Abstract
Objective:
Contrast-enhanced ultrasound (CEUS) is a well-established imaging modality which has been put into clinical use in recent years with the development of second-generation contrast agent and imaging devices, and its applications in the assessment of inflammatory arthritis, such as rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, have provoked abundant discussion and researches among radiologists and rheumatologists. To summarize the achievements of clinical studies on CEUS in the application of arthritis, and to keep up with the latest progresses of the imaging technique, we reviewed the literature in recent years, hoping to establish the role of CEUS in joint diseases.
Data Sources:
PubMed and EMBASE.
Study Selection:
We searched the database with the conditions “contrast-enhanced ultrasound AND arthritis” with the time limitation of recent 10 years. Clinical studies applying CEUS in inflammatory arthritis and review articles about development of CEUS in joint diseases in English were selected.
Results:
As it is proved by most studies in recent years, by delineating microvasculature within the inflamed joints, CEUS can indicate early arthritis with high sensitivity and specificity. Moreover, the imaging of CEUS has been proved to be consistent with histopathological changes of inflammatory arthritis. Quantitative analysis of CEUS permits further evaluation of disease activity. CEUS also plays a significant role in the therapeutic monitoring of the disease, which has been backed up by a number of studies.
Conclusions:
CEUS may be a new choice for the rheumatologists to evaluate inflammatory arthritis, because of its low price, ability to provide dynamic pictures, and high sensitivity to angiogenesis. It can also be applied in disease classification and therapeutic monitoring. More studies about CEUS need to be done to set up the diagnostic standards.
Keywords: Contrast-enhanced Ultrasound, Inflammatory Arthritis, Rheumatoid Arthritis, Ultrasound
INTRODUCTION
Inflammatory arthritis is caused by a series of different rheumatoid diseases and nonrheumatoid diseases. The three of most common types are rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis, of which the etiologies are still unknown to clinicians. The arthritis is marked by deformity of joints as a result of erosions of bones and cartilages, leading to disability and reduced work capacity, thus becoming a significant burden of both individuals and society.[1] Because of these severe consequences, early diagnosis of inflammatory disease seems to be crucial to optimal therapeutic management.[2] Contrast-enhanced ultrasound (CEUS), a new technology of ultrasound imaging, has been widely employed clinically nowadays, in the supplementary diagnosis of hepatic tumors, pancreatic lesions, and cardiovascular diseases, leveraging the conventional ultrasound platforms for clinical use. It can also be applied to the assessment of inflammatory arthritis, which has been reported by a series of studies.[3,4] Through showing the exact vascular patterns of the lesions, CEUS may be an excellent tool in the early diagnosis and therapeutic monitoring of inflammatory arthritis, offering a new way for the clinicians. Nevertheless, the diagnostic role of CEUS of this series of diseases is still under debate, and more clinical studies are required.
CLINICAL IMAGING TECHNIQUES OF INFLAMMATORY ARTHRITIS
Conventional radiography is the fundamental imaging method of inflammatory arthritis. With the development of modern imaging modalities, computed tomography (CT) and magnetic resonance imaging (MRI) are also used in this kind of disease, especially MRI, which can provide more detailed information about soft tissues than CT by applying a variety of scanning sequences. Moreover, the diagnostic value of ultrasound of arthritis is also verified by recent studies.
By showing some unspecific features of impaired joints, such as tissue swelling, dislocation of fat pads, narrowing of articular space, and bone destructions, conventional radiography can indicate the advanced phase of inflammatory arthritis and help assess the degree of the disease. According to the 1987 American College of Rheumatology (ACR) classification criteria of RA, conventional radiography was regarded as one of the essential techniques for the diagnosis and classification of RA.[5] Nevertheless, in early RA, it is hard to find obvious changes in the conventional radiography. For this reason, the 2016 version of ACR criteria laid stress on the important status of MRI and ultrasound in the early diagnosis of RA.[6]
MRI provides excellent imaging information about soft tissues, and it has been considered as the gold standard of lesions in synovium by clinicians.[7] Early and occult erosions can be detected by MRI, especially in small joints, such as carpal bones. By presenting multiplanar visualization, minor changes of soft tissues can be detected by MRI before clinical manifestation.[8] In a prospective study focusing on the diagnostic value of MRI in detecting early RA, a sensitivity of 96% and an accuracy of 94% were reported, which appeared to be much higher than the clinical assessment.[9] Additional information about vascularization of focal lesions can be obtained by contrast-enhanced MRI (CE-MRI).[10] This may indicate angiogenesis of early RA, showing high enhancement pattern.[11] Moreover, the use of quantitative MRI assessment of synovitis can be a valuable method in early diagnosis, as well as follow-up of RA.[12]
Ultrasound has been widely used in the evaluation of musculoskeletal diseases in recent years. In the meanwhile, its value and reliability have been corroborated by many studies and clinical practice.[13] Conventional B-mode ultrasound can be used to estimate the degree of synovial inflammation by measuring the thickness of synovial lining. Color Doppler ultrasound (CDUS) and power Doppler ultrasound (PDUS) can depict hyperemic part of the lesions, providing evidence about vascularization of the inner joints.[14] Ultrasound is also able to detect cartilage and bone erosions, as well as tenosynovitis.[15,16] In recent years, researchers have set up several semi-quantitative and quantitative scoring systems of musculoskeletal ultrasound for more accurate evaluation of inflammatory arthritis, and high associations between these systems and disease activity were confirmed.[13,17] Nevertheless, CDUS and PDUS have limited ability to detect the blood flow within small vessels. The signal intensities of small vessels are lower than noises from movements of peripheral tissues, which will be filtered out by the wall filter devices. This kind of slow blood flow is often occurred with the process of angiogenesis in the inflamed synovium, so CDUS and PDUS are not sensitive enough to diagnose inflammatory arthritis within the small joints.
METHODS OF CONTRAST-ENHANCED ULTRASOUND
Techniques of performing contrast-enhanced ultrasound
The contrast agents currently utilized in CEUS are microbubbles, made up by gas bubbles, and stabilized by a shell. These tiny microbubbles are smaller than red blood cells, which thereby can be introduced into the vascular system, and keep stable during the whole process of examination. With the stabilization from the shells, the agents are capable to achieve better backscatter enhancement and longer duration in the bloodstream. In combination of contrast-specific ultrasound modalities, intra-articular vascularization and parenchymal perfusion can be demonstrated by separating contrast agent signals from tissue-derived signals.[18] The second-generation agents which have been most commonly used are the following three ones: SonoVue®, Definity®/Luminity®, and Sonazoid®.[19] Some contrast-specific imaging techniques have been developed and applied in the CEUS imaging, the representatives of which are pulse inversion imaging and harmonic imaging. For instance, nonlinear harmonic B-mode imaging can identify the echo of contrast agents from that of solid tissues and can also provide a real-time imaging.
