Abstract
Despite advances in drug technology and improvements in technology, including peripheral nerve catheters and ultrasound, postoperative pain is still a significant problem in the clinical setting worldwide. Postoperative pain can have a critical negative impact with regard to physiological consequences to the body and therefore, the role of liposomal bupivacaine as an extended release bupivacaine with approximately 72 h of duration may have far-reaching and significant impact in clinical practice. Liposomal bupivacaine has a DepoFoam multivesicular liposome technology with particle suspension in an isotonic aqueous solution and consists of tiny lipid-based particles, which contain discrete water-filled chambers dispersed through a lipid matrix. Other advantages include a reduction in opioid consumption, while not requiring a catheter or any other device, as well as easy dilution with saline. This review summarizes current research with this novel agent in postsurgical pain, and discusses potential roles in chronic pain states. Further studies are warranted for its use in epidural and intrathecal administration. Moreover, this review will explore the expansion of liposomal bupivacaine's current clinical role.
Keywords: Analgesia, Exparel, liposomal bupivacaine, nerve blocks, postoperative pain
Introduction
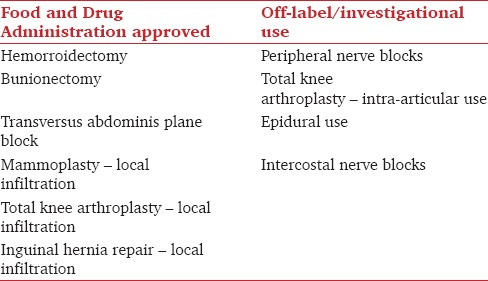
Inadequate postoperative pain control is associated with decreased patient satisfaction and delayed hospital discharge, which results in increased health-care costs and patient distress. With an increasing number of procedures being performed in the United States, inadequate pain control has the potential to have an ever significant impact on health-care costs.[1,2] Liposomal bupivacaine (Exparel (®)) was originally approved by the US Food and Drug Administration (FDA) in October 2011 for use as a local anesthetic by wound infiltration for hemorrhoidectomies and bunionectomies.[3] The maximum FDA-approved dosage of liposomal bupivacaine is 266 mg. Since the original approval, the FDA issued expanded the use of liposomal bupivacaine to “local surgical infiltration” on December 2015 [Table 1]. Under this expansion, liposomal bupivacaine for transversus abdominis plane (TAP) blocks, specifically, is an FDA approved use.[4] Liposomal bupivacaine is an extended release form of bupivacaine that may last as long as 3 days after a single infiltration.[5] Since its initial approval, it has been used to provide effective analgesia in thoracic, orthopedic, and abdominal surgeries. In addition, there have been several studies examining the safety and efficacy of liposomal bupivacaine on off-label peripheral nerve blocks.[6] The safety profile and efficacy of liposomal bupivacaine have not been fully established; however, to date, it appears that liposomal bupivacaine is an important drug for postoperative analgesia and has the potential to provide a significant tool for many types of surgeries.[7]
Table 1.
Summary of approved (i.e., surgical site local infiltration) versus off-label use of liposomal bupivacaine
Pharmacological Properties and Safety
Bupivacaine is the active compound in liposomal bupivacaine, which is an amide local anesthetic. The target site for local anesthetics is voltage-gated sodium ion channels, which alternate between several conformational states, including “activated” and “inactivated” states. Local anesthetics bind to the activated and inactivated states more readily. Local anesthetics bind the intracellular portion of voltage-gated sodium channels, and therefore, pKa of local anesthetics has a significant impact on the onset of blockage because only the nonionized form of the local anesthetic can cross inside the nerves. Bupivacaine has a pKa of 8.1 and it is, therefore, about 83% ionized at a physiologic pH of 7.4. Thus, a large percentage of the molecules are charged. As a result, bupivacaine has a slower onset than molecules with a pKa closer to physiologic pH of 7.4.[8]
The form of liposomal bupivacaine approved for use in the United States is based on DepoFoam technology, which consists of encapsulating drugs in a liposomal platform and releasing them over a desired period of 1–30 days. These multivesicular liposomes are made of biodegradable cholesterol, triglycerides, and phospholipids. The composition of drugs is not altered within these multivesicular liposomes.[9]
Unilamellar liposomes consist of a concentric lipid bilayer, which surrounds an aqueous compartment. In contrast, multivesicular liposomes do not have concentric bilayers; they consist of closely packed vesicles. This nonconcentric nature of multivesicular liposomes may be what allows the long duration of action of drugs.[10,11] Internal fusion and division occur in multivesicular liposomes, and this may allow internalized drugs to be released in a delayed fashion. This allows for a sustained drug delivery while avoiding systemic toxicity from high plasma drug levels.[9]
Bupivacaine has one of the longer half-lives of the commonly used local anesthetic drugs in modern-day anesthesia practices. It has a terminal half-life of 3.5 h.[8] The primary determinant of the duration of anesthesia is protein binding. Nearly 95% of bupivacaine is protein bound. This distinctively unique characteristic of bupivacaine allows for a long half-life. Extending the half-life with liposomes for a drug with an already-long half-life such as bupivacaine is a logical choice.[8] Bupivacaine is an amide anesthetic that is metabolized by hepatic carboxylesterases and cytochrome P450 enzymes. Only a trivial amount of amide local anesthetics is excreted through the renal system. As a result, a decrease in hepatic clearance or hepatic blood flow can predispose patients to the accumulation of these drugs in the plasma.
