Abstract
Background:
Acute kidney injury (AKI) is a severe disease in critically ill patients. Neutrophil infiltration into kidney was associated with the development of AKI, and P-selectin may be involved in the process of neutrophil recruitment in kidney. This study aimed to explore the potential effect of platelet-derived P-selectin on neutrophil recruitment in a mouse model of sepsis-induced AKI.
Methods:
A total of 30 C57BL/6 male mice were divided into five groups (n = 6 in each): sham group, sepsis group, anti-Ly6G group, anti-P-selectin group, and platelet depletion group. Sepsis was induced by cecal ligation and puncture. Serum creatinine concentration and platelet activity were measured by biochemical detector and flow cytometry, respectively. Histological and pathological features were analyzed using hematoxylin-eosin (H&E) and immunohistochemistry (IHC) staining, respectively. Myeloperoxidase (MPO) activity was detected with MPO assay. Unpaired t-test was used for data analysis.
Results:
Serum creatinine increased significantly in septic group compared to sham group (2.68 ± 0.27 mg/dl vs. 0.82 ± 0.19 mg/dl, t = 12.06, P = 0.0000) but attenuated in antibodies-treated animals compared to septic group (anti-Ly6G: 1.62 ± 0.30 mg/dl vs. 2.68 ± 0.27 mg/dl, t = 5.76, P = 0.0004; anti-P-selectin: 1.76 ± 0.31 mg/dl vs. 2.68 ± 0.27 mg/dl, t = 4.92, P = 0.0012; and platelet depletion: 1.93 ± 0.29 mg/dl vs. 2.68 ± 0.27 mg/dl, t = 4.14, P = 0.0032). Platelet amount significantly decreased compared to sham group (658.20 ± 60.64 × 109/L vs. 822.00 ± 48.60 × 109/L, t = 4.71, P = 0.0015) in septic mice, especially in platelet depletion group (240.80 ± 44.98 × 109/L vs. 822.00 ± 48.60 × 109/L, t = 19.63, P = 0.0000). P-selectin activity was significantly increased in septic group compared to sham group (16.54 ± 1.60% vs. 1.90 ± 0.29%, t = 15.64, P = 0.0000) but decreased significantly in platelet depletion group compared to septic group (3.62 ± 0.68% vs. 16.54 ± 1.60%, t = 12.89, P = 0.0002). IHC analysis shown that neutrophil infiltration increased in septic mice compared to sham group (36.67 ± 3.79% vs. 9.17 ± 1.61%, t = 11.58, P = 0.0003) and function-blocked groups (anti-Ly6G: 36.67 ± 3.79% vs. 15.33 ± 1.53%, t = 9.05, P = 0.0008; anti-P-selectin: 36.67 ± 3.79% vs. 21.33 ± 1.53%, t = 6.51, P = 0.0029; and platelet depletion: 36.67 ± 3.79% vs. 23.33 ± 3.06%, t = 4.75, P = 0.0090). MPO increased significantly in septic group compared to control (49.73 ± 1.83 ng/mg prot vs. 13.04 ± 2.16 ng/mg prot, t = 19.03, P = 0.0000) but decreased in function-blocked groups compared to septic group (anti-Ly6G: 26.52 ± 3.86 ng/mg prot vs. 49.73 ± 1.83 ng/mg prot, t = 9.59, P = 0.0000; anti-P-selectin: 33.06 ± 6.75 ng/mg prot vs. 49.73 ± 1.83 ng/mg prot, t = 4.85, P = 0.0013; and platelet depletion: 33.37 ± 2.25 ng/mg prot vs. 49.73 ± 1.83 ng/mg prot, t = 5.33, P = 0.0007).
Conclusion:
Platelets-derived P-selectin may be involved in the development of septic AKI through inducing neutrophil infiltration into kidney.