In most of the recent studies about inflammatory arthritis, the second generation of contrast agent, SonoVue, was commonly used. The agents were injected with the dosage of 4.8 ml[20,21,22,23] or 2.4 ml,[24] reconstituted by a 10 ml saline solution. And, a linear transducer, ranging from 4 to 12 MHz, was required for contrast imaging. The mechanical index (MI) was set at the low level of <0.15, to obviate the occurrence of bubble destructions, which can create a strong, transient echo, disturbing the normal imaging process. Relatively lower acoustic pressure was also selected for optimizing the detection of flow perfusion, set at about 45 kPa.[22] In recent studies, contrast-tuned imaging technology (CnTI) was emerged in evaluating the results of CEUS imaging. CnTI permitted the transmission of the frequencies of microbubbles and filtered out irrelevant signals caused by surrounding tissues. After bolus injection, an examination window was allowed for about 3–5 min,[20] and 2 min of examination window was also adopted by some studies.[21] For better stability, finger joints were immersed in the water during CEUS examinations in some researches.[25]
The joints selected for contrast-enhanced ultrasound
Small joints were more frequently chosen by the studies for the evaluation of inflammatory arthritis. For instance, all the small joints, metacarpophalangeal, metatarsophalangeal, proximal interphalangeal, wrist, elbow, and knee joints, were detected by Cai et al. to investigate the value of CEUS in the assessment of RA activity.[26] Some studies focused on one joint for each patient, such as wrist, knee, and elbow.[24,27] Other studies selected two kinds of joints or more, with an interval of 30 min for each examination, and also got positive results.[28]
There is also a research confirming that the vascularization can be shown in the inflamed sacroiliac joints with inflammatory arthritis by CEUS, which presents high negative predictive value of the evaluation of low back pain.[29] A recent study in 2016 paid attention to the special diagnostic value of CEUS in the patients of PsA with hip involvement. It turned out that CEUS might indicate coxitis, providing more information for disease activity and making treatment planning.[30] The studies proved that not only small joints but also large joints, such as hip joints, can be performed with CEUS for the appraisal of disease severity.
It is still hard to determine how many and which joint should be focused on for more accurate outcomes, and further validations are needed.
Quantitative analysis of contrast-enhanced ultrasound
As increased clinical application of CEUS, standardization of CEUS imaging is required for better evaluation of a variety of diseases,[31] including joint lesions. CEUS analysis can be subjective, or semi-quantitative, or quantitative. Because of the limitations of CEUS caused by personal experiences of the examiners and the different solutions of machines and imaging techniques, quantitative analysis of CEUS in the inflammatory arthritis may be of great value to achieve a more comprehensive assessment.
Recently, a commercial software, Qontrast® (Qontraxt, Amid and R&D, Bracco, Milan, Italy), was put into the use for quantitative analysis of CEUS. After an intravenous injection of the contrast microbubbles, a time-intensity curve (TIC) in the region of interest (ROI) is displayed by this software, showing an S-shaped wash-in and a nearly exponential wash-out.[32] [Figure 1].
Figure 1.
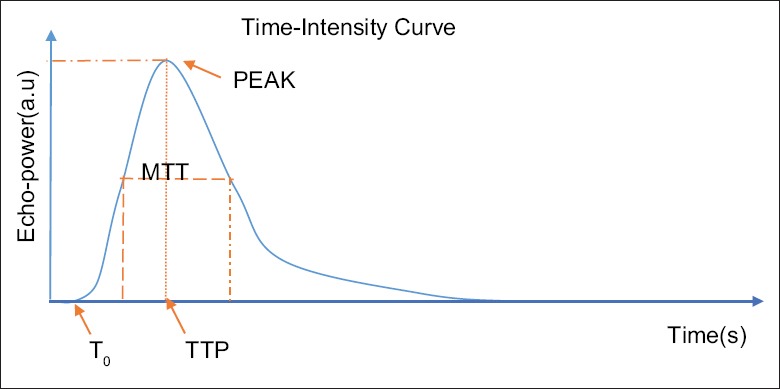
Time-intensity curve in the region of interest. T0: time zero offset; TTP: time to peak; MTT: mean transit time; PEAK: peak intensity; SI(t) = SI peak × [(t/TTP)β× TTP e-β × (t-TTP)].
The common parameters calculated by the software are listed as follows:[33] (1) time zero offset: the start of TIC curve uprising after the bolus injection, not directly relevant to perfusion kinetics, (2) time to peak: the time from the start to the maximum intensity, (3) peak intensity: maximal intensity of the ascending side, (4) area under the curve (AUC): the area under the TIC curve and is proportion to blood volume in parenchymal tissue, and (5) slope: the inclination of the curve.
Most of the studies applied Tp, MTT, and AUC of the TIC curve of ROI for the quantitative evaluation of CEUS.[22,24,34,35] Another study conducted by Klauser et al. applied SI ration to assess the procedure of the enhancement.[20]
SONOGRAPHIC FINDINGS AND HISTOPATHOLOGIC FEATURES OF CONTRAST-ENHANCED ULTRASOUND IN ARTHRITIS
Synovitis is the most essential pathological abnormality of inflammatory arthritis. It is of great significance to find out synovitis to establish the diagnosis of arthritis and undertaking early treatment.
The pathologic features of synovium in RA can be listed as follows: hyperplasia of synovial lining cells, infiltration of inflammatory cells, neoangiogenesis, and fibrin deposition.[36] These histologic characteristics are the foundations of the synovial changes of different imaging techniques, which can be seen directly. Moreover, recent studies showed that the images depicted by CEUS were highly related to the pathology of RA.
The vascular endothelium, one of the key mediators in RA, gives rise to the proliferation of capillaries from the preexistent vessels in the local synovium through several complicated pathways, leading to intra-articular vascularization. This process is called neoangiogenesis, which is a vital biochemical event causing the following pathophysiologic alterations in RA. And, the hypertrophied lining layers are nourished by these newly formed vessels.[37] The hypertrophied synovium, referred to as pannus, mediates the destruction of bones and cartilages, and causes joint instabilities and deformities, correlated with disease activity.[38] Pannus formation is the basic pathogenesis of RA and can be figured out by the detection of vascularization. In conclusion, the abnormal intra-articular vascularization and high density of capillaries are important indicators of RA.[39]
As for other types of arthritis, similar features have also been confirmed, such as vascularization, focal hyperemia, hypertrophic lining, and pannus, which are all found in the samples of PsA.[40] In PsA, researchers observed the proliferation of tortuous vessels from early phases to chronic courses.[40,41]
The contrast agent circulates through the capillary beds and persists in the blood pool, generating high-intensity signals which can be detected by the transducer, in that CEUS shows a great sensitivity to microvascularization. Based on this property of CEUS, the inflamed joints of different histologic features can be all detected by CEUS. Moreover, several studies have verified that CEUS findings were in accordance with histopathologic changes of inflammatory arthritis.
