Abstract
Background and Aims:
Opioid-free anesthesia decreases postoperative nausea and vomiting, emergence agitation, prolonged sedation, ileus, and urinary retention. The feasibility of the use of dexmedetomidine as sole analgesic agent has been shown in patients undergoing bariatric and gynecological laparoscopic surgery. We explored its use for robotic urological surgery.
Material and Methods:
Thirty patients were randomized to receive either dexmedetomidine (Group D) or fentanyl (Group F) along with total intravenous anesthesia with propofol. The hemodynamic parameters and number of doses of rescue analgesics used intraoperatively and postoperatively were noted. Recovery parameters at the end of surgery were also recorded.
Results:
The dose of intraoperative rescue fentanyl was not significantly different between groups (P = 0.13). The hemodynamic profile of patients in the two groups was comparable except the heart rate was significantly more in Group D after intubation and at 60 min. The mean arterial pressure was significantly lower after the initial loading dose of study drug in Group D. The recovery profiles were not significantly different between groups.
Conclusion:
The study reveals that dexmedetomidine has equal analgesic efficacy as fentanyl for intraoperative use and can be used as the sole analgesic agent in patients undergoing robotic urological surgery.
Keywords: Analgesia, dexmedetomidine, fentanyl, robotic surgical procedures, urology
Introduction
Opioid-free anesthesia has been shown to have multiple advantages. It causes decreased postoperative nausea and vomiting (PONV), decreases the incidence of emergence agitation, prolonged sedation, ileus, and urinary retention. Opioids are also known to cause acute hyperalgesia. Thus, high intraoperative doses may lead to increased requirement of opioids in the postoperative period amplifying the opioid-related side-effects. Many patient factors, e.g., morbid obesity, obstructive sleep apnea, day care surgery, etc., may also merit decreased or no use of opioids during anesthesia. The use of multimodal analgesia utilizing nonsteroidal anti-inflammatory agents, dexamethasone, tramadol, and/or ketamine has been shown to either decrease or avoid intraoperative and postoperative opioid use. Alpha-2 agonists are an attractive choice for providing intraoperative and postoperative analgesia. They are being used for anesthesia and analgesia in animals for many years. Dexmedetomidine, the most recent alpha-2 agonist, is used for sedation and analgesia in the Intensive Care Unit (ICU), as premedication, as an anesthetic adjunct for general and regional anesthesia and also for postoperative sedation and analgesia.
The use of various elements of multimodal analgesia may decrease the opioid consumption but may not eliminate it completely. Dexmedetomidine, on the other hand, has shown to be an opioid equivalent analgesic in a few recent studies in patients undergoing laparoscopic surgery.[1,2] However, whether the same results can be extrapolated for robotic surgeries remains to be explored. This study was thus planned to compare the analgesic efficacy of dexmedetomidine with fentanyl in patients undergoing robotic urological surgeries.
Material and Methods
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. After obtaining the approval of our institutional human ethics committee and individual written informed consent, thirty American Society of Anesthesiologists I or II patients undergoing robotic urological surgery using the da Vinci S system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) were randomly divided, through sealed envelope assignment, into two groups: Group F (received fentanyl for intraoperative analgesia) and Group D (received dexmedetomidine for intraoperative analgesia). Flow of patients for recruitment into the two groups is shown in Figure 1. All thirty patients met the inclusion criteria of age between 18 and 60 years with no contraindication to the study drugs (left ventricular failure, volume-depleted state, severe heart block, known hypersensitivity to study drugs, bronchial asthma, head injuries, and increased intracranial pressure). Exclusion criteria included body mass index >25, history of major cardiovascular and nervous system disorders, and allergy to study drugs. Informed written consent was taken from all the patients. Patients undergoing robotic radical cystectomy with ileal conduit formation were excluded from the study as the pain quality was expected to be different due to open dissection involved during conduit formation.
Figure 1.