Keywords: Acute Kidney Injury, Neutrophils, P-Selectin, Sepsis
INTRODUCTION
Acute kidney injury (AKI) is a severe disease in critically ill patients and is associated with high morbidity and mortality.[1] In addition, it is an independent risk factor for death.[2] Epidemiologically, the most common cause of AKI in critically ill patients is sepsis which accounts for up to 50% of all AKI cases.[3] Despite considerable research on sepsis-induced AKI (SAKI) has been done in the past few years, the pathophysiology of SAKI remains incompletely understood. Neutrophil infiltration of the renal tissue has been found to be involved in the development of ischemia-reperfusion injury (IRI)-induced AKI. Preischemic neutrophil depletion provided protection from renal failure.[4,5] Recently, it is also indicated that neutrophil recruitment into kidney was associated with SAKI. Neutrophil depletion protected the renal from SAKI as well.[6]
P-selectin is the largest selectin (140,000) and stored in α-granules of platelets and Weibel–Palade bodies of endothelial cells. Previous studies have revealed the role of P-selectin in IRI and sepsis model of AKI, and blocking P-selectin protects mice from AKI through attenuating neutrophil recruitment into kidney.[6,7] Research also indicated that platelet P-selectin, but not endothelial, is critical for neutrophil-mediated acute postischemic renal failure.[8] Whether platelet-derived P-selectin is involved in the development of SAKI has not been determined. In the present study, we attempt to investigate the role of platelet P-selectin in the neutrophil infiltration into the kidney in SAKI.
METHODS
Animals and study design
A total of thirty male 12-week-old C57BL/6 mice (weighing from 18 to 22 g) were recruited to this study. All the mice were randomly divided into five groups with six mice in each: (i) sham group (mice without intervention); (ii) sepsis group (mice with cecal ligation and puncture [CLP] without any treatment); (iii) anti-Ly6G group: 50 μg of anti-Ly6G antibody (Biorbyt, USA) i.p. after CLP to deplete neutrophils;[9] (iv) anti-P-selectin group: 100 μg of anti-P-selectin (Abcam, USA) i.p. after CLP to block P-selectin; and (v) platelet depletion group: 0.2 mg rat anti-mouse glycoprotein Ib-α antibody (Santa Cruz, USA) to deplete platelets. All animal experiments were done according to the ethical requirements from the ethics committee.
Sepsis induction by cecal ligation and puncture
CLP was performed as described previously.[10] Briefly, after induction of anesthesia through intraperitoneal injection of chloral hydrate, a skin incision of approximately 10 mm in the midline abdominal area was made, the peritoneal sheath was incised, and the cecum was carefully exteriorized. After the cecum had been ligated by polyglactin sutures, two transmural injuries were made to the ligated section using a 20-gauge needle to allow peritoneal dissemination of bacteria. The abdominal cavity was closed with two layers, followed by fluid resuscitation. Afterward, animals were put back to their cages. Sham animals underwent the same procedure, but CLP was omitted. Forty-eight hours after CLP, mice were euthanized. Blood samples were collected in heparinized tubes and stored at −80°C. Both kidneys were removed from the mice and either was kept at −80°C or fixed in 10% formalin.
Therapeutic intervention
In Group III, mice received 50 μg of anti-Ly6G antibody (b322983, Biorbyt, USA) through i.p. after surgical closure of the peritoneal sheath. In Group IV, mice received 100 μg of blocking antibody against P-selectin (ab54427, Abcam, USA). In Group V, mice received 0.2 mg rat anti-mouse glycoprotein Ib-α antibody (SC-7069, Santa Cruz, USA) through i.p. immediately after closure of the abdomen which has previously been shown to deplete platelets.[11]
Examination of platelet activity
Forty-eight hours after laparotomy, animals were euthanized and blood samples were taken by cardiac puncture. Whole blood was taken into anticoagulant tubes and centrifuged at 72 ×g for 5 min. Platelet-rich plasma (PRP) was isolated and 5 μl of PRP was added and fixed with 1% paraformaldehyde for 20 min. The CD62P (P-selectin)-FITC antibody (BD, USA) was added and incubated at room temperature for 20 min before resuspension with 1 ml of phosphate-buffered saline (PBS) and tested by flow cytometer.
Renal function and cytokine analysis
Blood sample's serum creatinine and IL-1β concentration was measured with ELISA assay Kit (R&D Systems, USA) according to the manufacturer's protocol.