In an animal experiment in 2012, different doses of ovalbumin were injected into the knee joint of 36 rabbits, establishing an animal model of RA of different disease activities. CEUS was performed to measure the synovial thickness, and the result was compared with histological examinations. Statistical correlation between thickness measured by CEUS and pathological scores was found, which proved that CEUS may be efficient in the diagnosis of RA in the animal models.[42]
Fiocco et al. examined the refractory knee joints of 32 patients with PsA by CEUS. The patients underwent arthroscopic biopsies and immunohistochemical staining of CD45, CD31, and CD105 markers a week later, which were used as effective indexes for vessel density. To analyzing the vascular perfusions of the examined joints, some parameters were evaluated: regional blood flow, peak of the C-signal intensity, beta (β) perfusion frequency, refilling time, and slope. The result demonstrated a statistically significant relevance between refilling time and CD31+ tissues. Peak and density of CD105+ vessels are also proved to be correlated with each other. Meanwhile, the morphologic changes of arthritis, such as formation of small and disorganized vessels, inflammatory cell infiltration, and synovial hypertrophy, were detected within the sampled tissues. This research revealed that the perfusion kinetics of CEUS, such as refilling time, peak intensity, regional blood flow, and slope, are associated with focal angiogenesis. It was supposed by the authors that CEUS could be a new choice for showing the vascular pattern of PsA, and quantitative analysis could be performed for more information.[41]
CEUS also helps differentiate synovitis and joint effusions, by demonstrating enhancement patterns of intra-articular vascularization in synovitis.[43] Apart from synovitis, other specific histological features of inflammatory arthritis, such as bursal involvement, tendon lesions, and bone erosions, can also be detected by CEUS.
Synovial hyperplasia, which cannot be delineated by PDUS, may be demonstrated clearly on CEUS. CEUS has the ability to show the impaired synovium and measure the synovial thickness for further evaluation.[44]
APPLICATIONS OF QUANTITATIVE ANALYSIS OF CONTRAST-ENHANCED ULTRASOUND IN INFLAMMATORY ARTHRITIS
Almost all the studies using the software mentioned above for quantitative analysis of CEUS affirmed the role of quantitative CEUS evaluation in monitoring the disease activity and confirmed the advantages of quantitative CEUS in the assessment of inflammatory arthritis.
A study performed by Klauser et al., focusing on the comparison between subjective CEUS and computer-aided quantitative CEUS analysis, found that the quantitative analysis of CEUS was significantly correlated with both subjective CEUS and subjective PDUS (P < 0.05), concluding that computer-aided quantitative CEUS might be an important objective tool in the evaluation of RA and other similar lesions.[20] As it has been mentioned above, a study conducted by Fiocco et al. compared quantitative CEUS with histopathological and morphologic evaluation of synovial microvasculation in patients with PsA and revealed a significant correlation between refilling time and CD31+ vessel density, also the peak and CD105+ density.
A study in 2015, written by Tamas et al., performed CEUS in the radiocarpal and intercarpal joints of the patients with early RA and wrist involvement, demonstrating that the maximum parameters of TIC curves decreased significantly after 6 months’ treatment, which had high baseline in the beginning. The research concluded that these parameters of quantitative CEUS analysis could be promoted in the clinical for diagnosing EA and monitoring the therapeutic effect as follow-up.[24] Zhang et al. also put forward a similar view by comparing quantitative parameters of CEUS and clinical laboratory tests in the patients with RA.[35] A research in 2014 applied quantitative CEUS to the evaluation of enthesitis of SpA. It concluded that quantitative analysis of CEUS is superior to conventional US in the assessment of enthesitis of SpA.[34]
The common strategy of quantitative CEUS analysis is to perform at level of the ROI. The TICs and kinetic perfusion parameters are obtained by analyzing several large regions in this way. Rizzo et al. considered that this ROI-based method could be operator dependent and might miss out some spatial information of CEUS; for the distribution of blood flow, signals were not homogeneous in the chosen region. Hence, they performed the CEUS at a pixel-based level on the patients with a variety of different causes of inflammatory arthritis. The kinetic parameters were derived from analyzing the information separately pixel by pixel, taken the whole inflamed area into account. The statistical analysis proved the pixel-based method to be more accurate and comprehensive in assessing RA, and it could figure out RA from other kinds of arthritis by picturing different kinetic perfusion patterns.[21]
Fiocco et al. also utilized this new approach of pixel-based level to process the data of CEUS imaging and analyze the vascular perfusion of synovium in the patients with PsA. A linear relationship was discovered between some of the parameters of quantitative CEUS and the frequencies of CD161+, interleukin (IL)-17+, IL-23+, and CD4+ Th17 cells. The research further illustrated that the quantitative CEUS of pixel-based level can be helpful in the management of synovitis.[45]
COMPARISONS WITH OTHER IMAGING TECHNIQUES
By delineating specific enhancement patterns of microvascularization, CEUS can get better accuracy in the detection of vascularity than nonenhanced US, and several studies in the past 10 years have proved its superiority.[26,43]
It has been confirmed that CEUS can detect the intra-articular vascularization of the joints affected by RA, and the diagnostic values are relatively high, compared to PDUS and conventional ultrasound. In a retrospective study, there were 22 patients with RA undergoing CEUS with low MI, along with conventional B-mode ultrasound. And, CEUS did better than B-mode US, in differentiating synovitis and joint effusions, by demonstrating enhancement pattern of intra-articular vascularization in synovitis.[43] Another research conducted by a group of German scientists, performed CEUS, PDUS, and conventional US in the patients of RA, and evaluated the efficacy of these methods, using MRI results as the standard. A higher correlation rate of CEUS with MRI scoring was demonstrated in this study, which indicated a superior sensitivity of CEUS in detecting synovitis.[46]
There are also researches indicating that CEUS can do better in diagnosing RA-affected joints and evaluating inflammatory activity than PDUS. A multicenter study figured out that the measurement of synovial thickness could be more accurate by means of CEUS, compared with PDUS, thus assisting the detection of vascularized synovial proliferation.[23]
Song et al. compared different imaging methods in assessing synovitis activity in the patients with knee osteoarthritis, including conventional B-mode ultrasound, PDUS, CEUS, and CE-MRI. Positive findings were found in 95% of CEUS, compared to 82% of MRI and 63% in PDUS, verifying that CEUS might have a higher sensitivity than both PDUS and CE-MRI in detecting synovitis activity.[47]
Nevertheless, some studies did not demonstrate the advantages of applying CEUS in the assessment of inflammatory arthritis. And, in the present study, the correlation between vascularization and disease progress has not been confirmed. The further application of CEUS in the role of managing inflammatory arthritis requires more researches.
