Abstract
Background:
Mounting evidence has demonstrated that hypoxia-inducible factor-1α (HIF-1α) could attenuate brain injuries after cerebral ischemia and reperfusion (CIR). However, few reports have addressed the therapeutic efficacies of a recombinant adenovirus vector containing HIF-1α (AdHIF-1α) gene after ischemia and reperfusion. The aim of this study was to examine the antiapoptotic and neuroprotective effects of AdHIF-1α gene for cerebral injuries after ischemia and reperfusion in rats.
Methods:
From February to December 2016, male Sprague-Dawley rats were randomly divided into normal, sham, CIR, AdHIF-1α, and recombinant adenovirus (Ad) groups. Middle cerebral artery occlusion model was established by Longa's method and reperfusion resumed at 2 h postocclusion. AdHIF-1α solution, Ad solution, and phosphate-buffered saline were injected into the right lateral ventricle of rats in AdHIF-1α, Ad, and CIR groups. Brain tissue sections were observed under fluorescent microscope to confirm the definite expression of recombinant adenovirus in Ad and AdHIF-1α groups. The expressions of HIF-1α protein were analyzed by immunohistochemical staining at 6 h, 24 h, and 72 h postreperfusion. Brain water content and neurological deficit scores were evaluated at 6 h, 24 h, and 72 h postreperfusion. Pathological brain injuries were examined after hematoxylin and eosin stain and nerve cell apoptosis was measured after terminal-deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) stain at 72 h postreperfusion. Comparisons were conducted with one-way analysis of variance by post hoc Scheffe's test among different experimental groups.
Results:
Green fluorescent protein was successfully expressed in brain tissue of Ad and AdHIF-1α groups from 24 h to 21 days postinjection. As detected by immunohistochemical staining, the expressions of HIF-1α protein were obviously enhanced in AdHIF-1α group than those in CIR and Ad groups at 24 h and 72 h postreperfusion, respectively. There were significant reductions of brain water content (78.83% ± 0.34% vs. 83.21% ± 0.50% and 83.35% ± 0.32%; 84.13% ± 0.24% vs. 89.76% ± 0.34% and 89.70% ± 0.18%; respectively; all P < 0.05) and neurological deficit scores (2.90 ± 0.74 vs. 3.50 ± 0.52 and 3.60 ± 0.53 at 24 h; 2.40 ± 0.84 vs. 3.60 ± 0.52 and 3.50 ± 0.53 at 72 h; respectively; all P < 0.05) in AdHIF-1α group versus CIR and Ad groups at 24 h and 72 h postreperfusion, respectively. The pathologic changes of AdHIF-1α group were milder than those in CIR and Ad groups at 72 h postreperfusion. The percentage of TUNEL-positive cells in cerebral subcortex decreased significantly in AdHIF-1α group versus CIR and Ad groups at 72 h postreperfusion (P < 0.05).
Conclusion:
AdHIF-1α has an obvious neuroprotective effect on ischemia and reperfusion in rat brains possibly through inhibiting the apoptosis of nerve cells.
Keywords: Apoptosis, Cerebral Ischemia, Hypoxia-inducible Factor-1α, Recombinant Adenovirus Vector, Reperfusion
INTRODUCTION
Cerebral ischemia and reperfusion (CIR) injuries resulting from stroke contribute to worse neurological outcomes and poor prognoses. These damages are triggered by a rapid cascade of pathological events, including inflammation, disruption of blood-brain barrier, and apoptosis of nerve cells. However, treatment strategies are extremely limited for ischemic stroke.[1] Therefore, it is imperative to formulate effective treatments of lessening impairments of nervous system and reducing nerve cell apoptosis.
Hypoxia-inducible factor-1 (HIF-1) serves as a pivotal transcriptional factor in responses to hypoxia. As a heterodimeric complex, it is composed of two subunits, i.e., an alpha subunit and a beta subunit. As an active and functional subunit under hypoxia, HIF-1α participates in the responses of tissues, organs, and cells.[2] Mounting evidence has demonstrated that HIF-1α could upregulate antiapoptotic genes and downregulate proapoptotic genes to promote cellular survival.[3,4] Apoptosis is one of the causative mechanisms for neuronal degeneration due to ischemia and reperfusion. Moreover, HIF-1α plays crucial roles in mediating apoptosis during acute and chronic neurological disorders.[5] Recombinant adenovirus containing green fluorescent protein (GFP) was employed as a carrier. And, recombinant adenovirus solution was injected into lateral ventricle of rats. Subsequently, the expression of GFP and the upregulation ofHIF-1α were detected in brain tissues. To further explore the potential neuroprotective effects of HIF-1α after ischemia and reperfusion, brain water content, neurological deficit scores, pathological changes of brain, and apoptosis of nerve cells were analyzed.
METHODS
Ethical approval
Animal handling was performed strictly in accordance with the relevant guidelines and approved by our Institutional Ethics Committee on Animal Experiments (No. 25).
