgesia in μ-opioid receptor knockout mice. European Journal of Pharmacology. 1998;355:R1–R3. doi: 10.1016/s0014-2999(98)00516-0. [DOI] [PubMed] [Google Scholar]
- Ling GSF, MacLeod JM, Lee S, Lockhart SH, Pasternak GW. Separation of morphine analgesia from physical dependence. Science. 1984;226:462–464. doi: 10.1126/science.6541807. [DOI] [PubMed] [Google Scholar]
- Ling GSF, Spiegel K, Lockhart SH, Pasternak GW. Separation of opioid analgesia from respiratory depression: evidence for different receptor mechanisms. J Pharmacol Exp Ther. 1985;232:149–155. [PubMed] [Google Scholar]
- Ling GSF, Spiegel K, Nishimura S, Pasternak GW. Dissociation of morphine’s analgesic and respiratory depressant actions. Eur J Pharmacol. 1983;86:487–488. doi: 10.1016/0014-2999(83)90203-0. [DOI] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang WL, Chen YF, Wei LN. Mυ opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Molecular Brain Research. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu J, Rossi GC, Majumdar S, Pasternak GW, Pan YX. Mediation of opioid analgesia by a truncated 6-transmembrane GPCR. J Clin Invest. 2015;125:2626–30. doi: 10.1172/JCI81070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Grinnell S, Le RV, Burgman M, Polikar L, Ansonoff M, Pintar J, Pan YX, Pasternak GW. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci USA. 2011;108:19776–19783. doi: 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone GF, Grinnell SG, Lu Z, Rossi GC, Le Rouzic V, Xu J, Majumdar S, Pan YX, Pasternak GW. Truncated mu opioid GPCR variant involvement in opioid-dependent and opioid-independent pain modulatory systems within the CNS. Proc Natl Acad Sci U S A. 2016;113:3663–8. doi: 10.1073/pnas.1523894113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Oladosu FA, Conrad MS, O’Buckley SC, Rashid NU, Slade GD, Nackley AG. Mu Opioid Splice Variant MOR-1K Contributes to the Development of Opioid-Induced Hyperalgesia. PLoS One. 2015a;10:e0135711. doi: 10.1371/journal.pone.0135711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladosu FA, Maixner W, Nackley AG. Alternative Splicing of G Protein-Coupled Receptors: Relevance to Pain Management. Mayo Clin Proc. 2015b;90:1135–51. doi: 10.1016/j.mayocp.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YX, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci USA. 2009;106:4917–4922. doi: 10.1073/pnas.0811586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharmacol Sci. 2001;22:67–70. doi: 10.1016/s0165-6147(00)01616-3. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan Y-X. Mu opioids and their receptors: Evolution of a concept. Pharmacol Rev. 2013;65:1257–1317. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GA, Dewey WL, Spaulding TC, Harris LS. Relationship of brain morphine levels to analgesic activity in acutely treated mice and rats and in pellet implanted mice. J Pharmacol Exp Ther. 1975;193:876–83. [PubMed] [Google Scholar]
- Pert CB, Snyder SH. Opiate receptor: demonstration in nervous tissue. Science. 1973;179:1011–1014. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- Portoghese PS. Stereochemical factors and receptor interactions associated with narcotic analgesics. J Pharmac Sciences. 1966;55:865–887. doi: 10.1002/jps.2600550902. [DOI] [PubMed] [Google Scholar]
- Roeckel LA, Le Coz GM, Gaveriaux-Ruff C, Simonin F. Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.06.029. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Pan Y-X, Cheng J, Pasternak GW. Blockade of morphine analgesia by an antisense oligodeoxynucleotide against the mu receptor. Life Sci. 1994;54:L375–L379. doi: 10.1016/0024-3205(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Samoshkin A, Convertino M, Viet CT, Wieskopf JS, Kambur O, Marcovitz J, Patel P, Stone LS, Kalso E, Mogil JS, Schmidt BL, Maixner W, Dokholyan NV, Diatchenko L. Structural and functional interactions between six-transmembrane mu-opioid receptors and beta2-adrenoreceptors modulate opioid signaling. Sci Rep. 2015;5:18198. doi: 10.1038/srep18198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, Pintar JE. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- Shabalina SA, Zaykin DV, Gris P, Ogurtsov AY, Gauthier J, Shibata K, Tchivileva IE, Belfer I, Mishra B, Kiselycznyk C, Wallace MR, Staud R, Spiridonov NA, Max MB, Goldman D, Fillingim RB, Maixner W, Diatchenko L. Expansion of the human mu-opioid receptor gene architecture: novel functional variants. Hum Mol Genet. 2009;18:1037–1051. doi: 10.1093/hmg/ddn439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K-F, Crain SM. Cholera toxin-B subunit blocks excitatory effects of opioids on sensory neuron action potentials indicating that GM1 ganglioside may regulate Gs-linked opioid receptor functions. Brain Research. 1990;531:1–7. doi: 10.1016/0006-8993(90)90751-v. [DOI] [PubMed] [Google Scholar]
- Simon EJ, Hiller JM, Edelman I. Stereospecific binding of the potent narcotic analgesic [3H]etorphine to rat-brain homogenate. Proc Natl Acad Sci USA. 1973;70:1947–1949. doi: 10.1073/pnas.70.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define μ receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Kourides I, Pasternak GW. Prolactin and growth hormone release by morphine in the rat: different receptor mechanisms. Science. 1982;217:745–747. doi: 10.1126/science.6285470. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Hartman A, Bilfinger TV, Magazine HI, Liu Y, Casares F, Goligorsky MS. Presence of the μ3 opiate receptor in endothelial cells - Coupling to nitric oxide production and vasodilation. J Biol Chem. 1995;270:30290–30293. doi: 10.1074/jbc.270.51.30290. [DOI] [PubMed] [Google Scholar]
- Terenius L. Characteristics of the “receptor” for narcotic analgesics in synaptic plasma membrane from rat brain. Acta Pharmacolet toxicol. 1973;33:377–384. doi: 10.1111/j.1600-0773.1973.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Mansour A, Akil H, Watson SJ. Cloning and pharmacological characterization of a rat μ opioid receptor. Neuron. 1993;11:903–913. doi: 10.1016/0896-6273(93)90120-g. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Childers S, Pasternak GW. An opiate-receptor gene family reunion. Trends Neurosci. 1994;17:89–93. doi: 10.1016/0166-2236(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Wang JB, Imai Y, Eppler CM, Gregor P, Spivak CE, Uhl GR. μ opiate receptor: cDNA cloning and expression. Proc Natl Acad Sci USA. 1993;90:10230–10234. doi: 10.1073/pnas.90.21.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin BL, Pasternak GW. Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc Natl Acad Sci USA. 1981;78:6181–6185. doi: 10.1073/pnas.78.10.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Faskowitz AJ, Rossi GC, Xu M, Lu Z, Pan YX, Pasternak GW. Stabilization of morphine tolerance with long-term dosing: association with selective upregulation of mu-opioid receptor splice variant mRNAs. Proc Natl Acad Sci U S A. 2015;112:279–84. doi: 10.1073/pnas.1419183112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu M, Brown T, Rossi GC, Hurd YL, Inturrisi CE, Pasternak GW, Pan YX. Stabilization of the mu opioid receptor by truncated single transmembrane splice variants through a chaperone-like action. J Bio Chem. 2013;288:21211–21227. doi: 10.1074/jbc.M113.458687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.