CLINICAL APPLICATIONS OF CONTRAST-ENHANCED ULTRASOUND IN INFLAMMATORY ARTHRITIS
Early arthritis
Several studies have suggested that histological involvement of joints can be found in the early stage of RA, and the tissue changes evolve with the disease activity.[48,49] A study compared the samples of synovial tissues of newly diagnosed patients with RA (< 1 year), with the samples from patients with RA of more than 5 years’ duration. And synovial lining hyperplasia and synovial inflammation were evident in both group.[48] Another research performed biopsy samplings on the unaffected knees of 20 patients with RA, who had arthritis elsewhere, with no symptoms of arthritis. Histological changes were observed in all samples.[49] It has been approved broadly that there are minor synovial changes in early RA indicating disease activity before clinical signs, and it is of great significance to detect these changes by a more convenient means.
Hyperemia of local synovial tissues is found in the early arthritis, as well as microvascular changes.[50] Angiogenesis is reported to be the earliest sign of RA and other inflammatory rheumatoid diseases. These are the features that can be shown clearly by CEUS. A study recruited 11 patients diagnosed with early RA. Synovial hypertrophy was found by gray-scale ultrasound in these patients. The wrist joints were evaluated by CEUS, and TIC was then analyzed. High baseline values of AUC and peak were detected in the focal lesions.[24]
Nevertheless, the most difficult problem is that there is no standard enhancement pattern of joints in CEUS to define the abnormal changes, because of the lack of relevant researches and data. More experiments or retrospective researches are needed for establishing the threshold between normal pattern and lesioned ones.
Disease activity
By showing the angiogenesis of the lesioned joints, inflammation in the joints can be detected by CEUS sensitively, which has been revealed by several studies about the topic.
An early study in 2002 performed contrast-enhanced Doppler ultrasound and unenhanced Doppler ultrasound in 198 finger joints of 46 patients with RA and 80 healthy joints of 10 volunteers. It turned out that enhancement pattern during arterial phase was shown in 49% of inactive RA, 98% of moderately active RA, and 100% of active RA. The author concluded that the CEUS improved the detection rate of intra-articular vascularization and might be useful in differentiating active RA with inactive RA (P < 0.001).[51]
The examinations of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are two important clinical laboratory tests in the assessment of inflammatory arthritis, which are correlated with disease activity. A study in 2015 performed quantitative CEUS in the small joints of 39 patients with RA, and compared the kinetic parameters with the level of CRP and ESR. The result confirmed the statistical association of the CEUS parameters, AUC and PI, with biochemical tests.[26]
The concentrations of vascular endothelial growth factor (VEGF) will rise up during the inflammatory process of RA within the affected joints.[52] The correlation of VEGF and disease activity has also been emphasized by early studies. The tissues with angiogenesis also express VEGF, and the level of VEGF is related to microvascular density.[53] Thus, the molecular imaging of VEGF or other markers may help assess the disease activity with higher accuracy and sensitivity. Therefore, VEGF-targeted microbubbles may permit depiction of the degree of vascular formation, thus assessing the disease activity.
Therapeutic monitoring
There are several studies monitoring therapeutic efficacy after a specific therapy of inflammatory arthritis by CEUS, applying quantitative or semi-quantitative analysis system, and the results showed that CEUS might be a sensitive method to monitor the disease progress and therapeutic efficacy of inflammatory arthritis.[24,27,54,55] [Table 1].
Table 1.
The researches focusing on the role of CEUS in the therapeutic monitoring of inflammatory arthritis
| Author | Disease | Therapy | Analysis method | Numbers of patients | Results |
|---|---|---|---|---|---|
| Tamas et al. | RA | Conventional synthetic drugs | Time-intensity curves parameters | 11 | Peak and AUC decreased following the treatment |
| Cozzi | PsA | Mud-bath therapy, TNF inhibitors | The count of swollen (ACR 66), tender (ACR 68), and AJC | 36 | A significant appearance delay and faster washout was observed in the therapeutic group |
| Bonifati et al. | PsA | Anti-TNF drugs | The count of swollen (ACR 66), tender (ACR 68), and AJC | 25 | A significant reduction of all clinical variables including CEUS |
| Song et al. | SpA and RA | Intra-articular glucocorticosteroids | The slope values by time-intensity analysis | 2 | A remarkable improvement of clinical and CEUS parameters in patient 1, and elevated parameters in patient 2 |
| Salaffi et al. | RA | Intra-articular injection of triamcinolone hexacetonide | Median values of the area underlying time-intensity curves | 18 | Synovitis activity was highly associated with changes of the value of the area underlying time-intensity curves. The values are also correlated to CRP |
| Ohrndorf et al. | RA | TNF-α block therapy | Enhancement, slope and semi-quantitative assessment | 15 | CEUS showed the best sensitivity in detecting the changes after the therapy among all the imaging techniques applied in the research |
| Maria-Magdalena Tămaş et al. | Early arthritis | Conventional synthetic drugs | Peak, slope, AUC of TIC | 11 | Peak and AUC significantly decreased during the treatment with the remission of the symptoms |
RA: Rheumatoid arthritis; TNF: Tumor necrosis factor; ACR: American College of Rheumatology; AJC: Active inflamed joints; AUC: Area under the curve; TIC: Time-intensity curve; CEUS: Contrast-enhanced ultrasound; CRP: C-reactive protein; PsA: Psoriatic arthritis.
Some new therapies such as biological attack can cause angiogenesis, by inducing immune reaction and inflammatory factors cascade. CEUS may play a vital part in monitoring the progress of these new therapeutic strategies, for its specificity in detecting the microvascularization.
LIMITATIONS
One of the most major limitations of CEUS in the assessment of inflammatory arthritis is that there is always only one joint can be once examined, and the examined joints have to be determined by grey-scale ultrasound and PDUS. Some studies perform CEUS for further evaluation after GS and PDUS find out the lesioned joint. If more joints need to be inspected, it will take relatively more time for performing CEUS in all those locations.
Another important limitation is the deficiency of standard of normal articular perfusion patterns of CEUS. The threshold of normal and inflammatory joints and the degree of inflammatory devastation are not well established. More researches on CEUS and arthritis should be carried out to verify the specificity and efficacy of CEUS and standardize its analysis system.[44]
ADVANTAGES
Ultrasound is remarkable for its low price, compared with CT and MRI. Although the price of CEUS is higher than conventional GS and CDUS, it is still more economical than MRI. Real-time imaging and availability of bedside application are also main characteristics of ultrasound, as with CEUS.