Histomorphometric analysis
The kidneys fixed with 10% of formalin were embedded in paraffin. Those tissues were sliced into 4 μm thick sections following by hematoxylin-eosin (H&E) staining. Histologic morphology was scored for the loss of brush borders (0–3), tubular vascularization (0–3), and cell infiltration (0–3) by a blinded investigator.
Determination of myeloperoxidase activity
Snap frozen kidneys were homogenized (20 mmol/L KPO4 buffer [pH 7.4]) and centrifugated at 17,000 ×g for 10 min at 4°C. Afterward, the supernatant was discarded and resuspended with 20 mmol/L KPO4 buffer (pH 7.4) and incubated for 20 min at 4°C. After another centrifugation, the supernatant was used to detect the activity of myeloperoxidase (MPO). The results were determined by bicinchoninic acid assay (Pierce, USA) and expressed as units of MPO per gram of supernatant protein.
Immunohistochemistry analysis
Four micrometers’ sections of paraffin embedded kidneys were blocked with 0.5% BSA for 30 min and incubated with anti-rabbit LY-6G (orb322983, Biorbyt, USA) antibody. After incubation of primary antibody, a goat anti-rabbit secondary antibody was added and incubated at 37°C for 60 min. Sections were washed with PBS and 3,3-diaminobenzidine chromogenic solutions was added to each slice, followed by hematoxylin staining and microscopic analysis by an experienced pathologist.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Statistical analysis was performed by SPSS version 19.0 (SPSS Inc., Palo Alto, California, USA). Unpaired t-test was used for comparison between each group. P < 0.05 was considered statistically significant.
RESULTS
Cecal ligation and puncture-induced acute kidney injury accompanied by inflammation
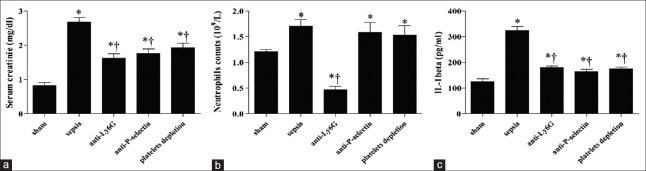
After 48 h of CLP, compared to the sham group, animals of the septic group showed increased neutrophils’ count (1.70 × 109/L vs. 1.21 × 109/L, t = 3.61, P = 0.0059) and IL-1β levels (324.92 ± 35.14 pg/ml vs. 125.54 ± 42.08 pg/ml, t = 10.47, P = 0.0000) in peripheral blood [Figure 1]. The serum creatinine levels in septic mice were significantly increased compared to control group (2.68 ± 0.27 mg/dl vs. 0.82 ± 0.19 mg/dl, t = 12.06, P = 0.0000), indicating that mice developed AKI at 48 h after CLP, but attenuated in antibodies-treated animals compared to septic group (anti-Ly6G: 1.62 ± 0.30 mg/dl vs. 2.68 ± 0.27 mg/dl, t = 5.76, P = 0.0004; anti-P-selectin: 1.76 ± 0.31 mg/dl vs. 2.68 ± 0.27 mg/dl, t = 4.92, P = 0.0012; and platelet depletion: 1.93 ± 0.29 mg/dl vs. 2.68 ± 0.27 mg/dl, t = 4.14, P = 0.0032) [Figure 1a]. After using depleting dose of anti-Ly6G antibody, P-selectin antibody, and rat anti-mouse glycoprotein Ib-α antibody, IL-1β levels significantly decreased compared to septic mice (179.01 ± 16.42 pg/ml vs. 324.92 ± 35.14 pg/ml, t = 8.41, P = 0.0000; 163.91 ± 20.47 pg/ml vs. 324.92 ± 35.14 pg/ml, t = 8.85, P = 0.0000; and 175.22 ± 14.84 pg/ml vs. 324.92 ± 35.14 pg/ml, t = 8.78, P = 0.0000, respectively) in function-blocked mice. Neutrophils were significantly attenuated in peripheral blood of anti-Ly6G-treated mice compared to septic mice (0.47 ± 0.15 × 109/L vs. 1.70 ± 0.29 × 109/L, t = 8.47, P = 0.0000) [Figure 1].
Figure 1.
