Abstract
Background:
One-lung ventilation (OLV) is a common ventilation technology during thoracic surgery that can cause serious clinical problems. We aimed to conduct a meta-analysis to compare oxygenation and intrapulmonary shunt during OLV in adults undergoing thoracic surgery with dexmedetomidine (Dex) versus placebo to assess the influence and safety of using Dex.
Methods:
Randomized controlled trials comparing lung protection in patients who underwent thoracic surgery with Dex or a placebo were retrieved from PubMed, EMBASE, MEDLINE, Cochrane Library, and China CNKI database. The following information was extracted from the paper: arterial oxygen partial pressure (PaO2), PaO2/inspired oxygen concentration (PaO2/FiO2, oxygenation index [OI]), intrapulmonary shunt (calculated as Qs/Qt), mean arterial pressure (MAP), heart rate (HR), tumor necrosis factor-α (TNF-α), interleukin (IL)-6, superoxide dismutase (SOD), and malondialdehyde (MDA).
Results:
Fourteen randomized controlled trials were included containing a total of 625 patients. Compared with placebo group, Dex significantly increased PaO2/FiO2 (standard mean difference [SMD] = 0.98, 95% confidence interval [CI] [0.72, 1.23], P < 0.00001). Besides, Qs/Qt (SMD= −1.22, 95% CI [−2.20, −0.23], P = 0.020), HR (SMD= −0.69, 95% CI [−1.20, 0.17], P = 0.009), MAP (SMD= −0.44, 95% CI [−0.84, 0.04], P = 0.030), the concentrations of TNF-α (SMD = −1.55, 95% CI [−2.16, −0.95], P <0.001), and IL-6 (SMD = −1.53, 95% CI [−2.37, −0.70], P = 0.0003) were decreased in the treated group, when compared to placebo group. No significant difference was found in MDA (SMD = −1.14, 95% CI [−3.48, 1.20], P = 0.340) and SOD (SMD = 0.41, 95% CI [−0.29, 1.10], P = 0.250) between the Dex group and the placebo group. Funnel plots did not detect any significant publication bias.
Conclusions:
Dex may improve OI and reduce intrapulmonary shunt during OLV in adults undergoing thoracic surgery. However, this conclusion might be weakened by the limited number of pooled studies and patients.
Keywords: Dexmedetomidine, Intrapulmonary Shunt, Meta-analysis, One-lung Ventilation, Oxygenation Index
INTRODUCTION
One-lung ventilation (OLV) is a common ventilation technique during thoracic surgery that can achieve double-lung isolation effectively, provide a good view and operating space for the surgeon, and protect normal lungs from hemorrhage or abscess caused by the affected lung.[1] However, OLV can cause an imbalance of the ventilation/perfusion (V/Q) ratio and increase intrapulmonary shunt. During OLV, alveolar oxygen tension in the nonventilated collapsed lung declines to the mixed venous level,[2] which leads to hypoxemia, acute hypoxic pulmonary vasoconstriction (HPV), and ischemia-reperfusion injury.[3,4] Lung ischemia/reperfusion injury is always related to enhanced oxidative stress, strengthened lipid peroxidation, and reduced antioxidant abilities, which cause damage to pulmonary capillary endothelial cells and alveolar epithelial cells leading to lung edema. In addition, long periods of inhalation of high concentrations of oxygen also cause oxidative stress and lead to lung damage.[5]
HPV is an important protective mechanism by which blood flow is diverted from the nonventilated lung toward a better-ventilated region, thereby maintaining adequate arterial oxygenation.[6] HPV decreases blood flow to the collapsed lung, and many anesthetics, such as inhalation anesthetics, may inhibit HPV and increase hypoxemia.[7] Therefore, the anesthesiologists aim to reduce intrapulmonary shunt and protect lung function with the help of various drugs and techniques.
Dexmedetomidine (Dex) is a new highly selective α2 adrenergic receptor agonist with strong sedative, analgesic, anti-inflammatory, and organ protection effects.[8,9,10] Dex can attenuate the increased sympathetic tone and result in low heart rate (HR), blood pressure, and myocardial oxygen consumption.[11] In the last few years, a number of studies have evaluated the effect of Dex on OLV. The results of these studies are markedly variable, and all studies appear to be underpowered.[12,13]
We used the existing data to evaluate oxygenation and intrapulmonary shunt during OLV in adults undergoing thoracic surgery with Dex versus placebo to determine the influence of perioperative Dex on thoracic surgery patients using a standard meta-analysis.
METHODS
Search strategy
Multiple databases, including the PubMed, EMBASE, MEDLINE, Cochrane Library, and China CNKI databases, were searched systematically from inception to April 2017. The following keywords were used to conduct a basic search: “dexmedetomidine,” “hypoxic,” “one-lung ventilation,” and “randomized controlled trial (RCT).” No language restriction was applied for the selection of articles. Chinese documents with a Jadad score of three points and above were included.
This meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. We have extensively searched all relevant published studies.
Inclusion criteria
Based on the principles of PICOS, the inclusion criteria of eligible studies were as follows: (1) inclusion of the American Society of Anesthesiologists I–III adults undergoing thoracic surgery with OLV, (2) comparison of perioperative Dex to normal saline (NS), (3) report of the perioperative indexes of arterial oxygen partial pressure (PaO2) and intrapulmonary shunt (calculated as Qs/Qt), and (4) being a randomized controlled trial published before April 2017.