Moreover, the contrast agents applied in CEUS are confined to vascular space, and will not leak into the synovial fluid, or be rapidly cleared out from vessels. And, there is no evidence showing side effects of the contrast agents of CEUS, compared with the contrast agents of CT and MRI, which are proved to have impacts on liver and renal function. Therefore, CEUS is supposed to be an ideal tool for vascular imaging, especially within small circulations.
NEW IMAGING MODALITIES FOR INFLAMMATORY ARTHRITIS
Apart from CEUS, there are some new imaging technologies combining optical and ultrasonic imaging, such as diffuse optical imaging (DOI) and photoacoustic imaging (PAI). These methods provide us with new ways to assess the inflammatory arthritis through detecting vascularization of the tissues.
Diffuse optical imaging
DOI is an important optical imaging modality and has a wide use in brain functioning imaging and breast tumor imaging. Studies have found its potential significance in the diagnosis of articular diseases.[56,57] Several studies have verified that DOI can show the detailed structures in the joints, such as synovium, bones, vessels, and tendons, all can be identified in the DOI. By discriminating oxygenated hemoglobin and deoxygenated hemoglobin, DOI can provide information about focal vascularization and metabolic functions of the anatomic structures.[58] This feature of DOI suggests that it may have an advantage in detecting arthritis.
Photoacoustic imaging
PAI, a sophisticated imaging modality, has been rapidly developing over the recent years.[59] With the detection of hemoglobin, tiny vasculature can be delineated, and hypoxia areas can also be found by PAI.[59,60] On account of this property of PAI, it is able to differentiate inflammatory arthritis and normal joints and may have further application in disease activity scoring and therapeutic monitoring. And, there are a number of animal experiments, as well as clinical studies confirming the assumption.[61] In the recent years, some studies carried out PAI in the finger joints of patients with arthritis and made comparisons with healthy people. The results were consistent with the animal experiments, and PAI was proved to be a very promising tool for the assessing and monitoring of inflammatory arthritis.[62,63,64]
Superb microvascular imaging
Superb microvascular imaging (SMI) is a brand-new imaging technique based on gray-scale and CDUS imaging, which has been a research hotspot in these 2 years. The major characteristics of SMI are the capacity of depicting microvasculatures marked with very low velocities, which cannot be detected by CDFI, or may be confused with artifacts arising from tissue motions.[65] Therefore, the diseases closely related to angiogenesis can get diagnosed by SMI in an early phase. The applications of SMI in breast, thyroid, and hepatic malignancies have been presented by recent researches.[65,66,67,68] Although the uses of SMI in rheumatoid diseases have not been involved in published researches, it can be supposed that SMI may show high sensitivity in the assessment of inflammatory arthritis.
AngioPLUS
AngioPLUS is also an innovative CDUS technique focusing on visualizing slow vessel flows. Malignant lesions and inflamed joints with microvascularization may be indicated via an advanced imaging algorithm of AngioPLUS, without the need for injecting contrast agent. AngioPLUS may also be a superior substitute for CEUS and worth clinical promotion.
CONCLUSIONS
There are a series of imaging modalities for the assessment and classification of inflammatory disease, including conventional radiography, CT, MRI, and ultrasound. All these techniques are proved to play an essential role in the overall management of the disease. In the last decade, the highly developed CEUS has been applied in the imaging assessment of arthritis by some researchers. And, the results showed the special value of CEUS in the early diagnosis of arthritis, grading the disease activity, and therapeutic monitoring. The principle of CEUS imaging in inflammatory arthritis is based on the demonstration of vascularization of the focal tissues. In addition, quantitative analysis of CEUS may further the diagnostic value. However, there are still limitations of CEUS for clinical application in terms of lacking of clinical standard and so on. Therefore, more researches need to be done on it. In parallel, some fast-developing imaging techniques, such as DOI, PAI, SMI, and AngioPLUS, may also provide new ways for this topic.
Financial support and sponsorship
This study was supported by grants from the International S and T Cooperation Program of China (No. 2015DFA30440), the Natural Science Foundation of China (No. 81301268), and Beijing Nova Plan (No. Z131107000413063).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: Estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1316–22. doi: 10.1136/annrheumdis-2013-204627. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–38. doi: 10.1016/S0140-6736(16)30173-8. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 3.De Zordo T, Mlekusch SP, Feuchtner GM, Mur E, Schirmer M, Klauser AS. Value of contrast-enhanced ultrasound in rheumatoid arthritis. Eur J Radiol. 2007;64:222–30. doi: 10.1016/j.ejrad.2007.07.011. doi: 10.1016/j.ejrad.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Ignee A, Atkinson NS, Schuessler G, Dietrich CF. Ultrasound contrast agents. Endosc Ultrasound. 2016;5:355–62. doi: 10.4103/2303-9027.193594. doi: 10.4103/2303- 9027.193594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal R, Rider LG, Ruperto N, Bayat N, Erman B, Feldman BM, et al. 2016 American College of Rheumatology/European League Against Rheumatism criteria for minimal, moderate, and major clinical response in adult dermatomyositis and polymyositis: An International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis. 2017;76:792–801. doi: 10.1136/annrheumdis-2017-211400. doi: 10.1136/annrheumdis-2017-211400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McQueen FM. Magnetic resonance imaging in early inflammatory arthritis: What is its role? Rheumatology (Oxford) 2000;39:700–6. doi: 10.1093/rheumatology/39.7.700. doi: 10.1093/rheumatology/39.7.700. [DOI] [PubMed] [Google Scholar]