Cecal ligation and puncture-induced acute kidney injury accompanied with inflammation. (a) Serum creatinine, (b) neutrophil, and (c) Interlukin-1β levels were measured in sham control, septic, neutrophil-depleted, P-selectin-blocked, and platelets-depleted mice using commercially available ELISA kits (*P < 0.01 vs. sham; †P < 0.01 vs. sepsis).
Platelet amount and activation are inhibited by rat anti-mouse glycoprotein Ib-α antibody
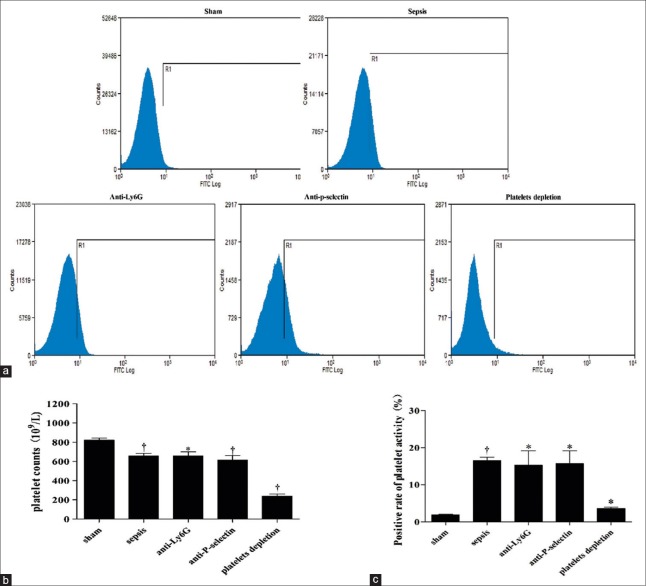
Compared to sham animals, the flow cytometry results showed that the amount of platelets significantly decreased (658.20 ± 60.64 × 109/L vs. 822.00 ± 48.60 × 109/L, t = 4.71, P = 0.0015) in septic mice 48 h after CLP and especially in platelet depletion group (240.80 ± 44.98 × 109/L vs. 822.00 ± 48.60 × 109/L, t = 19.63, P = 0.0000) [Figure 2a and 2b]. However, the platelet activation indicated by P-selectin increased significantly in septic, P-selectin-blocked, neutrophil-depleted, and platelet depletion mice compared to sham group (16.54 ± 1.60% vs. 1.90 ± 0.29%, t = 15.64, P = 0.0000; 15.68 ± 6.02% vs. 1.90 ± 0.29%, t = 3.52, P = 0.0244; 15.34 ± 6.60% vs. 1.90 ± 0.29%, t = 3.96, P = 0.0167; and 3.62 ± 0.68% vs. 1.90 ± 0.29%, t = 4.04, P = 0.0156, respectively) [Figure 2c].
Figure 2.
Platelet activity in sham control, septic, neutrophil-depleted, P-selectin-blocked, and platelets-depleted mice. (a) Data were showed by flow cytometry. (b) Platelets amount and (c) platelets activity were examined in different groups, and comparison was exhibited with bar graph (*P < 0.05 vs. sham, †P < 0.01 vs. sham).
Kidney morphology is protected by inhibition of neutrophil infiltration
To investigate the mentioned histological characteristics, H&E staining of the kidneys was performed. Kidneys from septic mice without intervention had massive tubular edema, leukocyte infiltration, and loss of tubular epithelial cells but attenuated in antibodies-treated mice [Figure 3a]. To quantify these changes, we used a previously published histological scoring system, results shown that the sepsis group had the highest score, significantly higher than the other four groups (sham group: 1.33 ± 0.57 vs. 6.33 ± 1.52, t = 5.303, P = 0.0061; anti-Ly6G group: 2.00 ± 1.00 vs. 6.33 ± 1.52, t = 4.11, P = 0.0147; anti-P-selectin group: 2.00 ± 1.00 vs. 6.33 ± 1.52, t = 4.11, P = 0.0147; and platelet depletion group: 2.00 ± 1.00 vs. 6.33 ± 1.52, t = 4.11, P = 0.0147) [Figure 3b].[12,13]
Figure 3.