Construction of recombinant adenovirus vector containinghypoxia-inducible factor- 1α gene
In accordance with the manufacturer's instructions, adenovirus vector containing HIF-1α (AdHIF-1α) was generated with AdEasy-1 adenoviral vector system (Stratagene, La Jolla, CA, USA). And, recombinant adenovirus contained GFP. Briefly, human HIF-1α gene obtained by polymerase chain reaction (PCR) was inserted into plasmid pMD19-T (Takra, Beijing, China) and HIF-1α cDNA sequences cloned into adenoviral shuttle plasmid pShuttle-cytomegalovirus (CMV, Jiran, Shanghai, China) of AdEasy-1 system. Adenoviral HIF-1α vector was constructed using the resultant pShuttle-CMV-HIF-1α for homologous recombination with adenoviral backbone plasmid pAdEasy-1 in BJ5183 (Jiran, Shanghai, China) bacterial cells. After selecting by kanamycin, it was identified by digesting with HindIII (Thermo, USA) restriction enzyme and amplifying in XL10-Gold competent bacteria (Weidi, Shanghai, China). Then, DNA of recombinant adenovirus vector was linearized with PacI. And, recombinant adenovirus was generated in HEK293 (Weidi, Shanghai, China) packaging cells for harvesting AdHIF-1α. In addition, viral stocks were propagated in HEK293 cells, purified by cesium chloride ultracentrifugation at 6755 × g for 20 h at 4°C and further titrated with agarose overlaying plaque assay. PCR was employed for conforming that HIF-1α genes were successfully transfected into recombinant adenovirus. The recombinant adenovirus vector containing HIF-1α gene was named as AdHIF-1α and noncarrier Ad.
Animals
Male Sprague-Dawley rats (Guiyang, China) weighing 220–250 g were provided by Laboratory Animal Center of Guizhou Medical University (No. SCSK [QIAN] 2012-0001). The rats were randomly divided into the following five groups: normal group (n = 36), sham group (n = 36), CIR group (n = 46), recombinant adenovirus group (Ad, n = 46), and recombinant adenovirus containing HIF-1α gene group (AdHIF-1α, n = 46).
Model of focal cerebral ischemia and reperfusion
The model of focal CIR was established by intraluminal occlusion.[6] First, the animals were anesthetized with 10% chloral hydrate (0.35 ml/100 g) and maintained in a supine position. After a midline neck incision, right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were sequentially dissected away from adjacent tissues. After a ligation of right ECA, clips were placed at CCA. A small incision was made at 2.0 mm away from CCA bifurcation and a nylon filament coated with silicone (a diameter of 0.31–0.32 mm) inserted for 18–22 mm from ECA into ICA. Then, silk suture was tightened around ECA stump and nylon filament. Reperfusion resumed after a removal of nylon filament at 2 h postocclusion. The rats of CIR, Ad, and AdHIF-1α groups were handled similarly while sham group underwent skin operation and simple suturing.
Stereotaxic injection of transgene
Recombinant adenovirus containing hypoxia-inducible factor-1α gene group
After middle cerebral artery occlusion (MCAO), the rats were immobilized on stereotaxic instrument (Stoelting, USA). A medial incision at 3.0 cm posterior to the middle point of ears was made for exposing sagittal suture, anterior fontanel, and right cranium surface. A total of 10 μl (108 plaque forming units [PFU] AdHIF-1α) recombinant adenovirus containing HIF-1α gene solution was drawn into a microsyringe. Injection point (2.0 mm right to sagittal suture and 1.5 mm anterior to the rear of anterior fontanel) in cranium was drilled with a dental drill and a microsyringe needle inserted 4.0 mm deep into right lateral ventricle. Recombinant adenovirus solution containing HIF-1α gene was injected at a rate of 1 μl/min. Needle was retracted after 10 min and cranial hole plugged with bone wax.
Cerebral ischemia and reperfusion group
Stereotaxic injection was performed as previously described.[6] Only 10 μl 0.1 mol/L phosphate-buffered saline (PBS) instead of AdHIF-1α solution was used.
Adenovirus group
Ten microliter (containing 108 PFU Ad) recombinant adenovirus was injected as previously described.[6]
Scoring of neurological functions
Neurological function of rats (n = 12)was evaluated at 6 h, 24 h, 72 h postreperfusion according to a modified 6-point scoring scale of Longa et al.[7]:0 = no deficit; 1 = difficulty of fully extending contralateral forelimb; 2 = reduced grip strength of left forepaw; 3 = mild circling to contralateral side; 4 = severe circling; 5 = falling to contralateral side. The higher neurological deficit scores, the more severe motor impairment.
Expression of recombinant adenovirus in proplexus
Two rats each from CIR, Ad, and AdHIF-1α groups were sacrificed, frozen sections prepared and detected under fluorescent microscope at day 1, 3, 7, 14, 21 postreperfusion. Brain tissue slices were prepared in triplicates (n = 2)and detected under fluorescent microscope to determine whether recombinant adenovirus containing GFP was expressed in brain tissue.