Exclusion criteria
We excluded studies with incomplete or unclear original data or those that included patients with serious liver and kidney dysfunction or respiratory failure, severe heart disease (bradycardia [HR <45 beats/min], arrhythmia, coronary heart disease, or heart failure), long-term alcohol, opioid, or sedative hypnotic drug addiction or dependency history, a history of neuropsychiatric diseases, or systemic diseases such as uncontrolled hypertension, diabetes, and severe bleeding disease.
Quality assessment
Two reviewers scanned the titles and abstracts independently, read the full-text articles, used a standardized form to extract the data, and solved disagreements through consultation and discussion with a third reviewer. If disagreements remained unresolved, the views of all members of the study team were sought. The Jadad standard was used to evaluate the quality of the studies. The randomization method received 1 or 2 (2, the correct description of the randomization method and 1, only described as random). The blinding method received between 0 and 2 (2, appropriateness of double-blind; 1, described as double blind; and 0, not mentioned). The analysis of reasons for withdrawal received 0 or 1 (1, detailed description and 0, no description). A total score of 3 or more indicated a high-quality study.
Assessment index
The following information was extracted during OLV from each trial: the primary outcomes were PaO2/inspired oxygen concentration (PaO2/FiO2, oxygenation index [OI]) and Qs/Qt, and the secondary outcomes were mean arterial pressure (MAP), HR, malondialdehyde (MDA), tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and superoxide dismutase (SOD).
Statistical analysis
We extracted data from all publications to calculate the standard mean difference (SMD) and associated 95% confidence interval (CI) for continuous outcomes. P < 0.05 was considered statistically significant. The presence of statistical heterogeneity of outcomes across trials was assessed using the I2 measure and was thought to be significant when P ≤ 0.10 and I2 > 50%. If heterogeneity was absent (P - value for heterogeneity >0.1), a fixed-effects model to calculate pooled effects was chosen; otherwise, a random-effects model was used.
We meta-analyzed all studies together and then performed subgroup analysis separating intravenous anesthesia combined with intravenous anesthesia and total intravenous anesthesia for OI, as inhalation anesthetics may inhibit HPV and increase hypoxemia.[7]
To explore potential sources of heterogeneity and assess the robustness of our results, we performed additional analyses for outcomes that were reported by at least five studies. We assessed the effect of each study on the overall effect size and heterogeneity by repeating the analysis after removing one study at a time.