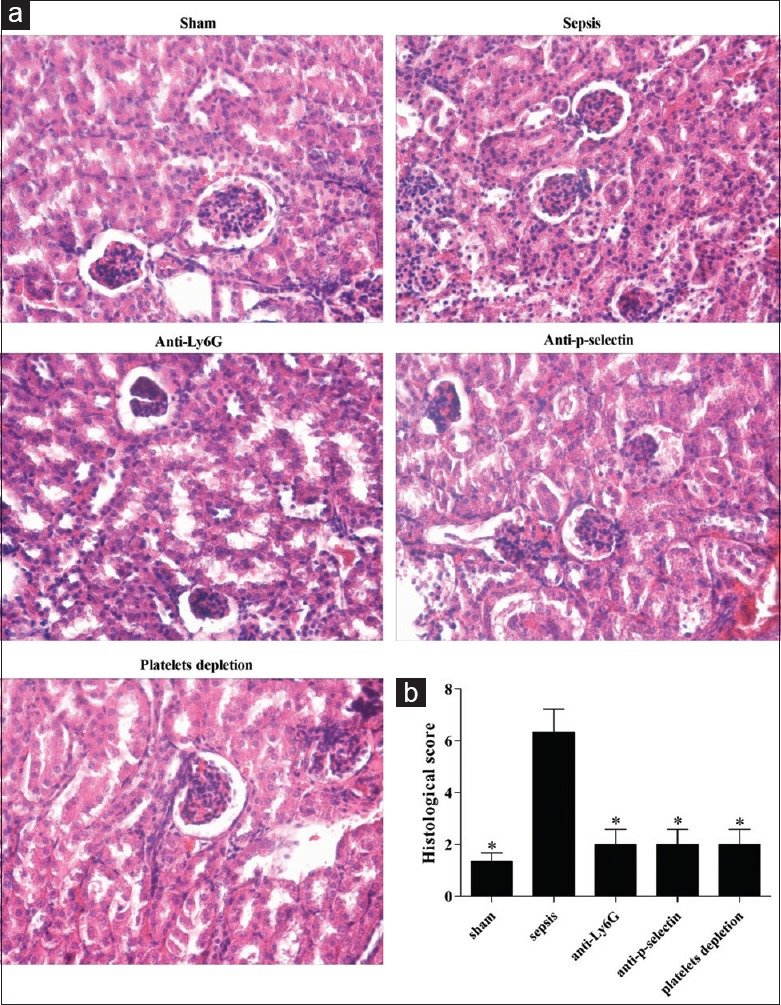
Histological feature of tissues in different groups was examined using H&E staining (original magnification, ×400). (a) Microscopic observation of histological traits in tissue samples obtained from different group of mice. (b) Semi-quantified analysis was shown with bar graph (*P < 0.01 vs. sepsis).
Neutrophil infiltration indicated by immunohistochemistry and myeloperoxidase activity
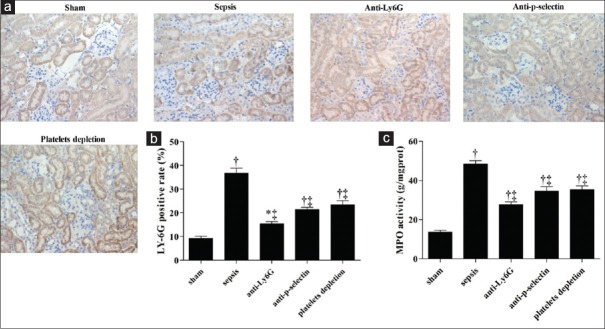
To identify the leukocytes, immunohistochemistry (IHC) assays were performed. The results indicated that neutrophil infiltration in kidney was dramatically increased by CLP but attenuated by antibodies. IHC analysis indicated that neutrophil infiltration into kidney significantly increased in septic mice compared to control group (36.67 ± 3.79% vs. 9.17 ± 1.61%, t = 11.58, P = 0.0003) and function-blocked groups (anti-Ly6G group: 36.67 ± 3.79% vs. 15.33 ± 1.53%, t = 9.05, P = 0.0008; anti-P-selectin group: 36.67 ± 3.79% vs. 21.33 ± 1.52%, t = 6.51, P = 0.0029; and platelet depletion group: 36.67 ± 3.79% vs. 23.33 ± 3.06%, t = 4.75, P = 0.0090) [Figure 4a and 4b]. Furthermore, MPO activity increased significantly in septic group compared to control (49.73 ± 1.83 ng/mg prot vs. 13.04 ± 2.16 ng/mg prot, t = 19.03, P = 0.0000) but decreased in function-blocked groups compared to septic group (anti-Ly6G: 26.52 ± 3.86 ng/mg prot vs. 49.73 ± 1.83 ng/mg prot, t = 9.59, P = 0.0000; anti-P-selectin: 33.06 ± 6.75 ng/mg prot vs. 49.73 ± 1.83 ng/mg prot, t = 4.85, P = 0.0013; and platelet depletion: 33.37 ± 2.25 ng/mg prot vs. 49.73 ± 1.83 ng/mg prot, t = 5.33, P = 0.0007) [Figure 4c].