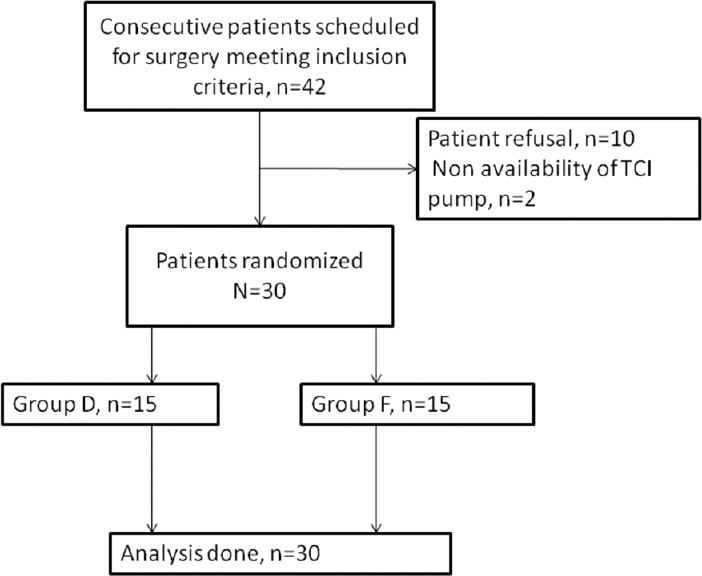
Flow of patients in the study
Patients were kept nil per oral after midnight before surgery. They were premedicated with 0.25 mg alprazolam orally on the morning of surgery at 6.00 a.m. In the operating room, standard monitors were attached to the patient (electrocardiogram, noninvasive blood pressure, and pulse oximeter) and baseline parameters were recorded. Intravenous access was established with a 16G intravenous cannula under local anesthesia. Randomization was done by closed envelope method. Drug to be used was decided by a computer-generated randomization sequence. Person not involved in study picked up the envelope and according to the drug chosen prepared the study drug in identical syringes. Each syringe was labeled with the patients’ number and the initials, and neither the treatment assignment nor the content of the syringe was known by the anesthesia staff involved in the patient management and data recording. To prepare the drug, in Group F, 400 mcg of fentanyl was diluted in 20 ml saline to make the infusion concentration of 20 mcg/ml, whereas in Group D, 100 mcg of dexmedetomidine was diluted in 20 ml saline to make the infusion concentration 5 mcg/ml.
Before the induction of anesthesia, loading dose of the study drug 0.1 ml/kg (which was equal to 2 mcg/kg for fentanyl and 0.5 mcg/kg for dexmedetomidine) was given intravenously over 10 min. Propofol was administered by an Orchestra® Base Primea target controlled infusion (TCI) pump (Fresenius Kabi, Brezins, France) using the Schnider pharmacokinetic mode. During induction, target effect site concentration was set at 4 mcg/ml. Loss of consciousness was assessed by lack of response to verbal command. After induction of anesthesia and checking adequate mask ventilation, atracurium 0.5 mg/kg was given intravenously to achieve muscle relaxation. Patients were manually ventilated by mask with oxygen-air mixture and trachea was intubated after 3 min. After intubation, patients’ lungs were mechanically ventilated with an oxygen-air mixture to maintain end-tidal CO2 between 35 and 40 mmHg.
Anesthesia was maintained with propofol by TCI system to target the effect site concentration of 2–3 mcg/ml, study drug infusion at the rate of 0.05 ml/kg/h (which was 1 mcg/kg for fentanyl and 0.25 mcg/kg for dexmedetomidine) and atracurium boluses as and when required.
Rescue analgesia in the form of boluses of 0.5 mcg/kg of fentanyl was given in case of hypertension and/or tachycardia by the attending anesthesiologist who was blinded to the group allotted. The primary aim of the study was dose of rescue analgesic used intraoperatively. Adverse hemodynamic events were noted based on the following values: hypertension (mean arterial pressure [MAP] >20% above the preoperative baseline value), hypotension (MAP <20% below the preoperative baseline value), tachycardia (heart rate [HR] >20% above the preoperative baseline value), and bradycardia (HR <50 beats/min). Appropriate management of bradycardia and hypotension was instituted in the form of atropine and ephedrine in suitable doses, respectively, in both groups. Approximately 5 min before the anticipated end of surgery (defined as the last surgical suture), both propofol and the study drug infusion were stopped. After the end of surgery, neuromuscular blockade was reversed using intravenous neostigmine (50 mcg/kg) and glycopyrrolate (10 mcg/kg). Trachea was extubated when adequate spontaneous ventilation (tidal volume >4 ml/kg) was established. HR, MAP, systolic pressure, and diastolic pressure were recorded intraoperatively.
Recovery profile was assessed by measuring time to tracheal extubation from stopping infusions, time to eye opening from stopping infusions, and Modified Observer's Assessment of Alertness/Sedation Scale (MOASS).
In the Post Anesthesia Care Unit, patients were observed for 2 h. Nausea and vomiting was treated by intravenous ondansetron, 0.1 mg/kg, to patients who requested. Inadequate analgesia was treated with fentanyl 0.5 mcg/kg.