Two authors independently assessed the methodological quality and risk of bias of the included articles using the Cochrane tool. The Cochrane tool assessed domains including selection bias, performance bias, detection bias, attrition bias, reporting bias, and other sources of bias, and for each individual domain, classified studies into low, unclear, and high risk of bias.
RevMan 5.3 software (The Cochrane Collaboration, Copenhagen, Denmark) was used for the standard meta-analysis. STATA version 12.0 (StataCorp LP, College Station, Texas, USA) was used for the sensitivity analysis.
RESULTS
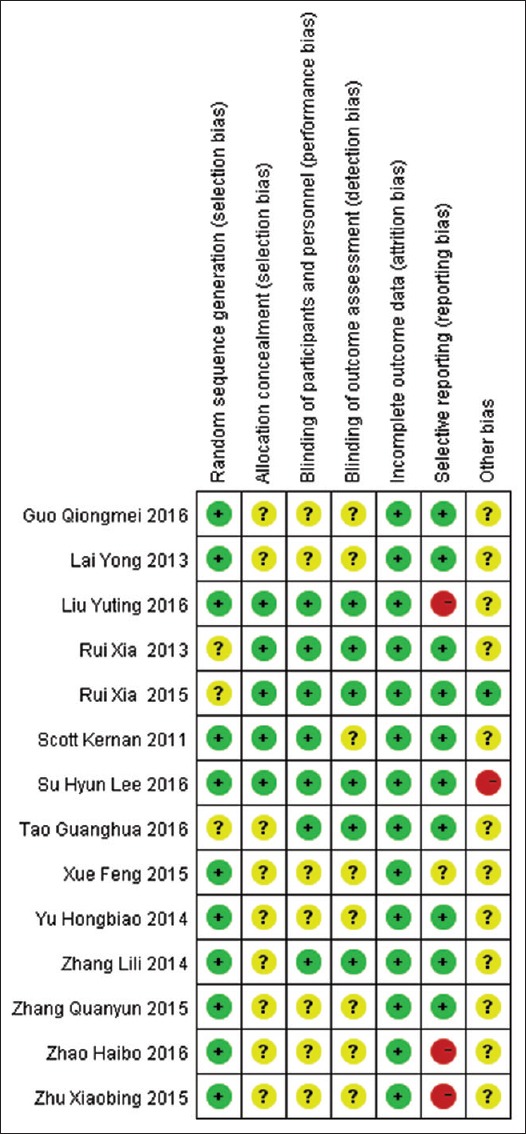
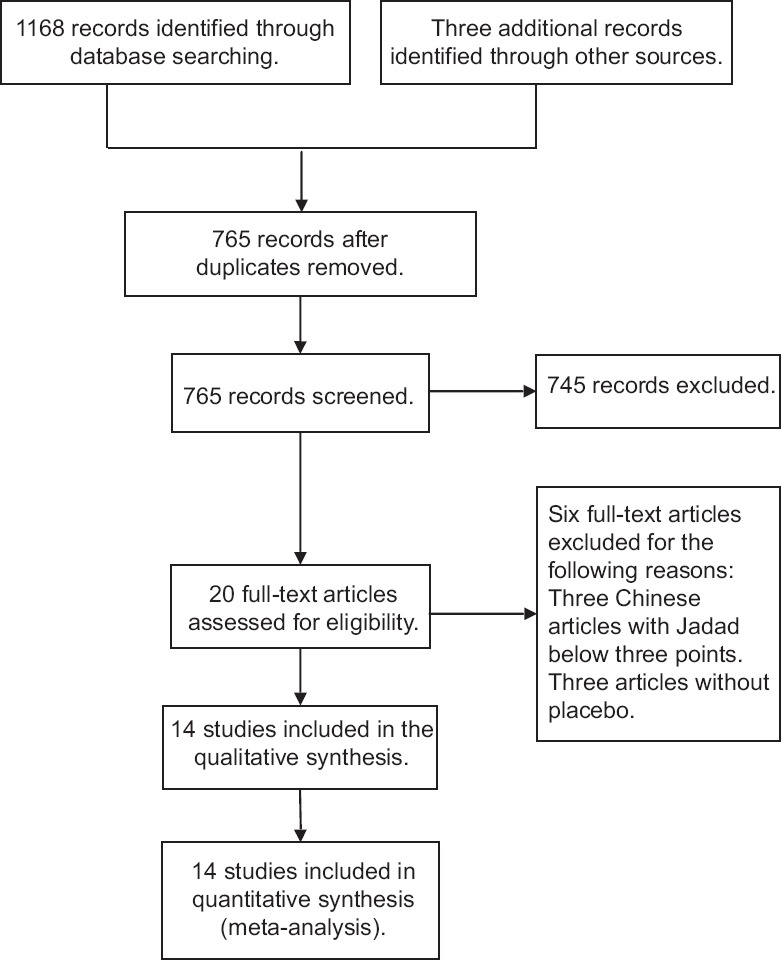
The systematic literature search identified 1171 relevant publications. After a review of titles and abstracts, twenty studies were selected as potentially eligible for inclusion in this systematic review. After reading the full-text articles, we selected 14 RCTs[12,13,14,15,16,17,18,19,20,21,22,23,24,25] including a total of 625 patients with 313 cases in the Dex group and 312 cases in the placebo group. Basic data are shown in Table 1. The methodological quality assessment was performed by Revman 5.3 [Figure 1]. One trial was classified as low risk of bias,[25] 12 as unclear risk of bias,[12,13,14,15,16,17,18,19,20,22,23,24] and one as high risk of bias.[21] The randomization procedure was adequately described in 11 trials,[13,15,16,17,18,19,20,21,22,23,24] and the concealment of treatment allocation was described in five trials.[12,17,21,22,25] Six studies were double blind,[12,14,17,20,21,25] whereas the blinding status was unclear for all other studies. No studies had an unclear or high risk of incomplete data on outcomes. Three trials were classified as having selective reporting,[17,18,23] ten did not have selective reporting,[12,14,15,16,19,20,21,22,24] and one was unclear[13] [Figure 1]. The complete search strategy is presented in Figure 2.
Table 1.
Basic data of enrolled studies comparing lung protection in patients who underwent thoracic surgery with Dex or a placebo
| Study ID | Patients’ age (years) | Number of patients | Management of Dex | Jadad | ||
|---|---|---|---|---|---|---|
| Dex | Placebo | Dex | Placebo | |||
| Xia R et al. 2013 | 53 ± 14 | 55 ± 15 | 23 | 22 | Dex: 1.0 µg·kg−1·h−1 10 min after induction, later 0.7 µg·kg−1·h−1 | 4 |
| Lee SH et al. 2016 | 68.4 ± 6.4 | 69.4 ± 8.7 | 25 | 25 | Dex: 1.0 µg·kg−1·h−1 10 min after the patient was hemodynamically stable 30 min after the initiation of OLV, later 0.5 µg·kg−1·h−1 | 5 |