Measurement of brain water content
After scoring neurological function, brain water content was measured by the wet-dry method.[8] After anesthesia, the rats were sacrificed by decapitation (n = 6) at 6 h, 24 h, and 72 h postreperfusion. Cerebral hemispheres were harvested freshly and packaged with preweighting foils. Dry weights were recorded after a 24 h drying at 105°C by blast drier (Jiecheng, China) right after measuring wet weights of cerebral hemispheres. Brain water content was calculated as ([wet weight − dry weight]/wet weight) × 100%.
Brain tissue fixation and sectioning
At 6 h, 24 h, and 72 h post-MCAO, all five experimental groups (n = 6) were placed under deep anesthesia with 10% chloral hydrate and perfused through left ventricle successively with 0.1 mmol/L phosphate buffer saline (0.1 mol/L PBS) and 250 ml 4% paraformaldehyde. Brain tissues of all experimental groups were harvested, fixed for 24 h in 4% paraformaldehyde, and embedded in paraffin. Serial coronal brain sections were prepared with a microtome (Leica, Germany) at a thickness of 4 μm.
Hematoxylin and eosin staining
At 72 h postreperfusion, all rats (n = 6) from each group were anesthetized with 10% chloral hydrate and perfused with 4% paraformaldehyde. Serial adjacent brain sections (a thickness of 4 μm) were sliced from coronal plane of wax-embedded tissue and stained with hematoxylin and eosin (H and E staining) for evaluating necrotic and viable neuronal cells.
Immunohistochemical detection of hypoxia-inducible factor-1α expression
Brain tissue sections (n = 6)were dewaxed, dehydrated, and blocked step by step with 3% hydrogen peroxide-formalin. After a drop-wise addition of 50 μl goat sera (Santa Cruz, USA), the sections were incubated with 50–100 μl primary HIF-1α antibody (Santa Cruz, USA) for 10 min at 37°C and hydro-incubated for 2 h at 37°C. After rinsing thrice with 50 μl 0.1 mol/L PBS, a reinforcing agent was added and hydro-incubated for 30 min. The sections were labeled with freshly prepared 3,3’-diaminobenzidine (DAB) (Abcam, UK), counter-stained with hematoxylin, dehydrated, and mounted with neutral gum. Brownish yellow stain was positive for HIF-1α expression. Moreover, HIF-1α- positive cells were measured from six visual fields with Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, Maryland, USA).
Staining assay of terminal-deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling
The extent of apoptosis in ischemic core and penumbra regions of paraffin-embedded coronal brain sections from all groups(n = 6)after 72 h was analyzed by terminal-deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining using an in situ Cell Death Detection Kit (Roche, USA) according to the manufacturer's instructions. Briefly, paraffin-embedded tissue sections were deparaffinized, rehydrated, treated with proteinase K working solution, and then permeabilized. Permeabilized tissue sections were incubated with TUNEL reaction mixture in a humid and dark atmosphere for 60 min at 37°C. Sections were stained for nuclei with 4’,6-diamidino-2-phenylindole (DAPI, Beyotime, China), cover-slipped with fluorescent mounting medium (Beyotime, China), and observed under fluorescent microscope (Zeiss, Germany).
Statistical analysis
All data were presented as mean ± standard deviation (SD) for numeric variables and as frequency (%) for categorical variables. Comparisons were conducted with one-way analysis of variance (ANOVA) by post hoc Scheffe's test among different experimental groups. Statistical analysis was performed with the SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
Expression of green fluorescent protein in proplexus
Since green fluorescence was detected under fluorescent microscope [Figure 1], recombinant adenoviruses containing GFP were successfully expressed in ependymal and adjacent cells of Ad and AdHIF-1α groups at 24 h postinjection. No green fluorescence signal was detected at any time point in CIR group. Moreover, green fluorescence remained positive for 21 days in Ad and AdHIF-1α groups.
Figure 1.
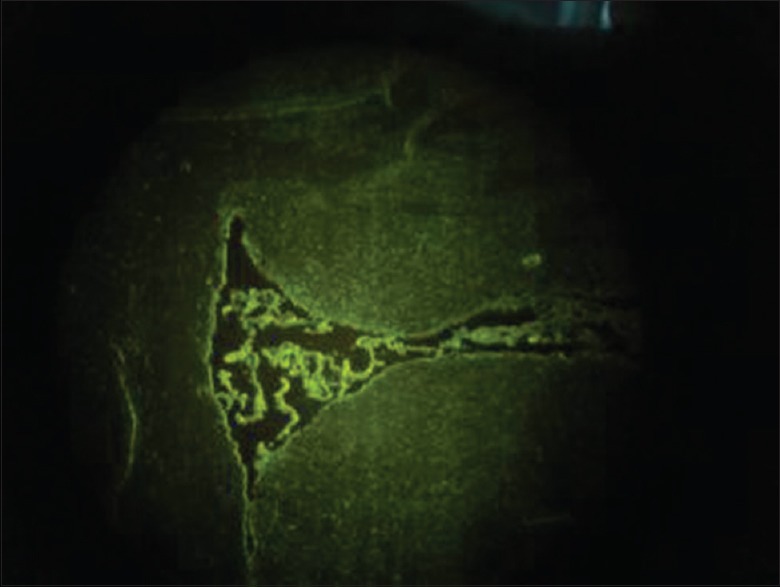
Green fluorescence under fluorescent microscope denoted recombinant adenovirus containing green fluorescent protein (GFP, original magnification, ×20).