Bupivacaine is highly potent and lipid soluble. This allows it to readily cross the blood-brain-barrier and cause central nervous system (CNS) toxicity. This potential for CNS toxicity may be further exacerbated by hypercarbia, hypercapnia, decrease in protein binding of a local anesthetic, and by systemic acidosis. When compared to lidocaine, bupivacaine has four times the relative potency for CNS toxicity. More potent agents such as bupivacaine can lead to adverse outcomes such as complete heart block and cardiovascular collapse.[8] Therefore, giving higher dosages of bupivacaine in hopes of prolonging anesthesia is potentially harmful. In contrast, liposomal bupivacaine has been shown to decrease the toxic dosages in vivo. In a study by Boogaerts et al., when compared to plain bupivacaine, rabbits required more than twice the dose of liposomal bupivacaine to produce seizures and ventricular arrhythmias.[12] Consequently, liposomal bupivacaine offers the advantage of potentially providing greater safety.[12] In addition to the known sodium channel-blocking properties, bupivacaine has the potential to block potassium channels. This has the potential of producing serious cardiac arrhythmias. Naseem et al. studied the effects of QTc prolongation after a single subcutaneous administration of liposomal bupivacaine in 4 dosages: 300 mg, 450 mg, 600 mg, and 750 mg. All four dosages did not produce any clinically significant prolongation in QTc. Interestingly, a slight shortening of the interval was observed, and the clinical significance of this is not known.[13]
The sole fraction of bupivacaine available to enter the CNS and the cardiovascular system is the free (ie. non-liposomal) formulation, because multilamellar liposomes do not cross the blood-brain barrier.[12] Liposomal bupivacaine is released into the plasma in two different ways after local tissue infiltration. Initially, plain bupivacaine in the “liposomal bupivacaine” solution is systemically absorbed. Subsequently, there is a gradual, sustained release of bupivacaine from multilamellar vesicles. This may explain why despite a large amount of drug deposition, less systemic toxicity is seen. Since liposomes limit the immediate release of bupivacaine, we postulate that liposomal bupivacaine is likely to cause less systemic toxicity with accidental intravascular injections.
Currently, the manufacturer recommends that liposomal bupivacaine be administered 20 min after lidocaine, and other formulations of bupivacaine should not be administered 96 h after administration of liposomal bupivacaine. Liposomal bupivacaine should not be allowed to come into contact with antiseptics such as chlorhexidine or povidone iodine in solution. If a topical antiseptic us used, the surgical site should be allowed to dry before administration of liposomal bupivacaine. Contact with antiseptics may cause the liposomes to disband, which can suddenly release toxic amounts of bupivacaine into the plasma.[14]
Bramlett et al. studied the pharmacokinetic parameters of liposomal bupivacaine. Patients undergoing total knee arthroplasty were treated with plain bupivacaine 150 mg, and varying quantities of liposomal bupivacaine (133 mg, 266 mg, 399 mg, and 532 mg). A dose-proportional increase was observed in Cmax (maximum observed plasma concentration) and a slightly greater than dose-proportional increase was observed in AUC0–∞ (area under the plasma concentration-time curve from time of study drug administration to infinity). Significant systemic plasma levels of bupivacaine persisted for up to 96 h after local infiltration with liposomal bupivacaine. However, systemic plasma concentration may not correlate with clinical effect.[15]
At this time, there are no adequate and well-controlled studies of liposomal bupivacaine in pregnant women. Animal reproduction studies have been conducted to evaluate bupivacaine. In these studies, subcutaneous administration of bupivacaine to rats and rabbits during organogenesis was associated with embryo-fetal deaths in rabbits at a dose equivalent to the maximum recommended human dose (MRHD).