| Xia R et al. 2015 | 55 ± 12 | 56 ± 11 | 25 | 24 | Dex: 1.0 µg·kg−1·h−1 10 min after induction, later 0.7 µg·kg−1·h−1 | 4 |
| Kernan S et al. 2011 | 53.8 ± 13.5 | 51.2 ± 17.8 | 9 | 10 | Dex: 0.3 µg/kg 10 min after 10 min of OLV, later 0.3 µg·kg−1·h−1 | 5 |
| Guo QM et al. 2016 | 62 ± 13 | 60 ± 12 | 20 | 20 | Dex: 1.0 µg·kg−1·h−1 10 min before induction, later 0.5 µg·kg−1·h−1 | 3 |
| Lai Y et al. 2013 | 48.3 ± 12.5 | 46.2 ± 15.6 | 11 | 11 | Dex: 0.3 µg/kg 10 min, later 0.3 µg·kg−1·h−1 | 3 |
| Liu YT et al. 2016 | 59 ± 5 | 60 ± 6 | 36 | 36 | Dex: 0.5 µg/kg 15 min before induction, later 0.3 µg·kg−1·h−1 | 5 |
| Tao GH et al. 2016 | 62.7 ± 6.4 | 62.7 ± 6.3 | 20 | 20 | Dex: 1.0 µg·kg−1·h−1 20 min before induction, later 0.5 µg·kg−1·h−1 | 3 |
| Xue F et al. 2015 | 59.6 ± 5.6 | 60.2 ± 6.2 | 30 | 30 | Dex: 0.6 µg/kg 10 min before induction, later 0.5 µg·kg−1·h−1 at the beginning of surgery | 3 |
| Yu HB et al. 2014 | 57 ± 9 | 58 ± 7 | 19 | 19 | Dex: 1.0 µg·kg−1· h−1 15 min before induction, later 0.5 µg·kg−1·h−1 | 3 |
| Zhang LL et al. 2014 | 57.3 ± 8.7 | 56.8 ± 7.9 | 20 | 20 | Dex: 0.6 µg/kg 10 min before induction, later 0.4 µg·kg−1·h−1 | 4 |
| Zhang QY et al. 2015 | 57 ± 14 | 55 ± 12 | 20 | 20 | Dex: 1.0 µg·kg−1·h−1 over 10 min before operation, later 0.5 µg·kg−1·h−1 | 3 |
| Zhao HB 2016 | 54.1 ± 5.3 | 53.4 ± 4.6 | 25 | 25 | Dex: 1.0 µg·kg−1·h−1 10 min after induction, later 0.5 µg·kg−1·h−1 | 3 |
| Zhu XB et al. 2015 | 40 ± 5 | 42 ± 7 | 30 | 30 | Dex: 1.0 µg·kg−1·h−1 10 min before induction, later 0.4 µg·kg−1·h−1 | 3 |
Dex: Dexmedetomidine; OLV: One-lung ventilation.
Figure 1.
Methodological quality assessment.
Figure 2.
Complete search strategy.
Effect of dexmedetomidine on the oxygenation index in patients undergoing thoracic surgery during one-lung ventilation
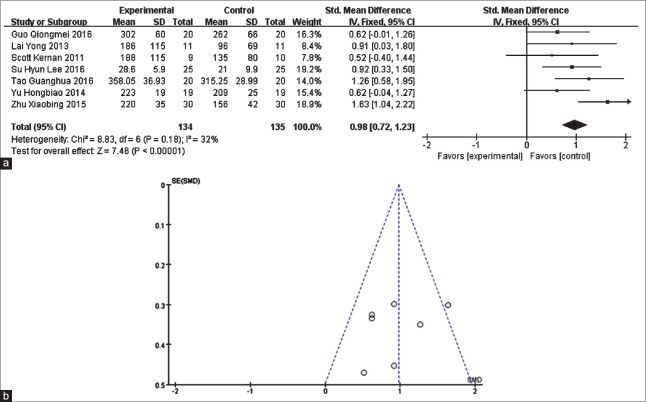
Seven studies[14,15,18,19,21,22,24] with 269 cases found that the use of Dex significantly improved the patient's OI during OLV (SMD = 0.98, 95% CI [0.72, 1.23], P < 0.00001) [Figure 3] compared with the placebo (NS).
Figure 3.
Meta-analysis of intraoperative oxygenation index. (a) Forest plot for intraoperative oxygenation index: dexmedetomidine vs. placebo. (b) Funnel plot of 7 reviewed studies on intraoperative oxygenation index when comparing dexmedetomidine with placebo.
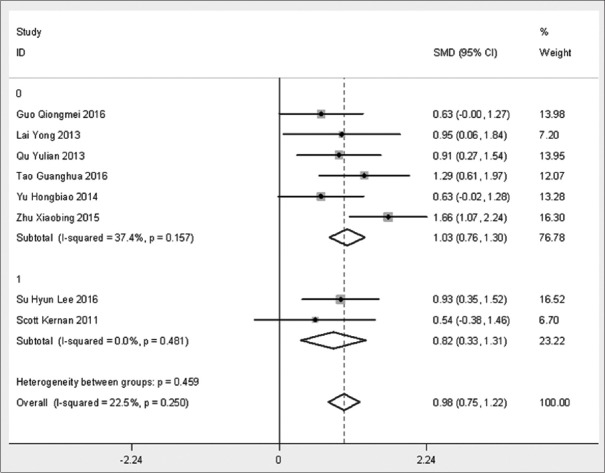
In the subgroup analysis [Figure 4], intravenous anesthesia combined with inhalation anesthesia (1, total intravenous anesthesia) produced OIs that were significantly improved in both groups (P < 0.0001 and P = 0.001), but there were no significant differences between the groups (P = 0.495).
Figure 4.
Subgroup analysis of intraoperative oxygenation index.