Immunohistochemical analysis of hypoxia-inducible factor-1α expression
For each group, quantitative analysis of HIF-1-positive cells was performed with Image-J software (National Institutes of Health, USA) [Figures 2 and 3]. No expression of HIF-1α was detected at any time point in normal and sham groups. HIF-1α expression was obviously stronger in AdHIF-1α group than those in CIR and Ad groups at 24 h and 72 h postreperfusion. And, HIF-1α expression was rare in CIR, Ad, and AdHIF-1α groups at 6 h postreperfusion.
Figure 2.
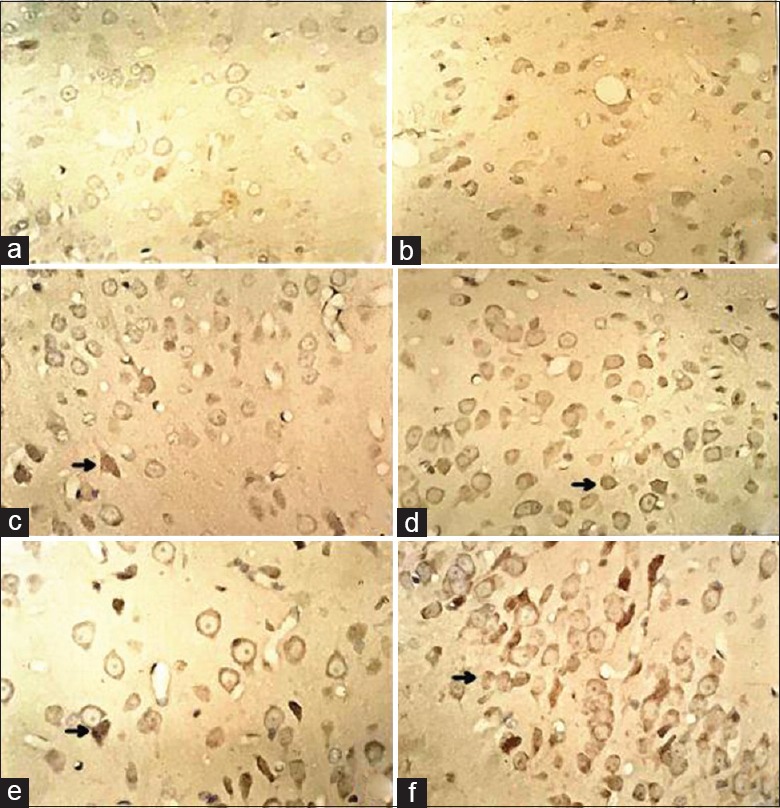
Immunohistochemical staining results of HIF-1α expression at each time point in Ad and AdHIF-1α groups. HIF-1α-positive cells with brownish yellow granules as arrows indicate were HIF-1α expressions (original magnification, ×400). Rare brownish yellow granules were found in CIR group. (a) HIF-1α expression in CIR group at 6 h postreperfusion; (b) HIF-1α expression in AdHIF-1α group at 6 h postreperfusion; (c) HIF-1α expression in CIR group at 24 h postreperfusion; (d) HIF-1α expression in AdHIF-1α group at 24 h postreperfusion; (e) HIF-1α expression in CIR group at 72 h postreperfusion; (f) HIF-1α expression in AdHIF-1α group at 72 h postreperfusion. HIF-1α: Hypoxia-inducible factor-1α; Ad: Recombinant adenovirus; AdHIF-1α: Recombinant adenovirus vector containing HIF-1α; CIR: Cerebral ischemia and reperfusion.
Figure 3.
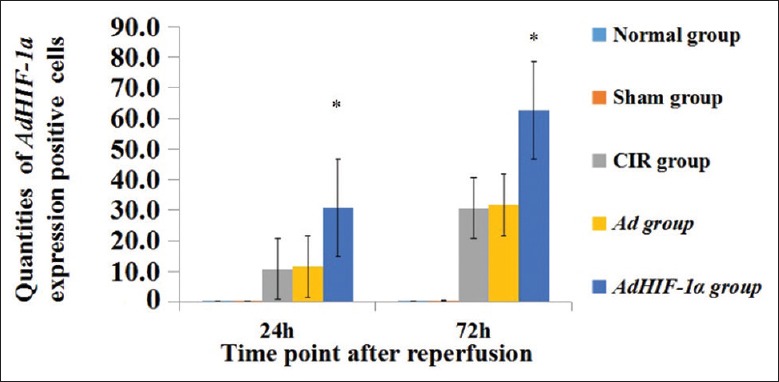
Counts of AdHIF-1α expression positive cells in each group at 24 h and 72 h postreperfusion. *P < 0.05, AdHIF-1α group versus CIR and Ad groups at 24 h and 72 h postreperfusion. The data are presented as mean ± standard deviation (n = 12). HIF-1α: Hypoxia-inducible factor-1α; Ad: Recombinant adenovirus; AdHIF-1α: Recombinant adenovirus vector containing HIF-1α; CIR: Cerebral ischemia and reperfusion.