Clinical Uses
At present, liposomal bupivacaine has many different applications that are outlined below. It appears that broader indications for its use will be presented to the FDA in the near future. As mentioned in the previous section, liposomal bupivacaine has consistently demonstrated a safety profile similar to that of plain bupivacaine. As a result, off-label use has accelerated. Currently, it is being studied in peripheral nerve blocks and neuraxial techniques. However, the largest body of data supporting its clinical use remains with surgical site infiltration.
Surgical site infiltration
Liposomal bupivacaine was first approved by the FDA in October 2011 for infiltration of surgical sites. It is currently only approved for postoperative pain control by local infiltration after bunionectomy and hemorrhoidectomy.[15] Several randomized trials have examined the efficacy and safety of liposomal bupivacaine. The efficacy of liposomal bupivacaine has been studied for local wound infiltration in mammoplasty, total knee arthroplasty, bunionectomy, hemorrhoidectomy, inguinal hernia repair, and TAP blocks. In the above studies, liposomal bupivacaine has been shown to achieve clinically meaningful lower cumulative pain scores, reduce opioid requirements, expedite discharge from the hospital, and reduce hospital cost.[6]
Smoot et al. performed a multicenter, randomized, double-blind study to compare the efficacy of liposomal bupivacaine compared to 0.5% bupivacaine with 1:200,000 epinephrine in patients undergoing bilateral, cosmetic, and submuscular augmentation mammoplasty. The control group received 600 mg liposomal bupivacaine while the treatment group received 200 mg of 0.5% bupivacaine with epinephrine divided in the implant pockets. Both groups also received rescue oxycodone as needed, and acetaminophen 1000 mg 3 times daily. The total amount of opioid consumed was significantly lower in the treatment group, but there was no significant difference in the pain scores between the two groups. The authors concluded that this study may have been underpowered.[16]
Golf et al. conducted a multicenter, randomized, double-blind Phase III clinical trial on patients undergoing bunionectomy. The treatment group received 180 mg of liposomal bupivacaine in 8 ml and control group normal saline 8 ml via local wound infiltration. Authors concluded that liposomal bupivacaine use led to decreased opioid use and extended pain relief when compared to placebo.[17]
Gorfine et al. performed a multicenter, randomized, double-blind, parallel group placebo-controlled Phase III study to compare the efficacy of liposomal bupivacaine compared to placebo in patients undergoing hemorrhoidectomy. The treatment group received 300 mg liposomal bupivacaine in 30 ml versus 30 ml of normal saline placebo in the control group. The study revealed a statistically significant reduction in pain through 72 h, there was a delay in time to first opioid use, decreased total opioid use, and an overall improved patient satisfaction in the control group when compared to placebo.[18]
Cohen et al. conducted a single-center, open-label sequential cohort study in patients undergoing open segmental colectomy with anastomosis. Patients received general anesthesia with multimodal anesthesia. The control group received patient-controlled analgesia with either morphine or hydromorphine while the treatment group received ketorolac 30 mg intravenous, liposomal bupivacaine 266 mg via local wound infiltration, and oral 1 g acetaminophen, 600 mg ibuprofen every 6 h for 3 days after surgery. The treatment group had significantly less use of opioids after surgery, median hospital day was shorter, and the average cost of hospitalization was shorter. However, this study did not directly compare placebo or nonliposomal bupivacaine with liposomal bupivacaine. Therefore, it is difficult to draw a definitive conclusion about the efficacy of liposomal bupivacaine alone.[19]
Transversus abdominis plane (TAP) blocks
TAP blocks have gained popularity as a regional anesthesia technique for intraabdominal surgery.[20] Recent studies show promise in using liposomal bupivacaine for TAP blocks and local surgical site infiltration after abdominal surgery. Keller et al. performed a pilot study in 50 consecutive patients undergoing elective laparoscopic colorectal resection by giving the experimental group a postinduction, preincision bilateral TAP block and local peritoneal infiltration at port-insertion sites with liposomal bupivacaine. This was compared to a control group with no TAP block or local infiltration. The experimental group had a significantly decreased intraoperative opioid requirement, decreased postanesthesia care unit pain scores, and shorter length of stay.[21] This study has important implications for the ability of liposomal bupivacaine in lowering health-care costs.