The study was planned as a pilot study as no previous published trial has compared the intraoperative analgesic efficacy of fentanyl and dexmedetomidine in robotic urological procedures. Sample size was calculated to detect a difference of 20 mcg in intraoperative rescue fentanyl use in both groups assuming that the standard deviation in fentanyl use would be 20 mcg with a power of 90% and a two-sided confidence interval of 95%. Statistical analysis was carried out using IBM@ SPSS@ statistics version 20 (IBM Corporations and its Licensors 1989, 2011, CA, USA). Data are presented as number (%) or mean ± standard deviation/median (minimum – maximum) as appropriate. The baseline continuous variables were compared between the two groups using Student's t-test for independent samples and categorical variables were compared using Fisher's exact test. The changes in hemodynamic parameters over a period between the two groups were also compared using repeated measures ANOVA. P < 0.05 was considered statistically significant.
Results
Forty-two patients were screened for inclusion in the study. The flow of patients is shown in Figure 1. Demographic characteristics (age, sex, height, and weight), baseline cardiovascular variables, volume of propofol and study drug used, duration of surgery, and anesthesia were comparable between the two groups [Table 1]. The intraoperative rescue fentanyl was used in 11 patients in each group. The dose of rescue fentanyl used intraoperatively was not significantly different in both groups [Table 2]. Intraoperative HR and MAP are being shown in Figures 2 and 3, respectively. The HR was significantly more in dexmedetomidine group after intubation and at 60 min of surgery than in the fentanyl group [Figure 2]. At all the other time points, there was no significant difference between the groups. The MAP was significantly lower after the initial loading dose of the study drug in the dexmedetomidine group than in the fentanyl group (P = 0.04) [Figure 3]. There was no significant difference at any other time points. The incidence of hypotension requiring treatment with ephedrine was not significantly different between the two groups. In Group F, median number of boluses of ephedrine used was 0 (range 0–2), and in Group D, it was 1 (range 0–3) (P = 0.52). One patient in Group F and two patients in Group D had bradycardia requiring treatment with atropine bolus.
Table 1.
Patient demographics and other intraoperative parameters in both groups
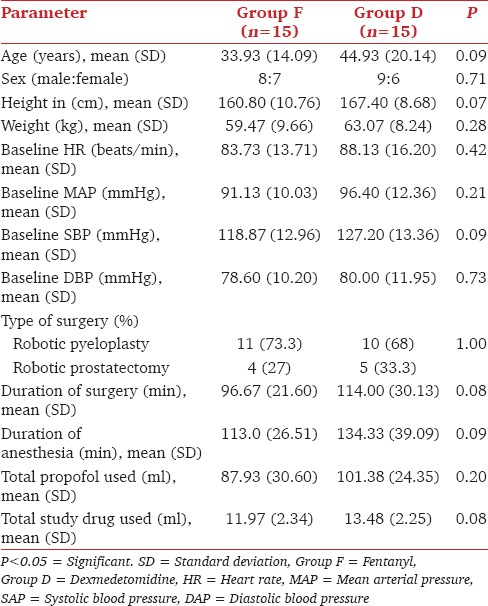
Table 2.
Comparison of dose of rescue fentanyl used in both groups

Figure 2.
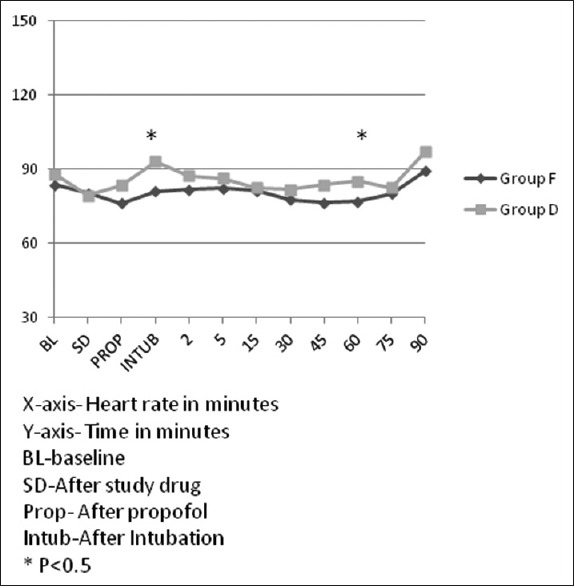
Heart rate trends in both groups
Figure 3.