Effect of dexmedetomidine on intrapulmonary shunt in patients undergoing thoracic surgery during one-lung ventilation
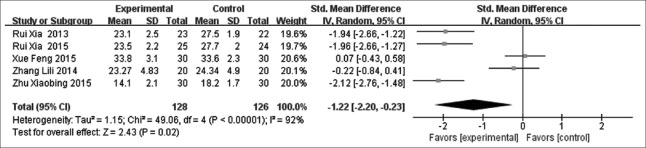
The results from five studies[12,13,20,23,25] demonstrated that Dex can decrease intrapulmonary shunt (SMD = −1.22, 95% CI [−2.20, −0.23], P = 0.02) [Figure 5]. Heterogeneity was high (P < 0.1 and I2 = 92%), so a random-effects model was chosen.
Figure 5.
Meta-analysis of intrapulmonary shunt.
Effect of dexmedetomidine on heart rate and mean arterial pressure in patients undergoing thoracic surgery during one-lung ventilation
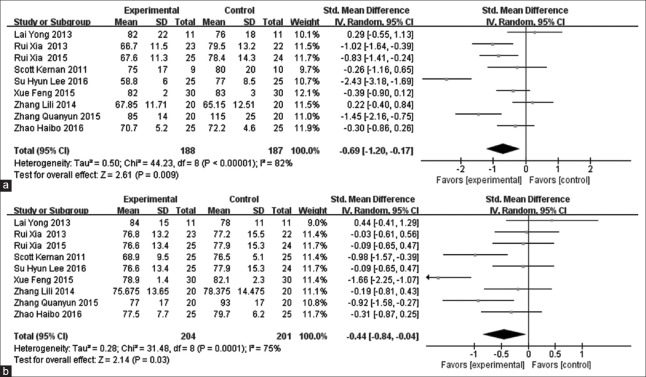
The pooling of data from nine studies[12,13,15,16,18,20,21,22,25] showed that Dex treatment was related to a significant decrease in perioperative HR (SMD = −0.69, 95% CI [−1.20, 0.17], P = 0.009) [Figure 6a] and MAP (SMD = −0.44, 95% CI [−0.84, 0.04], P = 0.030) [Figure 6b].
Figure 6.
Meta-analysis of intraoperative heart rate and mean arterial pressure. (a) Forest plot for heart rate: dexmedetomidine vs. placebo. (b) Forest plot for mean arterial pressure: dexmedetomidine vs. placebo.
Effect of dexmedetomidine on inflammatory factors in patients undergoing thoracic surgery during one-lung ventilation
There were four studies[17,18,19,24] that provided evidence that the concentration of TNF-α in the experimental group was lower than that in placebo group, and the result was statistically significant (SMD = −1.55, 95% CI [−2.16, −0.95], P < 0.001), which means the use of Dex could reduce the concentration of TNF-α in patients undergoing OLV. There were also statistically significant (SMD = −1.53, 95% CI [−2.37, −0.70], P = 0.0003) differences in the concentrations of IL-6 between the Dex group and the placebo group in a random-effects model (P = 0.006, I2 = 81%).[17,18,19] A total of 99 patients[18,25] were enrolled in a study comparing the concentration of MDA in the Dex group versus the placebo group. Heterogeneity (P < 0.00001, I2 = 96%) among studies was large, so a random-effects model was chosen. The meta-analysis showed that the effect of Dex on the concentration of MDA during OLV in patients was not statistically significant (SMD = −1.14, 95% CI [−3.48, 1.20], P = 0.340). We found that the differences in the concentration of SOD between the Dex group and the placebo group were not significant (SMD = 0.41, 95% CI [−0.29, 1.10], P = 0.250).[18,25] We chose a random-effects model due to high heterogeneity (P = 0.080, I2 = 67%).
Sensitivity analyses
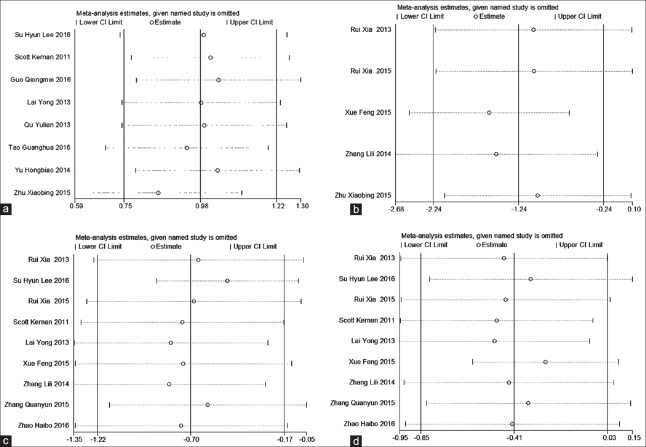
We conducted sensitivity analyses for the outcomes that included more than four studies (OI, intrapulmonary shunt, HR, and MAP). Removing one study at a time did not change the direction of the effect size for any of the outcomes [Figure 7].
Figure 7.
Sensitivity analyses of the intraoperative oxygenation index (a), intrapulmonary shunt (b), heart rate (c), and mean arterial pressure (d).
DISCUSSION
Despite its wide application in thoracic surgery, OLV can result in an imbalance of the intraoperative V/Q ratio, hypoxemia, and acute lung injury. The Qs/Qt ratio is approximately 2–5% in daily life and up to 10% after general anesthesia and lying on your side, but it can increase to 40–50% after OLV.[26] To date, it is uncertain whether there is well-established prophylaxis, although various preventive and protective measures have been studied, such as lung-protective ventilation strategies (lower tidal volumes and higher positive end-expiratory pressure)[27,28,29,30] and reducing the use of inhalation anesthetics.[31]
Dex is one of the interventions mentioned above because of its sedative, analgesic, anti-inflammatory, and organ protection effects that could contribute to decreasing lung injury.