- 8.Corvetta A, Giovagnoni A, Baldelli S, Ercolani P, Pomponio G, Luchetti MM, et al. MR imaging of rheumatoid hand lesions: Comparison with conventional radiology in 31 patients. Clin Exp Rheumatol. 1992;10:217–22. [PubMed] [Google Scholar]
- 9.Sugimoto H, Takeda A, Masuyama J, Furuse M. Early-stage rheumatoid arthritis: Diagnostic accuracy of MR imaging. Radiology. 1996;198:185–92. doi: 10.1148/radiology.198.1.8539375. doi: 10.1148/radiology.198.1.8539375. [DOI] [PubMed] [Google Scholar]
- 10.Wang XY, Yan F, Hao H, Wu JX, Chen QH, Xian JF. Improved performance in differentiating benign from malignant sinonasal tumors using diffusion-weighted combined with dynamic contrast-enhanced magnetic resonance imaging. Chin Med J. 2015;128:586–92. doi: 10.4103/0366-6999.151649. doi: 10.4103/0366-6999.151649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiser MF, Naegele M. Inflammatory joint disease: Static and dynamic gadolinium-enhanced MR imaging. J Magn Reson Imaging. 1993;3:307–10. doi: 10.1002/jmri.1880030142. doi: 10.1002/jmri.1880030142. [DOI] [PubMed] [Google Scholar]
- 12.Tehranzadeh J, Ashikyan O, Dascalos J. Magnetic resonance imaging in early detection of rheumatoid arthritis. Semin Musculoskelet Radiol. 2003;7:79–94. doi: 10.1055/s-2003-41342. doi: 10.1055/s-2003-41342. [DOI] [PubMed] [Google Scholar]
- 13.Hammer HB, Terslev L. Role of ultrasound in managing rheumatoid arthritis. Curr Rheumatol Rep. 2012;14:438–44. doi: 10.1007/s11926-012-0266-2. doi: 10.1007/s11926-012-0266-2. [DOI] [PubMed] [Google Scholar]
- 14.Boesen MI, Boesen M, Langberg H, Koenig MJ, Boesen A, Bliddal H, et al. Musculoskeletal colour/power Doppler in sports medicine: Image parameters, artefacts, image interpretation and therapy. Clin Exp Rheumatol. 2010;28:103–13. [PubMed] [Google Scholar]
- 15.Lillegraven S, Bøyesen P, Hammer HB, Østergaard M, Uhlig T, Sesseng S, et al. Tenosynovitis of the extensor carpi ulnaris tendon predicts erosive progression in early rheumatoid arthritis. Ann Rheum Dis. 2011;70:2049–50. doi: 10.1136/ard.2011.151316. doi: 10.1136/ard.2011.151316. [DOI] [PubMed] [Google Scholar]
- 16.Wakefield RJ, Gibbon WW, Conaghan PG, O’Connor P, McGonagle D, Pease C, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: A comparison with conventional radiography. Arthritis Rheum. 2000;43:2762–70. doi: 10.1002/1529-0131(200012)43:12<2762::AID-ANR16>3.0.CO;2-#. doi: 10.1002/1529-0131(200012)43:12<2762::AID-ANR16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Terslev L, Ellegaard K, Christensen R, Szkudlarek M, Schmidt WA, Jensen PS, et al. Head-to-head comparison of quantitative and semi-quantitative ultrasound scoring systems for rheumatoid arthritis: Reliability, agreement and construct validity. Rheumatology (Oxford) 2012;51:2034–8. doi: 10.1093/rheumatology/kes124. doi: 10.1093/rheumatology/kes124. [DOI] [PubMed] [Google Scholar]
- 18.Greis C. Technology overview: SonoVue (Bracco, Milan) Eur Radiol. 2004;14(Suppl 8):P11–5. doi: 10.1007/s10406-004-0076-3. [PubMed] [Google Scholar]
- 19.Dietrich CF. Comments and illustrations regarding the guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS) – Update 2008. Ultraschall Med. 2008;29(Suppl 4):S188–202. doi: 10.1055/s-2008-1027799. doi: 10.1055/s-2008-1027799. [DOI] [PubMed] [Google Scholar]
- 20.Klauser AS, Franz M, Bellmann Weiler R, Gruber J, Hartig F, Mur E, et al. Contrast-enhanced ultrasonography for the detection of joint vascularity in arthritis – Subjective grading versus computer-aided objective quantification. Ultraschall Med. 2011;32(Suppl 2):E31–7. doi: 10.1055/s-0031-1281671. doi: 10.1055/s-0031-1281671. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo G, Raffeiner B, Coran A, Ciprian L, Fiocco U, Botsios C, et al. Pixel-based approach to assess contrast-enhanced ultrasound kinetics parameters for differential diagnosis of rheumatoid arthritis. J Med Imaging (Bellingham) 2015;2:034503. doi: 10.1117/1.JMI.2.3.034503. doi: 10.1117/1.JMI.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiocco U, Stramare R, Coran A, Grisan E, Scagliori E, Caso F, et al. Vascular perfusion kinetics by contrast-enhanced ultrasound are related to synovial microvascularity in the joints of psoriatic arthritis. Clin Rheumatol. 2015;34:1903–12. doi: 10.1007/s10067-015-2894-1. doi: 10.1007/s10067-015-2894-1. [DOI] [PubMed] [Google Scholar]
- 23.Klauser A, Demharter J, De Marchi A, Sureda D, Barile A, Masciocchi C, et al. Contrast enhanced gray-scale sonography in assessment of joint vascularity in rheumatoid arthritis: Results from the IACUS study group. Eur Radiol. 2005;15:2404–10. doi: 10.1007/s00330-005-2884-9. doi: 10.1007/s00330-005-2884-9. [DOI] [PubMed] [Google Scholar]
- 24.Tamas MM, Bondor CI, Rednic N, Ghib LJ, Rednic S. The evolution of time-intensity curves of contrast enhanced ultrasonography in early arthritis patients with wrist involvement. Med Ultrason. 2015;17:345–51. doi: 10.11152/mu.2013.2066.173.mmt. doi: 10.11152/mu.2013.2066.173.mmt. [DOI] [PubMed] [Google Scholar]
- 25.Stramare R, Raffeiner B, Ciprian L, Scagliori E, Coran A, Perissinotto E, et al. Evaluation of finger joint synovial vascularity in patients with rheumatoid arthritis using contrast-enhanced ultrasound with water immersion and a stabilized probe. J Clin Ultrasound. 2012;40:147–54. doi: 10.1002/jcu.21887. doi: 10.1002/jcu.21887. [DOI] [PubMed] [Google Scholar]
- 26.Cai XH, Yang SP, Shen HL, Lin LQ, Zhong R, Wu RM, et al. Application of contrast-enhanced ultrasonography and ultrasonography scores in rheumatoid arthritis. Int J Clin Exp Med. 2015;8:20056–64. [PMC free article] [PubMed] [Google Scholar]