Hutchins et al. conducted a prospective randomized controlled observer-blinded study comparing bilateral TAP blocks with plain bupivacaine to bilateral TAP blocks with liposomal bupivacaine in patients undergoing robot-assisted hysterectomies. Patients in the liposomal bupivacaine had significantly decreased nausea, lower maximal pain scores at all time periods studied, and significantly decreased total opioid use in the first 72 h after injections.[22] Gasanova et al. compared surgical site infiltration with liposomal bupivacaine with TAP blocks using plain bupivacaine in patients undergoing open total abdominal hysterectomies. They found that group with surgical site infiltration had significantly lower pain scores at rest and with coughing, and significantly lower opioid requirements between 24 and 48 h.[23] The abovementioned studies indicate that liposomal bupivacaine may offer superior pain relief when compared to plain bupivacaine in both TAP blocks and local surgical site infiltration. Studies comparing liposomal bupivacaine for TAP blocks and local infiltration will shed light on this topic. Another recent study compared TAP block infiltrated with with liposomal bupivacaine to continuous epidural analgesia with plain bupivacaine. The authors found similar postoperative pain scores and opioid consumption.[24] As mentioned previously, liposomal bupivacaine is approved by the FDA for TAP block use.
Intraarticular use
Bramlett et al. performed a multicenter, randomized, double-blinded, Phase II dose-ranging study evaluating the pharmacokinetics, safety, and efficacy of liposomal bupivacaine in patients undergoing total knee arthroplasty. The treatment group received liposomal bupivacaine dosed at 133 mg, 266 mg, 399 mg, or 523 mg, injected into the deep tissues before implantation. The control group received plain bupivacaine 150 mg injected into the deep tissues before implantation. The authors concluded that the total consumption of opioids, mean pain scores, time for a return to normal daily activities, and time to resumption to work were not statistically different.[15] However, Barrington et al. have argued that the size of needle and technique for wound infiltration may play a major role in the efficacy of liposomal bupivacaine. They have argued that smaller needles and distribution of the drug in small amounts may be better.[25]
Epidural use
Boogaerts et al. conducted one of the first studies in 1997 of epidural administration liposomal bupivacaine, and compared the efficacy and safety of liposomal bupivacaine to plain bupivacaine.[26] The patients received 10 ml of either 0.5% plain bupivacaine with 1:200,000 epinephrine or 0.5% liposomal bupivacaine. The median duration of analgesia was 3.2 in the control group, and 6.25 h in the treatment group. The authors concluded that there was no increased incidence of cardiotoxicity, neurotoxicity, or motor block with liposomal bupivacaine use.