Intrapulmonary shunt is always measured as a standard for HPV.[32] The OI provides an integrated assessment of both intrapulmonary shunt and mean airway pressure. The OI has been used as an indicator of the severity of lung injury.[33]
This study attempted to explain whether Dex can improve arterial oxygenation and intrapulmonary shunt during OLV in adults undergoing thoracic surgery. There were few previous systematic reviews or guidances on the use of Dex for OLV. This meta-analysis showed that the OI in the Dex group was significantly higher than in the placebo group. In addition, the Qs/Qt was reduced compared to the placebo group. Therefore, during thoracic surgery, Dex plays an important role in lung protection through improving pulmonary oxygenation and reducing intrapulmonary shunt.
The mechanism through which Dex improves oxygenation and reduces intrapulmonary shunt during OLV may be related to several factors.[18] (1) Dex can reduce the amount of propofol, which is a beneficial to HPV and oxygenation. This meta-analysis included 14 randomized controlled trials, including ten studies of total intravenous anesthesia with propofol anesthesia and four studies that used propofol combined with inhalation anesthesia for the maintenance of anesthesia. With the use of Dex, the dosage of propofol decreased.[13,15,24] Recent studies have shown that propofol can inhibit HPV in a dose-dependent manner, and the mechanism of this inhibition is probably that propofol has antagonistic effects on calcium channels dependent on pulmonary artery smooth muscle cells with L-type voltage. Propofol reduces the sensitivity of cells to calcium, thus weakening calcium mobilization in pulmonary artery smooth muscle cells in anoxic condition. It can be concluded that, after intravenous infusion of Dex, the decreasing demand for propofol has a beneficial effect on HPV. (2) Dex can reduce the dose of inhaled anesthetics, thus playing a beneficial role in HPV and oxygenation functions. There were four studies included in this meta-analysis that chose inhalation anesthesia for maintenance of anesthesia. It has been shown that inhaled anesthetics can inhibit HPV and increase intrapulmonary shunt. (3) There is a direct effect of Dex on strengthening HPV. Dex can directly enhance HPV through targeting the alpha-2B receptor in vascular smooth muscle and inducing vasoconstriction. Therefore, Dex-related pulmonary vasoconstriction can improve the V/Q ratio and oxygenation function. (4) Dex can increase the concentration of nitric oxide in the blood to reduce intrapulmonary shunt and increase PaO2.[14]
Inflammation is another important cause of lung injury. Tissue hypoxia in the collapsed lung may cause hypoxia-induced inflammation, and subsequent re-expansion may lead to reoxygenation-induced injury through the production of reactive oxygen species.[2] A good question is whether Dex can reduce the inflammatory response of lung tissue. This meta-analysis showed that the concentrations of TNF-α and IL-6 were significantly lower in the Dex group than in the placebo group, but MDA and SOD were not significantly different between the two groups. Therefore, whether Dex can inhibit the inflammatory reaction and reduce lung injury needs further study.
In this analysis, Dex was used in a bolus of 1.0 μg/kg followed by a continuous infusion at 0.3–0.7 μg·kg−1·h−1 for hours in most studies [Figure 1]. However, the usage time and the effect of the drug varied from study to study. It was reported that Dex at a concentration 5–15 times lower than the clinical plasma target concentration (0.4–1.2 ng/ml) could induce vasoconstriction.[34] In addition, Dex at the clinical concentration can reduce the redistribution of pulmonary blood flow from the ventilated lung to the nonventilated lung.[12] Therefore, further high-quality, randomized, controlled trials are needed to explore the better management of Dex.
Pooling of data showed that Dex treatment was related to a significant decrease in the incidence of perioperative HR and MAP, which confirmed that Dex can inhibit sympathetic nerve excitability and thus play a role in decreasing HR and MAP.
There are some potential limitations associated with the included studies that should be considered when interpreting the results of our study. (1) The number of pooled studies was limited. (2) The number of patients in most randomized studies included in our meta-analysis was limited, which increases the risk of underestimating adverse effects and of overestimating treatment effects. (3) Bias existed because of the different doses and infusion times with Dex. (4) Nine studies did not mention the existence of allocation concealment, and 8 studies did not describe blinding procedures.