- 27.Cozzi F, Raffeiner B, Beltrame V, Ciprian L, Coran A, Botsios C, et al. Effects of mud-bath therapy in psoriatic arthritis patients treated with TNF inhibitors. Clinical evaluation and assessment of synovial inflammation by contrast-enhanced ultrasound (CEUS) Joint Bone Spine. 2015;82:104–8. doi: 10.1016/j.jbspin.2014.11.002. doi: 10.1016/j.jbspin.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi D, Tokunaga D, Oda R, Fujiwara H, Ikeda T, Ikoma K, et al. Maximum intensity projection with magnetic resonance imaging for evaluating synovitis of the hand in rheumatoid arthritis: Comparison with clinical and ultrasound findings. Clin Rheumatol. 2014;33:911–7. doi: 10.1007/s10067-014-2526-1. doi: 10.1007/s10067-014-2526-1. [DOI] [PubMed] [Google Scholar]
- 29.Klauser A, Halpern EJ, Frauscher F, Gvozdic D, Duftner C, Springer P, et al. Inflammatory low back pain: High negative predictive value of contrast-enhanced color Doppler ultrasound in the detection of inflamed sacroiliac joints. Arthritis Rheum. 2005;53:440–4. doi: 10.1002/art.21161. doi: 10.1002/art.21161. [DOI] [PubMed] [Google Scholar]
- 30.Löffler C, Sattler H, Uppenkamp M, Bergner R. Contrast-enhanced ultrasound in coxitis. Joint Bone Spine. 2016;83:669–674. doi: 10.1016/j.jbspin.2015.10.012. doi: 10.1016/j.jbspin.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Yang WL, Wei WB, Li DJ. Quantitative parameter character of choroidal melanoma in contrast-enhanced ultrasound. Chin Med J. 2012;125:4440–4. doi: 10.3760/cma.j.issn.0366- 6999.2012.24.021. [PubMed] [Google Scholar]
- 32.Greis C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS) Clin Hemorheol Microcirc. 2011;49:137–49. doi: 10.3233/CH-2011-1464. doi: 10.3233/CH-2011-1464. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich CF, Averkiou MA, Correas JM, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012;33:344–51. doi: 10.1055/s-0032-1313026. doi: 10.1055/s-0032-1313026. [DOI] [PubMed] [Google Scholar]
- 34.Mouterde G, Aegerter P, Correas JM, Breban M, D’Agostino MA. Value of contrast-enhanced ultrasonography for the detection and quantification of enthesitis vascularization in patients with spondyloarthritis. Arthritis Care Res (Hoboken) 2014;66:131–8. doi: 10.1002/acr.22195. doi: 10.1002/acr.22195. [DOI] [PubMed] [Google Scholar]
- 35.Zhang LY, Xiang X, Tang YJ, Leng QY, Qiu L. Correlation between quantitative results of contrast-enhanced ultrasonography and clinical and laboratory indexes for synovium of patients with rheumatoid arthritis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45:1001–4. [PubMed] [Google Scholar]
- 36.Pitzalis C, Kelly S, Humby F. New learnings on the pathophysiology of RA from synovial biopsies. Curr Opin Rheumatol. 2013;25:334–44. doi: 10.1097/BOR.0b013e32835fd8eb. doi: 10.1097/BOR.0b013e32835fd8eb. [DOI] [PubMed] [Google Scholar]
- 37.Weber AJ, De Bandt M. Angiogenesis: General mechanisms and implications for rheumatoid arthritis. Joint Bone Spine. 2000;67:366–83. [PubMed] [Google Scholar]
- 38.Ishikawa H, Hirata S, Andoh Y, Kubo H, Nakagawa N, Nishibayashi Y, et al. An immunohistochemical and immunoelectron microscopic study of adhesion molecules in synovial pannus formation in rheumatoid arthritis. Rheumatol Int. 1996;16:53–60. doi: 10.1007/BF01816436. doi: 10.1007/BF01816436. [DOI] [PubMed] [Google Scholar]
- 39.Knight AD, Levick JR. The density and distribution of capillaries around a synovial cavity. Q J Exp Physiol. 1983;68:629–44. doi: 10.1113/expphysiol.1983.sp002753. doi: 10.1113/expphysiol.1983.sp002753. [DOI] [PubMed] [Google Scholar]
- 40.Reece RJ, Canete JD, Parsons WJ, Emery P, Veale DJ. Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum. 1999;42:1481–4. doi: 10.1002/1529-0131(199907)42:7<1481::AID-ANR23>3.0.CO;2-E. doi: 10.1002/1529-0131(199907)42:7<1481::AID-ANR23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 41.Fiocco U, Cozzi L, Chieco-Bianchi F, Rigon C, Vezzù M, Favero E, et al. Vascular changes in psoriatic knee joint synovitis. J Rheumatol. 2001;28:2480–6. [PubMed] [Google Scholar]
- 42.Qiu L, Jiang Y, Luo Y, Zhang L, Xu H. Antigen-induced arthritis in rabbits: A comparative study between high-resolution ultrasound and contrast-enhanced ultrasound and pathologic findings. Rheumatol Int. 2012;32:1569–80. doi: 10.1007/s00296-011-1817-y. doi: 10.1007/s00296-011-1817-y. [DOI] [PubMed] [Google Scholar]
- 43.Kleffel T, Demharter J, Wohlgemuth W, Schalm J, Bohndorf K, Kirchhof K. Comparison of contrast-enhanced low mechanical index (Low MI) sonography and unenhanced B-mode sonography for the differentiation between synovitis and joint effusion in patients with rheumatoid arthritis. Rofo. 2005;177:835–41. doi: 10.1055/s-2005-858194. doi: 10.1055/s-2005-858194. [DOI] [PubMed] [Google Scholar]
- 44.Rednic N, Tamas MM, Rednic S. Contrast-enhanced ultrasonography in inflammatory arthritis. Med Ultrason. 2011;13:220–7. [PubMed] [Google Scholar]
- 45.Fiocco U, Stramare R, Martini V, Coran A, Caso F, Costa L, et al. Quantitative imaging by pixel-based contrast-enhanced ultrasound reveals a linear relationship between synovial vascular perfusion and the recruitment of pathogenic IL-17A-F IL-23+ CD161+ CD4+ T helper cells in psoriatic arthritis joints. Clin Rheumatol. 2017;36:391–9. doi: 10.1007/s10067-016-3500-x. doi: 10.1007/s10067-016-3500-x. [DOI] [PubMed] [Google Scholar]
- 46.Ohrndorf S, Hensch A, Naumann L, Hermann KG, Scheurig-Münkler C, Meier S, et al. Contrast-enhanced ultrasonography is more sensitive than grayscale and power Doppler ultrasonography compared to MRI in therapy monitoring of rheumatoid arthritis patients. Ultraschall Med. 2011;32(Suppl 2):E38–44. doi: 10.1055/s-0031-1281770. doi: 10.1055/s-0031-1281770. [DOI] [PubMed] [Google Scholar]
- 47.Song IH, Althoff CE, Hermann KG, Scheel AK, Knetsch T, Schoenharting M, et al. Knee osteoarthritis. Efficacy of a new method of contrast-enhanced musculoskeletal ultrasonography in detection of synovitis in patients with knee osteoarthritis in comparison with magnetic resonance imaging. Ann Rheum Dis. 2008;67:19–25. doi: 10.1136/ard.2006.067462. doi: 10.1136/ard.2006.067462. [DOI] [PubMed] [Google Scholar]