Viscusi et al. performed a Phase I, double-blind, randomized, dose-escalating study of the effects of a single L3–L4 epidural administration of liposomal bupivacaine. Healthy volunteers in the treatment received liposomal bupivacaine 89 mg, 155 mg, 266 mg, and volunteers in the control group received plain bupivacaine 50 mg. The half-lives of liposomal bupivacaine were similar and 3 times longer than plain bupivacaine and the maximum plasma concentration of bupivacaine was statistically significantly lower with all liposomal bupivacaine administrations. The proportion of subjects with the motor block was lower with liposomal bupivacaine groups, and sensory numbness to cold was about 6 times longer with liposomal bupivacaine 266 mg when compared to plain bupivacaine.[27]
Peripheral nerve blocks
Recently, the manufacturer of liposomal bupivacaine has submitted an application to the FDA for an expansion of indications for liposomal bupivacaine. This application includes multiple clinical trials completed by the manufacturer.[9] Ilfeld recruited 14 healthy volunteers for a dose-response study with liposomal bupivacaine in subjects undergoing femoral nerve blocks. Subjects were given anywhere from 0 mg to 80 mg of liposomal bupivacaine in 30 ml of NS. Two separate dosages were given to each subject – one on each limb. Interestingly, the authors noted that as the dosage of liposomal bupivacaine was escalated, the magnitude of motor block decreased. There was also significant intersubject variability. However, the healthy volunteers may not have been an appropriate representation of the surgical population. In addition, the small sample size may have caused the results to be skewed.[28]
There is a paucity of data over the efficacy of liposomal bupivacaine in peripheral nerve blocks. However, preliminary data from the manufacturer, Pacira Pharmaceuticals, Inc San Diego, CA., who sponsored these trials do suggest that liposomal bupivacaine may have a similar safety profile as plain bupivacaine. In a separate study, Ilfeld et al. evaluated the safety of liposomal bupivacaine in peripheral nerve blocks. The authors pooled data from 6 Phase I to III trials and concluded that myotoxicity and neurotoxicity do not significantly differ between plain bupivacaine and liposomal bupivacaine.[9]
Rice et al. conducted a prospective, randomized, controlled trial in 108 patients undergoing thoracic procedures. A total of 108 patients undergoing video-assisted thoracotomy, robot-assisted thoracotomy, and open thoracotomy were randomized to receive a thoracic epidural or a unilateral, five-level posterior intercostals nerve block with a total of 266 mg of liposomal bupivacaine. The median hospital stay for the liposomal bupivacaine group was 3 days, and 4 days for the thoracic epidural group. In addition, there were no significant differences in postoperative pain, perioperative complications, and opioid utilization between the two groups. The authors concluded that liposomal bupivacaine is safe and provides effective analgesia for patients undergoing thoracic surgery and that it may be considered an alternative to thoracic epidurals.[3]
Conclusions
Currently, liposomal bupivacaine is only approved for use in local surgical site infiltration in patients undergoing bunionectomy and hemorrhoidectomy. Although its efficacy has yet to be thoroughly studied in peripheral nerve blocks, it does appear to have a similar safety profile to plain bupivacaine. Therefore, more studies are warranted, and liposomal bupivacaine use appears to be promising for extended postoperative pain relief with peripheral nerve blocks.
Bupivacaine is currently labeled as pregnancy category C drug, as subcutaneous administration of bupivacaine to rats and rabbits during organogenesis were associated with embryo-fetal deaths in rabbits at a dose equivalent to the MRHD. Therefore, liposomal bupivacaine is also pregnancy category C drug per the FDA. Liposomal bupivacaine has a limited role during labor, but may be of benefit if patients continue to have pain after cesarean deliveries, and may be equivalent or better than TAP blocks.
Liposomal bupivacaine may also prove to be useful in the management of chronic pain patients. Nociception for facet joint arthropathy is carried by the median branches of dorsal rami. Radiofrequency ablation (RFA) of median branches of the dorsal rami is very effective in providing pain relief for several months. However, before RFA, clinicians typically perform diagnostic and therapeutic “median branch blocks” by injecting a local anesthetic such as plain lidocaine or bupivacaine, which provide relief for minutes to a few hours until they are seen at a follow-up visit in the clinic for more definitive treatment.[29] Liposomal bupivacaine has the potential to provide significant relief for 2–3 days – just before these patients are seen for follow-up and definitive RFA. Liposomal bupivacaine has been reasonably safe in peripheral nerve blocks and its use may benefit patients with chronic headaches and migraines, specifically for blocking greater/lesser occipital nerves, auriculotemporal nerves, zygomaticotemporal, and supraorbital nerves used for scalp blocks. In addition, liposomal bupivacaine has the potential to provide extended relief with trigger point injections commonly used in pain medicine.
Initially, liposomal bupivacaine was limited to local surgical site infiltration of bunionectomies and hemorrhoidectomies at a maximum dose of 266 mg. The FDA has recently expanded the approved use of this formulation to “local surgical site infiltration.” This has made TAP blocks and other infiltrations now as approved uses. The most robust data for the use of this formulation are its application in surgical site infiltration in abdominal surgeries. We anticipate that liposomal bupivacaine will have a greater role after abdominal surgeries, especially because a recent pilot study revealed that it may help reduce hospital length of stay.
Liposomal bupivacaine has a similar safety profile to plain bupivacaine, which will continue to promote off-label investigation. It has shown promise in peripheral nerve blocks, but higher powered trials are warranted to fully evaluate its efficacy. Further studies are warranted for its use in epidural and intrathecal administration. In addition, this formulation may have important applications in outpatient pain management.