In summary, Dex may improve arterial oxygenation and intrapulmonary shunt during OLV in adults undergoing thoracic surgery. However, we still need more powerful evidence to confirm this conclusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Campos JH. Current techniques for perioperative lung isolation in adults. Anesthesiology. 2002;97:1295–301. doi: 10.1097/00000542-200211000-00036. [DOI] [PubMed] [Google Scholar]
- 2.Tojo K, Goto T, Kurahashi K. Protective effects of continuous positive airway pressure on a nonventilated lung during one-lung ventilation: A prospective laboratory study in rats. Eur J Anaesthesiol. 2016;33:776–83. doi: 10.1097/EJA.0000000000000460. doi: 10.1097/EJA.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 3.Karzai W, Schwarzkopf K. Hypoxemia during one-lung ventilation: Prediction, prevention, and treatment. Anesthesiology. 2009;110:1402–11. doi: 10.1097/ALN.0b013e31819fb15d. doi: 10.1097/ALN.0b013e31819fb15d. [DOI] [PubMed] [Google Scholar]
- 4.Misthos P, Katsaragakis S, Theodorou D, Milingos N, Skottis I. The degree of oxidative stress is associated with major adverse effects after lung resection: A prospective study. Eur J Cardiothorac Surg. 2006;29:591–5. doi: 10.1016/j.ejcts.2005.12.027. doi: 10.1016/j.ejcts.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Gao S, Wang Y, Zhao J, Su A. Effects of dexmedetomidine pretreatment on heme oxygenase-1 expression and oxidative stress during one-lung ventilation. Int J Clin Exp Pathol. 2015;8:3144–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction: Physiology and anesthetic implications. Anesthesiology. 2015;122:932–46. doi: 10.1097/ALN.0000000000000569. doi: 10.1097/ALN.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 7.Wang JY, Russell GN, Page RD, Jackson M, Pennefather SH. Comparison of the effects of sevoflurane and isoflurane on arterial oxygenation during one lung ventilation. Br J Anaesth. 1998;81:850–3. doi: 10.1093/bja/81.6.850. doi: 10.1093/bja/81.6.850. [DOI] [PubMed] [Google Scholar]
- 8.Can M, Gul S, Bektas S, Hanci V, Acikgoz S. Effects of dexmedetomidine or methylprednisolone on inflammatory responses in spinal cord injury. Acta Anaesthesiol Scand. 2009;53:1068–72. doi: 10.1111/j.1399-6576.2009.02019.x. doi: 10.1111/j.1399-6576.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 9.Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, et al. Dexmedetomidine vs. midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA. 2012;307:1151–60. doi: 10.1001/jama.2012.304. doi: 10.1001/jama. 2012.304. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao X, et al. Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats. Anesthesiology. 2012;116:1035–46. doi: 10.1097/ALN.0b013e3182503964. doi: 10.1097/ALN.0b013e3182503964. [DOI] [PubMed] [Google Scholar]
- 11.Blake DW. Dexmedetomidine and hemodynamic responses to simulated hemorrhage in experimental heart failure. Anesth Analg. 2000;91:1112–7. doi: 10.1097/00000539-200011000-00012. doi: 10.1213/00000539-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Xia R, Yin H, Xia ZY, Mao QJ, Chen GD, Xu W. Effect of intravenous infusion of dexmedetomidine combined with inhalation of isoflurane on arterial oxygenation and intrapulmonary shunt during single-lung ventilation. Cell Biochem Biophys. 2013;67:1547–50. doi: 10.1007/s12013-013-9659-8. doi: 10.1007/s12013-013-9659-8. [DOI] [PubMed] [Google Scholar]
- 13.Xue F, Chen ZL, Liu CC, Wang SD. Effects of dexmedetomidine on intrapulmonary shunt and arterial oxygenation during one-lung ventilation (in Chinese) Chin J New Drugs Clin Remedies. 2015;34:861–5. doi: 10.14109/j.cnki.xyylc.2015.11.011. [Google Scholar]
- 14.Tao GH, Liu XL, Luo W, Zhang WL, Liu WZ, Zhu YH, et al. Application of dexmedetomidine combined with parecoxib sodium in one lung ventilation in pulmonary lobectomy (in Chinese) Lab Med Clin. 2016;13:3348–51. doi: 10.3969/j.issn.1672-9455.2016.23.025. [Google Scholar]
- 15.Lai Y, Li YL, Liu YY, Peng XM, Wang H, Zou P. Dexmedetomidine improves oxygenation during one-lung ventilation in balanced anesthesia with propofol-fentanyl in adults (in Chinese) J Southern Med Univ. 2013;33:1087–90. doi: 10.3969/j.issn.1673-4254.2013.07.32. [PubMed] [Google Scholar]
- 16.Zhang QY, Zhang XQ, Meng FZ. Effect of dexmedetomidine combined with continuous positive airway pressure on lung function during one-lung ventilation in undergoing radical surgery for esophageal carcinoma (in Chinese) J Qiqihar Univ Med. 2015;36:5156–9. [Google Scholar]
- 17.Liu YT, Liu YG, Wei L. Effect of dexmedetomidine in patients with radical resection of one-lung ventilation on serum inflammatory response of esophageal cancer (in Chinese) J Clin Anesthesiol. 2016;32:1053–6. [Google Scholar]