- 48.de Hair MJ, van de Sande MG, Ramwadhdoebe TH, Hansson M, Landewé R, van der Leij C, et al. Features of the synovium of individuals at risk of developing rheumatoid arthritis: Implications for understanding preclinical rheumatoid arthritis. Arthritis Rheumatol. 2014;66:513–22. doi: 10.1002/art.38273. doi: 10.1002/art.38273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pando JA, Duray P, Yarboro C, Gourley MF, Klippel JH, Schumacher HR. Synovitis occurs in some clinically normal and asymptomatic joints in patients with early arthritis. J Rheumatol. 2000;27:1848–54. [PubMed] [Google Scholar]
- 50.Schumacher HR, Jr, Bautista BB, Krauser RE, Mathur AK, Gall EP. Histological appearance of the synovium in early rheumatoid arthritis. Semin Arthritis Rheum. 1994;23(6 Suppl 2):3–10. doi: 10.1016/0049-0172(94)90079-5. doi: 10.1016/0049- 0172(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 51.Klauser A, Frauscher F, Schirmer M, Halpern E, Pallwein L, Herold M, et al. The value of contrast-enhanced color Doppler ultrasound in the detection of vascularization of finger joints in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:647–53. doi: 10.1002/art.10136. doi: 10.1002/art.10136. [DOI] [PubMed] [Google Scholar]
- 52.Lee YH, Bae SC. Correlation between circulating VEGF levels and disease activity in rheumatoid arthritis: A meta-analysis. Z Rheumatol. 2016 doi: 10.1007/s00393-016-0229-5. Epub ahead of print] doi: 10.1007/s00393-016-0229-5. [DOI] [PubMed] [Google Scholar]
- 53.Malemud CJ. Growth hormone, VEGF and FGF: Involvement in rheumatoid arthritis. Clin Chim Acta. 2007;375:10–9. doi: 10.1016/j.cca.2006.06.033. doi: 10.1016/j.cca.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 54.Bonifati C, Elia F, Graceffa D, Ceralli F, Maiani E, De Mutiis C, et al. Clinical and contrast-enhanced ultrasound echography outcomes in psoriatic arthritis patients after one year of continuous therapy with anti-TNF drugs. ISRN Dermatol 2014. 2014 doi: 10.1155/2014/932721. 932721. doi: 10.1155/2014/932721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song IH, Rediner U, Hermann KG, Burmester GR, Backhaus M. Contrast-enhanced ultrasound for the monitoring of the efficacy of intra-articular glucocorticosteroids in 2 patients with inflammatory knee joint synovitis. Ultraschall Med. 2012;33:E352–4. doi: 10.1055/s-0032-1312949. doi: 10.1055/s-0032-1312949. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Wang JZ, Yuan Z, Sobel ES, Jiang H. Computer-aided classification of optical images for diagnosis of osteoarthritis in the finger joints. J Xray Sci Technol. 2011;19:531–44. doi: 10.3233/XST-2011-0312. doi: 10.3233/XST-2011-0312. [DOI] [PubMed] [Google Scholar]
- 57.Yuan Z, Zhang Q, Sobel ES, Jiang H. Tomographic x-ray-guided three-dimensional diffuse optical tomography of osteoarthritis in the finger joints. J Biomed Opt. 2008;13:044006. doi: 10.1117/1.2965547. doi: 10.1117/1.2965547. [DOI] [PubMed] [Google Scholar]
- 58.Yuan Z, Zhang Q, Sobel ES, Jiang H. High-resolution x-ray guided three-dimensional diffuse optical tomography of joint tissues in hand osteoarthritis: Morphological and functional assessments. Med Phys. 2010;37:4343–54. doi: 10.1118/1.3467755. doi: 10.1118/1.3467755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Xie X, Ku G, Wang LV, Stoica G. Noninvasive imaging of hemoglobin concentration and oxygenation in the rat brain using high-resolution photoacoustic tomography. J Biomed Opt. 2006;11:024015. doi: 10.1117/1.2192804. doi: 10.1117/1.2192804. [DOI] [PubMed] [Google Scholar]
- 60.Li C, Wang LV. Photoacoustic tomography and sensing in biomedicine. Phys Med Biol. 2009;7;54:R59–97. doi: 10.1088/0031-9155/54/19/R01. doi: 10.1088/0031-9155/54/19/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chamberland D, Jiang Y, Wang X. Optical imaging: New tools for arthritis. Integr Biol (Camb) 2010;2:496–509. doi: 10.1039/b926506f. doi: 10.1039/b926506f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Wang Y, Yuan Z. Dual-modality imaging of the human finger joint systems by using combined multispectral photoacoustic computed tomography and ultrasound computed tomography. Biomed Res Int 2016. 2016 doi: 10.1155/2016/1453272. 1453272. doi: 10.1155/2016/1453272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xi L, Jiang H. Integrated photoacoustic and diffuse optical tomography system for imaging of human finger joints in vivo. J Biophotonics. 2016;9:213–7. doi: 10.1002/jbio.201500197. doi: 10.1002/jbio.201500197. [DOI] [PubMed] [Google Scholar]
- 64.Xu G, Rajian JR, Girish G, Kaplan MJ, Fowlkes JB, Carson PL, et al. Photoacoustic and ultrasound dual-modality imaging of human peripheral joints. J Biomed Opt. 2013;18:10502. doi: 10.1117/1.JBO.18.1.010502. doi: 10.1117/1.JBO.18.1.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee DH, Lee JY, Han JK. Superb microvascular imaging technology for ultrasound examinations: Initial experiences for hepatic tumors. Eur J Radiol. 2016;85:2090–5. doi: 10.1016/j.ejrad.2016.09.026. doi: 10.1016/j.ejrad.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 66.Xiao XY, Chen X, Guan XF, Wu H, Qin W, Luo BM. Superb microvascular imaging in diagnosis of breast lesions: A comparative study with contrast-enhanced ultrasonographic microvascular imaging. Br J Radiol. 2016;89:20160546. doi: 10.1259/bjr.20160546. doi: 10.1259/bjr.20160546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Machado P, Segal S, Lyshchik A, Forsberg F. A novel microvascular flow technique: Initial results in thyroids. Ultrasound Q. 2016;32:67–74. doi: 10.1097/RUQ.0000000000000156. doi: 10.1097/RUQ.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 68.Ma Y, Li G, Li J, Ren WD. The diagnostic value of Superb Microvascular Imaging (SMI) in detecting blood flow signals of breast lesions: A preliminary study comparing SMI to color Doppler flow imaging. Medicine (Baltimore) 2015;94:e1502. doi: 10.1097/MD.0000000000001502. doi: 10.1097/MD.0000000000001502. [DOI] [PMC free article] [PubMed] [Google Scholar]