Reduction of neurological deficit scores and water content after therapy
No neurological dysfunction was shown in normal and sham groups at any time point. However, neurological deficits were notable in CIR, Ad, and AdHIF-1α groups starting at 6 h postreperfusion. The neurological deficit scores of AdHIF-1α group declined significantly as compared with CIR and Ad groups (2.90 ± 0.74 vs. 3.50 ± 0.52 and 3.60 ± 0.53; 2.40 ± 0.84 vs. 3.60 ± 0.52 and 3.50 ± 0.53, respectively; all P < 0.05) at 24 h and 72 h [Figure 4]. It hinted at a neuroprotective effect of AdHIF-1α gene therapy. And, such a therapy could also decrease water content of infarcted brain due to ischemia and reperfusion. Brain water contents increased in CIR, Ad, and AdHIF-1α groups as compared to those in normal and sham groups at 6 h, 24 h, and 72 h postreperfusion. However, brain water content declined significantly in AdHIF-1α group versus CIR and Ad groups at 24 h and 72 h postreperfusion (78.83% ± 0.34% vs. 83.21% ± 0.50% and 83.35% ± 0.32%; 84.13% ± 0.24% vs. 89.76% ± 0.34% and 89.70% ± 0.18%, respectively; all P < 0.05; Figure 5).
Figure 4.
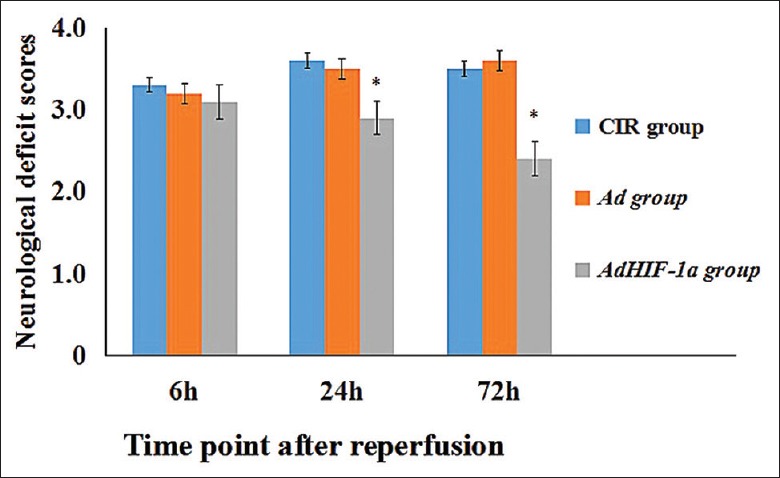
Effect of AdHIF-1α therapy on neurological functions. The neurological deficit scores in CIR, AdHIF-1α, and Ad groups were evaluated at 6 h, 24 h, and 72 h postreperfusion. The data are presented as mean ± standard deviation (n = 12). *P < 0.05, AdHIF-1α group versus CIR and Ad groups at 24 h and 72 h postreperfusion. HIF-1α: Hypoxia-inducible factor-1α; Ad: Recombinant adenovirus; AdHIF-1α: Recombinant adenovirus vector containing HIF-1α; CIR: Cerebral ischemia and reperfusion.
Figure 5.
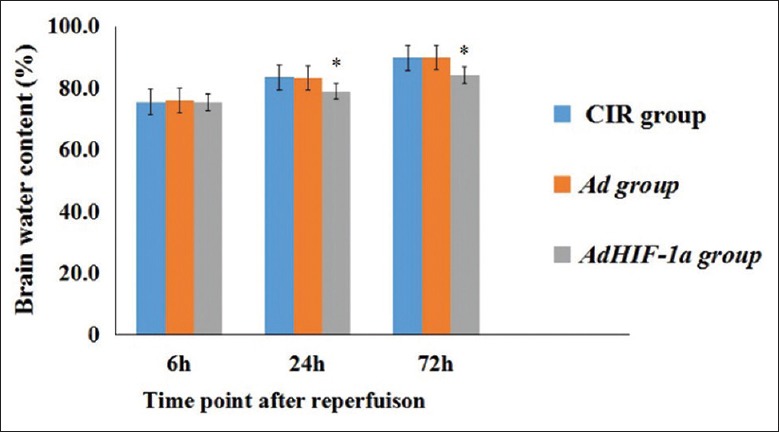
Brain water content of AdHIF-α group decreased significantly versus CIR and Ad groups at 24 h and 72 h postreperfusion (P < 0.05). *P < 0.05, AdHIF-1α group versus CIR and Ad groups at 24 h and 72 h postreperfusion. The data are expressed as mean ± standard deviation (n = 6). HIF-1α: Hypoxia-inducible factor-1α; Ad: Recombinant adenovirus; AdHIF-1α: Recombinant adenovirus vector containing HIF-1α; CIR: Cerebral ischemia and reperfusion.