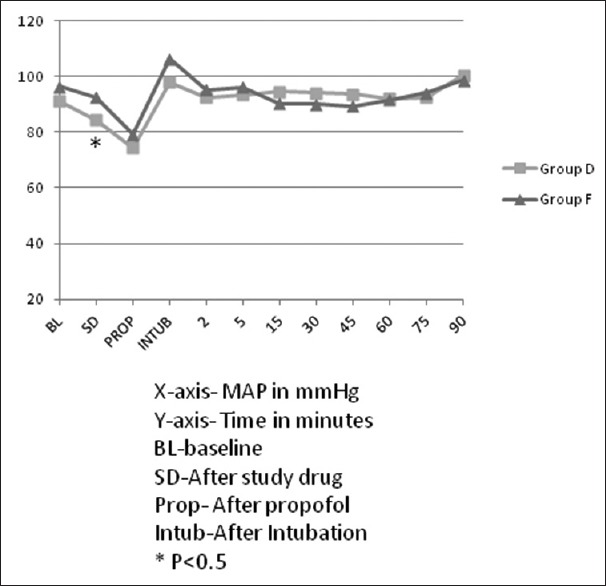
Mean arterial pressure trends in both groups
The time to eye opening and tracheal extubation were longer in Group D [Table 3], but the difference was not statistically significant. In both groups, the MOASS was 4 in 6 (40%) patients and 5 in 9 (60%) patients (P = 1.0).
Table 3.
Comparison between the two groups for recovery parameters

In the postoperative period, the hemodynamic parameters and the dose of fentanyl used for analgesia were not significantly different in both groups.
Discussion
Laparoscopic surgery has a different pain profile than normal open surgery. Pain during laparoscopic surgery is a result of both somatic and visceral afferents. It is difficult to conclude with present evidence whether pain during and after robotic surgery is different from laparoscopic surgery or not. However, it is quite evident that the other complications of laparoscopic surgery, e.g., PONV, shoulder tip pain and intestinal ileus are common in both kinds of procedures.[3] The use of opioids can add to the side effect profile of patients undergoing robotic and laparoscopic surgery by further aggravating PONV and ileus and causing prolonged sedation. Large bolus doses of opioids intraoperatively can also lead to postoperative hyperalgesia and increased consumption of rescue analgesics.[4] The use of opioid sparing and opioid-free analgesic techniques have the propensity to decrease and even avoid many of these side effects and lead to early oral intake, early ambulation, earlier hospital discharge, and lesser readmission rates to the hospital in the postoperative period.[5] Multimodal analgesic techniques such as use of acetaminophen, pregabalin, cyclooxygenase-II inhibitors, nonsteroidal anti-inflammatory drugs, local anesthetics, beta-blockers, dexamethasone either alone or in combination, have been shown to decrease the requirement of opioids intraoperatively and postoperatively.[6] However, none of these drugs are effective as the sole analgesic, and thus the need for evaluating newer drugs as replacement of opioids in the intraoperative period is still continuing. Alpha-2 agonists such as clonidine and dexmedetomidine have shown promising results in this context.[7] Many recent studies have evaluated the use of dexmedetomidine in patients undergoing various laparoscopic surgeries under balanced or total intravenous anesthesia (TIVA).[1,2,8,9] Feld et al. compared the intraoperative hemodynamics and bispectral index scores (BISs) and postoperative analgesic requirement of patients undergoing laparoscopic bariatric surgery under desflurane anesthesia with either fentanyl or dexmedetomidine as intraoperative analgesic.[8] They found that the patients in dexmedetomidine group used lesser desflurane and had better hemodynamic profile than the patients receiving fentanyl for intraoperative analgesia. In a more recent study, obese patients undergoing laparoscopic bariatric surgery receiving opioid-free TIVA with propofol, dexmedetomidine, and ketamine had a large reduction in relative risk of PONV compared with patients receiving opioid-based balanced anesthesia.[10] This study, however, has not mentioned about the intraoperative analgesia quality in both groups. The postoperative opioid use for analgesia was similar in both the groups in the study. Bakan et al. also showed that opioid-free TIVA with dexmedetomidine, lidocaine, and propofol infusions when compared with opioid-based TIVA with remifentanil and propofol infusions, is associated with lower fentanyl requirements in the early postoperative period (first 2 h) after laparoscopic cholecystectomy.[11] Dexmedetomidine has also been shown to be a suitable alternative analgesic agent to remifentanil in patients undergoing gynecological laparoscopic surgery.[9] However, no previous study has compared the analgesic efficacy of dexmedetomidine and fentanyl in patients undergoing robotic surgery. The difference in pain profile in robotic and laparoscopic surgeries is yet to be confirmed by randomized controlled trials. Pain scores have been shown to be either equivalent[12] or less[13] in robotic approaches as compared to laparoscopic surgeries although evidence presently is probably inadequate to convince either way.