Figure 4.
Pathological character of tissues in different groups was examined using immunohistochemistry staining (original magnification, ×400). (a) Representative images of neutrophil alterations in kidney tissues. (b) Statistical analysis of Ly6G positive rate and (c) myeloperoxidase (MPO) activity was shown with bar graph (*P < 0.05 vs. sham, †P < 0.01 vs. sham, ‡P < 0.01 vs. sepsis).
DISCUSSION
In our study, we investigated the role of platelet P-selectin in the neutrophil infiltration into the kidney in SAKI in a specialized mouse model. Our data confirmed that neutrophil infiltration into kidney plays an important role in the development of SAKI. Sepsis can activate platelets which consequently contribute to the development of AKI through inducing neutrophil recruitment into kidney mediated by P-selectin.
Neutrophils are important inflammatory cells and crucial for the elimination of pathogens.[14] In our research, at 48 h after CLP, neutrophils were significantly increased in peripheral blood and kidney tissue samples. Treatment with anti-Ly6G antibody significantly attenuated neutrophil counts in peripheral blood as well as neutrophil infiltration into kidney. Simultaneously, the serum creatinine decreased. Neutrophil recruitment into kidney participated in the development of AKI and inhibition of neutrophil infiltration in kidney may protect the mice against AKI.[6,7]
Platelets were considered to be essential contributor in hemostatic process of body. In 2015, Mederle et al.[15] described an immunological response of platelets in the development of sepsis and SAKI. Moreover, an increased level of platelet microparticles (MPs) was found in septic patients with renal dysfunction. The platelet-derived MPs were correlated with creatinine concentrations and serum blood urea nitrogen, suggesting a potential role for these platelet MPs in the development of renal failure.[16] In 2015, Schwarzenberger et al.[11] found that platelets are important mediators in renal injury induced by primary endothelial lesions. Nonetheless, the exact mechanism has not been elucidated in the process of renal injury. In the present study, we observed that platelet counts in peripheral blood significantly decreased 48 h after CLP, especially in platelet depletion mice. Other current clinical investigations showed that platelets’ count is decreased in sepsis and related to the severity of sepsis.[17,18] However, in flow cytometry, the activity of platelets indicated by P-selectin expression showed in flow cytometry increased significantly in septic mice except in rat anti-mouse glycoprotein Ib-α antibody-treated mice, which can deplete platelets.
P-selectin, a type-1 transmembrane glycoprotein, is stored in α-granules of platelets and in Weibel–Palade bodies of endothelial cells.[19,20] In addition, it can be released from stored position to the cell surface soon after exposure to stimuli.[21,22] P-selectin can induce the leukocyte infiltration through endothelial vessel cells. In 2000, Singbartl et al.[7] showed that, in the IRI model of kidney failure, inhibition of neutrophil infiltration by injection of anti-P-selectin antibodies has been found to ameliorate renal function. Research confirmed that platelet P-selectin plays a crucial role in postischemic neutrophil infiltration into kidney. However, whether platelet-derived P-selectin participated in SAKI has not been determined.