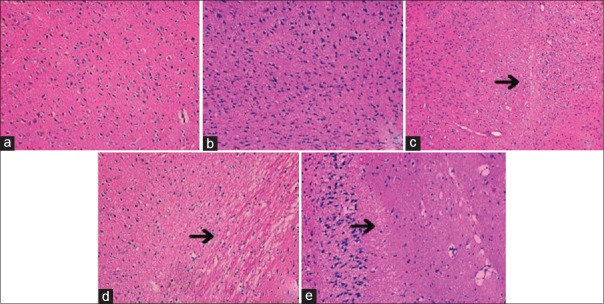
Histopathological changes of cerebral subcortex at 72 h postreperfusion
As demonstrated in Figure 6, normal and sham groups were tightly aligned with distinct nucleoli. And, Ad and CIR groups exhibited marked pathological abnormalities with loosely arranged neurons, pyknotic nucleus, and dark-hued staining. By contrast, histopathological alterations were dramatically reduced by gene therapy in AdHIF-1α group.
Figure 6.
Injury extent of subcortex in five groups (hematoxylin and eosin staining, original magnification, ×200). Arrows indicated pathological injury in CIR, Ad, and AdHIF-1α groups at 72 h postreperfusion. (a) Normal group; (b) sham group; (c) CIR group; (d) Ad group; (e) AdHIF-1α group. HIF-1α: Hypoxia-inducible factor-1α; Ad: Recombinant adenovirus; AdHIF-1α: Recombinant adenovirus vector containing HIF-1α; CIR: Cerebral ischemia and reperfusion.
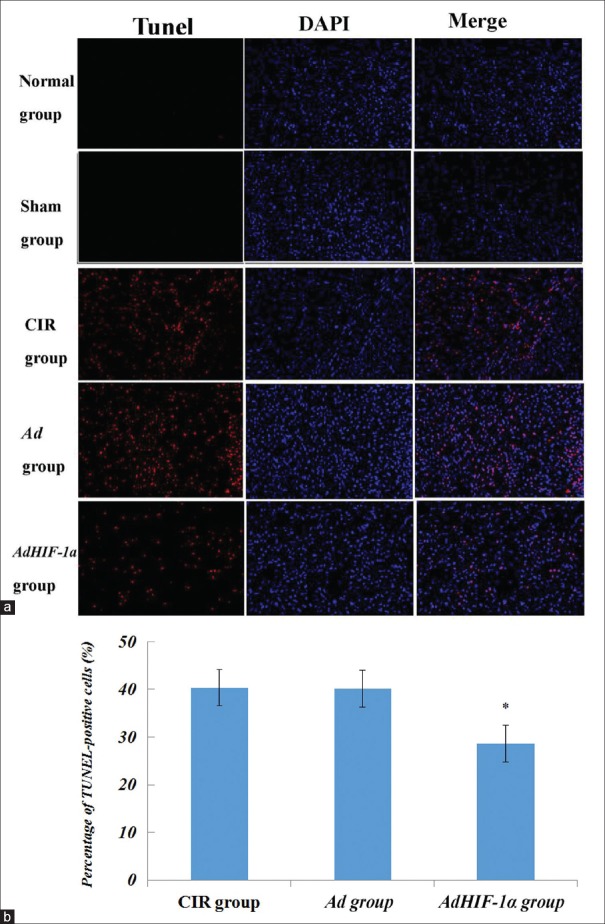
Apoptosis of nerve cells
After gene therapy, TUNEL assay was performed on paraffin-embedded coronal cerebral sections from various rat groups sacrificed at 72 h postreperfusion. Red florescence in ischemic core and penumbra regions of ipsilateral cerebral subcortex indicated TUNEL-positive cells [Figure 7]. And, blue fluorescence denoted DAPI staining of nuclei. Representative images shown were superimposed images of TUNEL and DAPI stains.
Figure 7.
Terminal-deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay for apoptosis of nerve cells. (a) Apoptosis after middle cerebral artery occlusion performed by TUNEL assay on paraffin-embedded coronal cerebral sections of rats from various groups sacrificed at 72 h postmiddle cerebral artery occlusion. Red fluorescence in ischemic core and penumbra regions of ipsilateral subcortex indicated TUNEL-positive cells. Blue fluorescence denoted 4’,6-diamidino-2-phenylindole staining of nuclei. Representative images shown were superimposed images of TUNEL and respective 4’,6-diamidino-2-phenylindole stains. The arrows indicated TUNEL-positive cells. (b) TUNEL-positive cells were counted at five arbitrarily selected microscopic fields at a magnification of × 200. The percentage of apoptotic cells is expressed as mean ± standard deviation (n = 6), *P < 0.05, AdHIF-1α group versus Ad, CIR groups. HIF-1α: Hypoxia-inducible factor-1α; Ad: Recombinant adenovirus; AdHIF-1α: Recombinant adenovirus vector containing HIF-1α; CIR: Cerebral ischemia and reperfusion; TUNEL: Terminal-deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling.