- 18.Zhao HB. Effect of pre-injection of dexmedetomidine on inflammatory response in patients undergoing thoracotomy during one lung ventilation (in Chinese) Shanxi Med J. 2016;45:1128–30. doi: 10.3877/cma.j.issn.1674-0785.2015.10.027. [Google Scholar]
- 19.Yu HB, Li G, Yang Y, Yan L, Xu GP. Effects of dexmedetomidine on activity of nuclear factor kappa B in neutrophil granulocytes during one-lung ventilation in patients undergoing pulmonary lobectomy (in Chinese) Chin J Anesthesiol. 2014;34:1293–6. doi: 10.3760.cma.j.issn.0254-1416.2014.11.001. [Google Scholar]
- 20.Zhang LL, Zhang Y, Li Y, Weng LJ, Chen Q, Jiang LL. Effects of dexmedetomidine on intrapulmonary shunt and oxygen pressure of the artery during one-lung ventilation undergoing esophageal cancer resection (in Chinese) Acta Univ Med Anhui. 2014;49:1291–4. [Google Scholar]
- 21.Lee SH, Kim N, Lee CY, Ban MG, Oh YJ. Effects of dexmedetomidine on oxygenation and lung mechanics in patients with moderate chronic obstructive pulmonary disease undergoing lung cancer surgery: A randomised double-blinded trial. Eur J Anaesthesiol. 2016;33:275–82. doi: 10.1097/EJA.0000000000000405. doi: 10.1097/EJA.0000000000000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kernan S, Rehman S, Meyer T, Bourbeau J, Caron N, Tobias JD. Effects of dexmedetomidine on oxygenation during one-lung ventilation for thoracic surgery in adults. J Minim Access Surg. 2011;7:227–31. doi: 10.4103/0972-9941.85645. doi: 10.4103/0972-9941.85645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu XB, Wu L, Liu ZQ, Qi ZC, Wang GB, Wang C. Effects of dexmedetomidine on perioperative tip perfusion index and intrapulmonary shunt in patients during one-lung ventilation (in Chinese) Chin J Clin. 2015;9:1875–8. doi: 10.3877/cma.j.issn.1674-0785.2015.10.027. [Google Scholar]
- 24.Guo QM, Zhou CH, Chen H, Zhao YL. Effects of dexmedetomidine on plasma IL-8, IL-10 levels and lung AQP1 expression in patients undergoing one lung ventilation (in Chinese) J Clin Anesthesiol. 2016;32:245–7. doi: 10.3969/j.issn.1007-3205.2006.03.014. [Google Scholar]
- 25.Xia R, Xu JJ, Yin H, Wu HZ, Xia ZY, Zhou DW, et al. Intravenous infusion of dexmedetomidine combined isoflurane inhalation reduces oxidative stress and potentiates hypoxia pulmonary vasoconstriction during one-lung ventilation in patients. Mediators Inflamm 2015. 2015 doi: 10.1155/2015/238041. 238041. doi: 10.1155/2015/238041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kammerer T, Speck E, von Dossow V. Anesthesia in thoracic surgery. Anaesthesist. 2016;65:397–412. doi: 10.1007/s00101-016-0173-4. doi: 10.1007/s00101-016-0173-4. [DOI] [PubMed] [Google Scholar]
- 27.Blank RS, Colquhoun DA, Durieux ME, Kozower BD, McMurry TL, Bender SP, et al. Management of one-lung ventilation: Impact of tidal volume on complications after thoracic surgery. Anesthesiology. 2016;124:1286–95. doi: 10.1097/ALN.0000000000001100. doi: 10.1097/ALN.0000000000001100. [DOI] [PubMed] [Google Scholar]
- 28.Brassard CL, Lohser J, Donati F, Bussières JS. Step-by-step clinical management of one-lung ventilation: Continuing professional development. Can J Anaesth. 2014;61:1103–21. doi: 10.1007/s12630-014-0246-2. doi: 10.1007/s12630-014-0246-2. [DOI] [PubMed] [Google Scholar]
- 29.Zhang XR, DU YC, Jiang HY, Xu JY, Xu YJ. Experimental study of acute lung injury induced by different tidal volume ventilation in rats. Chin Med J. 2005;118:777–80. [PubMed] [Google Scholar]
- 30.Lai TS, Wang ZH, Cai SX. Mesenchymal stem cell attenuates neutrophil-predominant inflammation and acute lung injury in an in vivo rat model of ventilator-induced lung injury. Chin Med J. 2015;128:361–7. doi: 10.4103/0366-6999.150106. doi: 10.4103/0366-6999.150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarzkopf K, Schreiber T, Preussler NP, Gaser E, Hüter L, Bauer R, et al. Lung perfusion, shunt fraction, and oxygenation during one-lung ventilation in pigs: The effects of desflurane, isoflurane, and propofol. J Cardiothorac Vasc Anesth. 2003;17:73–5. doi: 10.1053/jcan.2003.13. doi: 10.1053/jcan.2003.13. [DOI] [PubMed] [Google Scholar]
- 32.Chow MY, Goh MH, Boey SK, Thirugnanam A, Ip-Yam PC. The effects of remifentanil and thoracic epidural on oxygenation and pulmonary shunt fraction during one-lung ventilation. J Cardiothorac Vasc Anesth. 2003;17:69–72. doi: 10.1053/jcan.2003.12. doi: 10.1053/jcan.2003.12. [DOI] [PubMed] [Google Scholar]
- 33.Naik BI, Colquhoun DA, Shields IA, E Davenport R, Durieux ME, Blank RS. Value of the oxygenation index during 1-lung ventilation for predicting respiratory complications after thoracic surgery. J Crit Care. 2017;37:80–84. doi: 10.1016/j.jcrc.2016.09.001. doi: 10.1016/j.jcrc.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Talke P, Lobo E, Brown R. Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology. 2003;99:65–70. doi: 10.1097/00000542-200307000-00014. [DOI] [PubMed] [Google Scholar]