Financial support and sponsorship
Dr. Richard D. Urman received research funding for unrelated work from Mallinckrodt and Cara Pharmaceuticals.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kaye AD, Jones MR, Kaye AM, Ripoll JG, Galan V, Beakley BD, et al. Prescription Opioid Abuse in Chronic Pain: An Updated Review of Opioid Abuse Predictors and Strategies to Curb Opioid Abuse: Part 1. Pain Physician. 2017;20(2S):S93–S109. [PubMed] [Google Scholar]
- 2.Kaye AD, Jones MR, Kaye AM, Ripoll JG, Jones DE, Galan V, et al. Prescription Opioid Abuse in Chronic Pain: An Updated Review of Opioid Abuse Predictors and Strategies to Curb Opioid Abuse (Part 2) Pain Physician. 2017;20(2S):S111–S133. [PubMed] [Google Scholar]
- 3.Rice DC, Cata JP, Mena GE, Rodriguez-Restrepo A, Correa AM, Mehran RJ. Posterior intercostal nerve block with liposomal bupivacaine: An alternative to thoracic epidural analgesia. Ann Thorac Surg. 2015;99:1953–60. doi: 10.1016/j.athoracsur.2015.02.074. [DOI] [PubMed] [Google Scholar]
- 4.Woodcock J. Attn: David Stack. Chief Executive Officer & Chairman. Letter to Pacira Pharmaceuticals, Inc. 2015 [Google Scholar]
- 5.Liu SS, Buvanendran A, Rathmell JP, Sawhney M, Bae JJ, Moric M, et al. Predictors for moderate to severe acute postoperative pain after total hip and knee replacement. Int Orthop. 2012;36:2261–7. doi: 10.1007/s00264-012-1623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong YC, Kaye AD, Urman RD. Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol. 2014;28:15–27. doi: 10.1016/j.bpa.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 7.King CH, Beutler SS, Kaye AD, Urman RD. Pharmacologic Properties of Novel Local Anesthetic Agents in Anesthesia Practice. Anesthesiol Clin. 2017;35:315–25. doi: 10.1016/j.anclin.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Barash PG, Cullen BF, Stoelting KR, Cahalan M, Stock C, Ortega R. Clinical anesthesiology. In: Lin Y, Liu SS, editors. Local Anesthetics. 7th ed. New York: Lippincott Williams; 2013. pp. 561–82. [Google Scholar]
- 9.Ilfeld BM, Viscusi ER, Hadzic A, Minkowitz HS, Morren MD, Lookabaugh J, et al. Safety and side effect profile of liposome bupivacaine (Exparel) in peripheral nerve blocks. Reg Anesth Pain Med. 2015;40:572–82. doi: 10.1097/AAP.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 10.Ye Q, Asherman J, Stevenson M, Brownson E, Katre NV. DepoFoam technology: A vehicle for controlled delivery of protein and peptide drugs. J Control Release. 2000;64:155–66. doi: 10.1016/s0168-3659(99)00146-7. [DOI] [PubMed] [Google Scholar]
- 11.Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam™: A sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet. 2006;45:1153–76. doi: 10.2165/00003088-200645120-00002. [DOI] [PubMed] [Google Scholar]
- 12.Boogaerts J, Declercq A, Lafont N, Benameur H, Akodad EM, Dupont JC, et al. Toxicity of bupivacaine encapsulated into liposomes and injected intravenously: Comparison with plain solutions. Anesth Analg. 1993;76:553–5. doi: 10.1213/00000539-199303000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Naseem A, Harada T, Wang D, Arezina R, Lorch U, Onel E, et al. Bupivacaine extended release liposome injection does not prolong QTc interval in a thorough QT/QTc study in healthy volunteers. J Clin Pharmacol. 2012;52:1441–7. doi: 10.1177/0091270011419853. [DOI] [PubMed] [Google Scholar]
- 14.Pacira Pharmaceuticals, Inc. Exparel (bupivicaine liposomal injectable suspension) [prescribing information] San Diego, CA: Pacira Pharmaceuticals, Inc; 2012. [Web 11 April 2013]. Available from: http://www.pacira.com/products/exparel.php . [Google Scholar]