DISCUSSION
In the present study, the antiapoptotic effect of HIF-1α gene therapy effectively reduced neurological deficit scores and cerebral edema at 24 h and 72 h postreperfusion and inhibited pathological damages and apoptosis of nerve cells. Thus, HIF-1α gene therapy might become an alternative regimen for ischemic stroke. The findings were in accordance with previous reports that HIF-1α might play a crucial role in the development of apoptosis.[9,10,11] However, few reports have explored the antiapoptotic effect of HIF-1α gene therapy using recombinant adenovirus as a carrier on CIR injuries in MACO model.
With the advantages of diverse recipient cells, high transfection efficiency, high titer, and ease of concentrating and storage, the construction of recombinant adenovirus containing HIF-1α utilizes AdEasy System. And, the recombinant adenovirus contains complete GFP expression system. To ascertain the transgene expression, the recombinant adenovirus containing GFP was injected into right lateral ventricle and definite GFP expression confirmed in right cortex. GFP was visualized in ependymal and adjacent cells at 24 h postinjection. The expression of GFP peaked at 72 h and persisted for around 20 days postinjection. The expression of HIF-1α protein was detected by immunohistochemistry in AdHIF-1α group. There was significant increase of HIF-1α expression versus Ad and CIR groups at 24 h and 72 h postreperfusion. These results confirmed that the recombinant adenovirus was successfully transfected into cerebral cells and HIF-1α gene was expressed in ependymal and adjacent cells. The results were agreed with those of previous studies.[12,13] It also confirmed that recombinant adenovirus was only expressed locally. It had a limited duration of expression so that collateral circulation could be reestablished by angiogenesis. Local effect of recombinant adenovirus and a short duration of expression make it more suitable for treating CIR.
Ischemic stroke due to cerebral artery occlusion causes an infarction of brain tissues. Brain cells are particularly vulnerable to hypoxic insults. An acute shortage of oxygen and glucose leads to transient or permanent decrease of cerebral blood and triggers a cascade of events, including excitotoxicity, oxidative stress, inflammation, and apoptosis.[14] And, ischemic reperfusion results in massive apoptosis of nerve cells.[15,16] In the present study, an elevated number of apoptotic cells were detected in cerebral tissues of MACO rats. And, HIF-1α therapy markedly reduced cellular apoptosis in brain tissues due to CIR. Previous studies have implicated HIF-1α as a master transcriptional regulator of various genes under hypoxia. And, it might participate in the regulations of angiogenesis, glucose metabolism, anti-apoptosis, and cellular survival during hypoxia.[17,18] It could also upregulate such genes as glucose transporters, erythropoietin, and vascular endothelial growth factor.[19] The underlying mechanisms will be elucidated by further studies. HIF-1α stimulated the proliferation of blood vessels to maintain an adequate oxygen blood supply and boosted the capable of oxygen-carrying capacity, glucose transport and glycolysis of blood.[20] Here, HIF-1α gene therapy significantly reduced hypoxic injuries, lessened cerebral edema, and cell apoptosis and improved neurological function in rat's ischemia-reperfusion model. Due to a small sample size, larger future studies are warranted for further validations of the results.