In our study, blockage of P-selectin using anti-P-selectin antibody leads to inhibition of neutrophil infiltration into kidneys as the platelet depletion did. We supposed that platelet depletion decreased the expression of P-selectin and facilitated attenuation of P-selectin-induced neutrophil infiltration and protection of the septic mice from AKI. Our results indicate that platelet-derived P-selectin is involved in the development of SAKI through inducing recruitment of neutrophils in kidney.
Considering the leading clinical condition associated with AKI, namely, sepsis, major surgery, and heart failure are all associated with hypoperfusion, it is thought that macrohemodynamic changes attributed to all AKI. However, an increasing amount of evidence recently suggested that the absence of hypoperfusion can cause SAKI. Microcirculatory dysfunction, inflammation, and bioenergetic adaptive response to injury have been suggested to be involved in the development of SAKI, and a “unifying theory”[23] has been proposed to emphasize the relationship between the three main alterations. Although we did not monitor blood pressure in our present research, we investigated the changes of IL-1β and found its serum level increased significantly after induction of sepsis by CLP. This result could support the “unifying theory” that in case of inflammation, not only inflammatory cells but also inflammatory cytokines are involved in the development of SAKI.
Unfortunately, there are some limitations in our research. First, P-selectin is not only stored in α-granules of platelets but also in Weibel–Palade bodies of endothelial cells, and in sepsis, endothelial cells were also activated[24] and released P-selectin.[13] Therefore, we will confirm whether endothelial cell-derived P-selectin participate in the neutrophil infiltration into kidney and involved in AKI in the next study. However, we can at least conclude that platelet P-selectin plays an important role in SAKI. Second, there is a variety of functional inflammatory molecules and immune mediators on the surface of platelets. Depletion of platelets using rat anti-mouse glycoprotein Ib-α antibody not only decreases the expression of P-selectin but also affects the expression of inflammation-related molecules involved in sepsis.[25,26]
Even if there are some limitations above mentioned, our findings still provide new insights into the role of P-selectin in SAKI. In conclusion, platelet depletion is likely to improve the outcome of SAKI through reducing expression of the P-selectin which contributed to decrease neutrophil infiltration in kidney. This may provide some valuable information about therapeutic strategy for SAKI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Clec’h C, Gonzalez F, Lautrette A, Nguile-Makao M, Garrouste-Orgeas M, Jamali S, et al. Multiple-center evaluation of mortality associated with acute kidney injury in critically ill patients: A competing risks analysis. Crit Care. 2011;15:R128. doi: 10.1186/cc10241. doi: 10.1186/cc10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoste EA, Schurgers M. Epidemiology of acute kidney injury: How big is the problem? Crit Care Med. 2008;36(4 Suppl):S146–51. doi: 10.1097/CCM.0b013e318168c590. doi: 10.1097/ccm.0b013e318168c590. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S. The epidemiology of acute renal failure in the world. Curr Opin Crit Care. 2006;12:538–43. doi: 10.1097/01.ccx.0000247448.94252.5a. doi: 10.1097/01.ccx.0000247448.94252.5a. [DOI] [PubMed] [Google Scholar]
- 4.Hellberg PO, Källskog TO. Neutrophil-mediated post-ischemic tubular leakage in the rat kidney. Kidney Int. 1989;36:555–61. doi: 10.1038/ki.1989.230. doi: org/10.1038/ki.1989.230. [DOI] [PubMed] [Google Scholar]
- 5.Klausner JM, Paterson IS, Goldman G, Kobzik L, Rodzen C, Lawrence R, et al. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol. 1989;256(5 Pt 2):F794–802. doi: 10.1152/ajprenal.1989.256.5.F794. [DOI] [PubMed] [Google Scholar]
- 6.Herter JM, Rossaint J, Spieker T, Zarbock A. Adhesion molecules involved in neutrophil recruitment during sepsis-induced acute kidney injury. J Innate Immun. 2014;6:597–606. doi: 10.1159/000358238. doi: 10.1159/000358238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singbartl K, Green SA, Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J. 2000;14:48–54. doi: 10.1096/fasebj.14.1.48. [DOI] [PubMed] [Google Scholar]