- 15.Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19:530–6. doi: 10.1016/j.knee.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Smoot JD, Bergese SD, Onel E, Williams HT, Hedden W. The efficacy and safety of DepoFoam bupivacaine in patients undergoing bilateral, cosmetic, submuscular augmentation mammaplasty: A randomized, double-blind, active-control study. Aesthet Surg J. 2012;32:69–76. doi: 10.1177/1090820X11430831. [DOI] [PubMed] [Google Scholar]
- 17.Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam ® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28:776–88. doi: 10.1007/s12325-011-0052-y. [DOI] [PubMed] [Google Scholar]
- 18.Gorfine SR, Onel E, Patou G, Krivokapic ZV. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: A multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum. 2011;54:1552–9. doi: 10.1097/DCR.0b013e318232d4c1. [DOI] [PubMed] [Google Scholar]
- 19.Cohen SM. Extended pain relief trial utilizing infiltration of Exparel(®), a long-acting multivesicular liposome formulation of bupivacaine: A phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567–72. doi: 10.2147/JPR.S38621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lissauer J, Mancuso K, Merritt C, Prabhakar A, Kaye AD, Urman RD. Evolution of the transversus abdominis plane block and its role in postoperative analgesia. Best Pract Res Clin Anaesthesiol. 2014;28:117–26. doi: 10.1016/j.bpa.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Keller DS, Tahilramani RN, Flores-Gonzalez JR, Ibarra S, Haas EM. Pilot study of a novel pain management strategy: Evaluating the impact on patient outcomes. Surg Endosc. 2015 Aug 15; doi: 10.1007/s00464-015-4459-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Hutchins J, Delaney D, Vogel RI, Ghebre RG, Downs LS, Jr, Carson L, et al. Ultrasound guided subcostal transversus abdominis plane (TAP) infiltration with liposomal bupivacaine for patients undergoing robotic assisted hysterectomy: A prospective randomized controlled study. Gynecol Oncol. 2015;138:609–13. doi: 10.1016/j.ygyno.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasanova I, Alexander J, Ogunnaike B, Hamid C, Rogers D, Minhajuddin A, et al. Transversus abdominis plane block versus surgical site infiltration for pain management after open total abdominal hysterectomy. Anesth Analg. 2015;121:1383–8. doi: 10.1213/ANE.0000000000000909. [DOI] [PubMed] [Google Scholar]
- 24.Ayad S, Babazade R, Elsharkawy H, Nadar V, Lokhande C, Makarova N, et al. Comparison of transversus abdominis plan infiltration with liposomal bupivacaine versus continuous epidural analgesia versus intravenous opioid analgesia. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrington JW, Halaszynski TM, Sinatra RS Expert Working Group on Anesthesia and Orthopaedics Critical Issues in Hip and Knee Replacement Arthroplasty FT. Perioperative pain management in hip and knee replacement surgery. Am J Orthop (Belle Mead NJ) 2014;43(4 Suppl):S1–16. [PubMed] [Google Scholar]
- 26.Boogaerts JG, Lafont ND, Declercq AG, Luo HC, Gravet ET, Bianchi JA, et al. Epidural administration of liposome-associated bupivacaine for the management of postsurgical pain: A first study. J Clin Anesth. 1994;6:315–20. doi: 10.1016/0952-8180(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 27.Viscusi ER, Candiotti KA, Onel E, Morren M, Ludbrook GL. The pharmacokinetics and pharmacodynamics of liposome bupivacaine administered via a single epidural injection to healthy volunteers. Reg Anesth Pain Med. 2012;37:616–22. doi: 10.1097/AAP.0b013e318269d29e. [DOI] [PubMed] [Google Scholar]
- 28.Ilfeld BM. Liposome bupivacaine in peripheral nerve blocks and epidural injections to manage postoperative pain. Expert Opin Pharmacother. 2013;14:2421–31. doi: 10.1517/14656566.2013.844791. [DOI] [PubMed] [Google Scholar]
- 29.MacVicar J, Borowczyk JM, MacVicar AM, Loughnan BM, Bogduk N. Cervical medial branch radiofrequency neurotomy in New Zealand. Pain Med. 2012;13:647–54. doi: 10.1111/j.1526-4637.2012.01351.x. [DOI] [PubMed] [Google Scholar]