Financial support and sponsorship
This work was supported by grants from the Health and Family Planning Commission of Guizou (No. 2014-41), the Science and Technology Foundation of Guizhou (No. 2007-2096), and the Special Governor's Fund for Outstanding Technological and Educational Talents in Guizhou (No. 2006-60).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Guan W, Kozak A, Fagan SC. Drug repurposing for vascular protection after acute ischemic stroke. Acta Neurochir Suppl. 2011;111:295–8. doi: 10.1007/978-3-7091-0693-8_49. doi: 10.1007/978-3-7091-0693-8_49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh N, Sharma G, Mishra V. Hypoxia inducible factor-1: Its potential role in cerebral ischemia. Cell Mol Neurobiol. 2012;32:491–507. doi: 10.1007/s10571-012-9803-9. doi: 10.1007/s10571-012-9803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masoud GN, Li W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–89. doi: 10.1016/j.apsb.2015.05.007. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erler JT, Cawthorne CJ, Williams KJ, Koritzinsky M, Wouters BG, Wilson C, et al. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004;24:2875–89. doi: 10.1128/MCB.24.7.2875-2889.2004. doi: 10.1128/MCB.24.7.2875-2889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q, Bian H, Guo L, Zhu H. Berberine preconditioning protects neurons against ischemia via sphingosine-1-phosphate and hypoxia-inducible factor-1. Am J Chin Med. 2016;44:927–41. doi: 10.1142/S0192415X16500518. doi: 10.1142/S0192415X16500518. [DOI] [PubMed] [Google Scholar]
- 6.Calik MW, Carley DW. Intracerebroventricular injections of dronabinol, a cannabinoid receptor agonist, does not attenuate serotonin-induced apnea in Sprague-Dawley rats. J Negat Results Biomed. 2016;15:8. doi: 10.1186/s12952-016-0052-1. doi: 10.1186/s12952-016-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 8.Gotoh O, Sano A, Koide T, Takakura K. Ischemic brain edema following occlusion of the middle cerebral artery in the rat. Stroke. 1985;16:101–9. doi: 10.1161/01.str.16.1.101. doi: 10.1161/01.STR.16.1.101. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Lee JH, Yoo SY, Hur J, Kim HS, Kwon SM. Hypoxia inhibits cellular senescence to restore the therapeutic potential of old human endothelial progenitor cells via the hypoxia-inducible factor-1a-TWIST-p21 axis. Arterioscler Thromb Vasc Biol. 2013;33:2407–14. doi: 10.1161/ATVBAHA.113.301931. doi: 10.1161/ATVBAHA.113.301931. [DOI] [PubMed] [Google Scholar]
- 10.Cheng YL, Park JS, Manzanero S, Choi Y, Baik SH, Okun E, et al. Evidence that collaboration between HIF-1alpha and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol Dis. 2014;62:286–95. doi: 10.1016/j.nbd.2013.10.009. doi: 10.1016/j.nbd.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Zhao TZ, Zou YJ, Zhang JH, Feng H. Hypoxia Induces autophagic cell death through hypoxia-inducible factor 1alpha in microglia. PLoS One. 2014;9:e96509. doi: 10.1371/journal.pone.0096509. doi: 10.1371/journal.pone.0096509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H, Lin TN, Cheung WM, Nian GM, Tseng PH, Chen SF, et al. Cyclooxygenase-1 and bicistronic cyclooxygenase-1/prostacyclin synthase gene transfer protect against ischemic cerebral infarction. Circulation. 2002;105:1962–9. doi: 10.1161/01.cir.0000015365.49180.05. doi: 10.1161/01.CIR.0000015365.49180.05. [DOI] [PubMed] [Google Scholar]
- 13.Geddes BJ, Harding TC, Hughes DS, Byrnes AP, Lightman SL, Conde G, et al. Persistent transgene expression in the hypothalamus following stereotaxic delivery of a recombinant adenovirus: Suppression of the immune response with cyclosporin. Endocrinology. 1996;137:5166–9. doi: 10.1210/endo.137.11.8895393. doi: 10.1210/endo.137.11.8895393. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar S, Mukherjee A, Swarnakar S, Das N. Nanocapsulated ascorbic acid in combating cerebral ischemia reperfusion- induced oxidative injury in rat brain. Curr Alzheimer Res. 2016;13:1363–73. doi: 10.2174/1567205013666160625082839. doi: 10.2174/1567205013666160625082839. [DOI] [PubMed] [Google Scholar]
- 15.Zhen Y, Ding C, Sun J, Wang Y, Li S, Dong L. Activation of the calcium-sensing receptor promotes apoptosis by modulating the JNK/p38 MAPK pathway in focal cerebral ischemia-reperfusion in mice. Am J Transl Res. 2016;8:911–21. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu T, Zhan L, Liang D, Hu J, Lu Z, Zhu X, et al. Hypoxia-inducible factor 1a mediates neuroprotection of hypoxic postconditioning against global cerebral ischemia. J Neuropathol Exp Neurol. 2014;73:975–86. doi: 10.1097/NEN.0000000000000118. doi: 10.1097/NEN.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 17.Liu BN, Han BX, Liu F. Neuroprotective effect of pAkt and HIF-1 alpha on ischemia rats. Asian Pac J Trop Med. 2014;7:221–5. doi: 10.1016/S1995-7645(14)60025-0. doi: 10.1016/S1995-7645(14)60025-0. [DOI] [PubMed] [Google Scholar]
- 18.Wan J, Wu W. Hyperthermia induced HIF-1alpha expression of lung cancer through AKT and ERK signaling pathways. J Exp Clin Cancer Res. 2016;35:119. doi: 10.1186/s13046-016-0399-7. doi: 10.1186/s13046-016-0399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang T, Yao Q, Cao F, Liu Q, Liu B, Wang XH. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: Insight into the cytotoxicity and antiangiogenesis. Int J Nanomedicine. 2016;11:6679–92. doi: 10.2147/IJN.S109695. doi: 10.2147/IJN.S109695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mistry IN, Smith PJ, Wilson DI, Tavassoli A. Probing the epigenetic regulation of HIF-1α transcription in developing tissue. Mol Biosyst. 2015;11:2780–5. doi: 10.1039/c5mb00281h. doi: 10.1039/c5mb00281h. [DOI] [PubMed] [Google Scholar]