- 8.Singbartl K, Forlow SB, Ley K. Platelet, but not endothelial, P-selectin is critical for neutrophil-mediated acute postischemic renal failure. FASEB J. 2001;15:2337–44. doi: 10.1096/fj.01-0199com. doi: 10.1096/fj.01-0199com. [DOI] [PubMed] [Google Scholar]
- 9.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 10.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, et al. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–7. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzenberger C, Sradnick J, Lerea KM, Goligorsky MS, Nieswandt B, Hugo CP, et al. Platelets are relevant mediators of renal injury induced by primary endothelial lesions. Am J Physiol Renal Physiol. 2015;308:F1238–46. doi: 10.1152/ajprenal.00535.2014. doi: 10.1152/ajprenal.00535.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leelahavanichkul A, Yasuda H, Doi K, Hu X, Zhou H, Yuen PS, et al. Methyl-2-acetamidoacrylate, an ethyl pyruvate analog, decreases sepsis-induced acute kidney injury in mice. Am J Physiol Renal Physiol. 2008;295:F1825–35. doi: 10.1152/ajprenal.90442.2008. doi: 10.1152/ajprenal.90442.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerolle N, Nochy D, Guérot E, Bruneval P, Fagon JY, Diehl JL, et al. Histopathology of septic shock induced acute kidney injury: Apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36:471–8. doi: 10.1007/s00134-009-1723-x. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 14.Ocuin LM, Bamboat ZM, Balachandran VP, Cavnar MJ, Obaid H, Plitas G, et al. Neutrophil IL-10 suppresses peritoneal inflammatory monocytes during polymicrobial sepsis. J Leukoc Biol. 2011;89:423–32. doi: 10.1189/jlb.0810479. doi: 10.1189/jlb.0810479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mederle K, Meurer M, Castrop H, Höcherl K. Inhibition of COX-1 attenuates the formation of thromboxane A2 and ameliorates the acute decrease in glomerular filtration rate in endotoxemic mice. Am J Physiol Renal Physiol. 2015;309:F332–40. doi: 10.1152/ajprenal.00567.2014. doi: 10.1152/ajprenal.00567. [DOI] [PubMed] [Google Scholar]
- 16.Tokés-Füzesi M, Woth G, Ernyey B, Vermes I, Mühl D, Bogár L, et al. Microparticles and acute renal dysfunction in septic patients. J Crit Care. 2013;28:141–7. doi: 10.1016/j.jcrc.2012.05.006. doi: 10.1016/j.jcrc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Koyama K, Madoiwa S, Tanaka S, Koinuma T, Wada M, Sakata A, et al. Evaluation of hemostatic biomarker abnormalities that precede platelet count decline in critically ill patients with sepsis. J Crit Care. 2013;28:556–63. doi: 10.1016/j.jcrc.2012.10.069. doi: 10.1016/j.jcrc.2012.10.069. [DOI] [PubMed] [Google Scholar]
- 18.Mavrommatis AC, Theodoridis T, Orfanidou A, Roussos C, Christopoulou-Kokkinou V, Zakynthinos S. Coagulation system and platelets are fully activated in uncomplicated sepsis. Crit Care Med. 2000;28:451–7. doi: 10.1097/00003246-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Geng JG, Bevilacqua MP, Moore KL, McIntyre TM, Prescott SM, Kim JM, et al. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343:757–60. doi: 10.1038/343757a0. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- 20.McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–9. doi: 10.1172/JCI114175. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berman CL, Yeo EL, Wencel-Drake JD, Furie BC, Ginsberg MH, Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986;78:130–7. doi: 10.1172/JCI112542. doi: 10.1172/JCI112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foreman KE, Vaporciyan AA, Bonish BK, Jones ML, Johnson KJ, Glovsky MM, et al. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–55. doi: 10.1172/JCI117430. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11. doi: 10.1097/SHK.0000000000000052. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–45. doi: 10.1189/jlb.0607373. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 25.de Stoppelaar SF, van ‘t Veer C, van der Poll T. The role of platelets in sepsis. Thromb Haemost. 2014;112:666–77. doi: 10.1160/TH14-02-0126. doi: 10.1160/TH14-02-0126. [DOI] [PubMed] [Google Scholar]
- 26.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759–67. doi: 10.1182/blood-2013-11-462432. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]