This pilot study has demonstrated that dexmedetomidine when used as a sole analgesic agent, provides equivalent intraoperative analgesia as fentanyl in patients undergoing robotic urological surgery under TIVA. The intraoperative rescue fentanyl used in both groups was not significantly different. The hemodynamic parameters in both groups were also comparable at most of the time points. Both these results indicate a similar analgesic profile of dexmedetomidine and fentanyl for intraoperative analgesia for robotic urological surgery. The use of intravenous dexmedetomidine has unique hemodynamic profile. Bradycardia is a well-known adverse effect of dexmedetomidine.[14] The mechanism involved in the development of bradycardia seems to be combination of baroreflex-mediated reduction in HR, coinciding with the transient increase in blood pressure, reduced sympathetic tone (centrally mediated), and increased vagal tone.[15] Bradycardia is also a well-known side effect seen in patients undergoing urological laparoscopic surgery.[16] The incidence of bradycardia with the use of either of the drugs was, however, not significantly different in this study. This could be explained by the fact that the loading dose of dexmedetomidine was given over 10 min in this study. The incidence of bradycardia has shown to be less by slow administration or omission of the loading dose.[17] The difference in hemodynamic trends was seen only immediately postendotracheal intubation when the mean HR was significantly more in the patients receiving dexmedetomidine. In doses used in this study, dexmedetomidine may not be sufficient to suppress laryngoscopy and tracheal intubation-induced increase in HR. This difference was present only for a short duration (<2 min) and the difference was not significant in the MAP trends. Dexmedetomidine has been shown to attenuate the hemodynamic responses to laryngoscopy and endotracheal intubation when used as an adjunct to opioids.[18] In the present study, dexmedetomidine (0.5 μg/kg) was administered without any opioids which may not be sufficient to abolish the sympathetically mediated HR response associated with laryngoscopy and tracheal intubation.
The clinical effects of dexmedetomidine are due to the activation of presynaptic alpha-2 receptors in the locus coeruleus region of the brainstem. Activation of these receptors inhibits the release of norepinephrine, which is responsible for the analgesia, sedation, and hypnosis seen with use of dexmedetomidine. Dexmedetomidine has been shown to increase the time to recovery from anesthesia in patients receiving TIVA as compared to patients receiving inhalation anesthesia.[19] The mechanism behind this increase may have both pharmacodynamic and pharmacokinetic components. In this study, since there was no patient group which received inhalation anesthesia, this comparison is not possible. However, since the recovery profile (i.e., time to eye opening, time to tracheal extubation, and MOASS) in patients receiving either dexmedetomidine and fentanyl with propofol was similar, it can be elucidated that both these drugs when used in the doses described above may be expected to provide comparable recovery parameters when used as part of TIVA. Analogous results, i.e., dexmedetomidine based anesthesia providing equivalent recovery profiles as fentanyl-based general anesthesia has been shown in previous studies in patients undergoing laparoscopic and open surgery.[10,20]
The postoperative analgesic efficacy of dexmedetomidine in patients undergoing laparoscopic surgery has been evaluated previously.[1,8] Feld et al. compared the postoperative analgesic effect of fentanyl (0.5 μg/kg bolus followed by 0.5 μg/kg/h infusion) and dexmedetomidine (0.5 μg/kg bolus followed by 0.4 μg/kg/h infusion) in patients undergoing bariatric surgery.[8] Pain scores, morphine use in patient-controlled analgesia pump, HR, and blood pressure were lower in the dexmedetomidine group than in fentanyl group. In contrast to the above study, the postoperative hemodynamic parameters and the rescue analgesic requirement were similar (not less in dexmedetomidine) between the two groups in this study. This may be because of the lower dose of dexmedetomidine used in this study (0.5 μg/kg bolus followed by 0.25 μg/kg/h infusion) and because of different patient (obese) and surgical (laparoscopic bariatric) profile.
The study has a few limitations. The sample size could not be calculated as no previous study has evaluated the intraoperative analgesic efficacy of either of the drugs in patients undergoing robotic urological surgery. Intraoperative BIS monitoring could have been done to provide a more objective method for maintaining level of anesthesia.
Conclusions
This pilot study showed that dexmedetomidine used as a sole analgesic agent in the intraoperative period can provide equivalent analgesia to fentanyl. Both dexmedetomidine and fentanyl showed similar hemodynamic parameters, analgesic requirement and recovery profile in our study. Further studies with a larger sample size are needed to find if statistically significant difference exists.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: The effect on recovery outcome variables. Anesth Analg. 2008;106:1741–8. doi: 10.1213/ane.0b013e318172c47c. [DOI] [PubMed] [Google Scholar]
- 2.Jung HS, Joo JD, Jeon YS, Lee JA, Kim DW, In JH, et al. Comparison of an intraoperative infusion of dexmedetomidine or remifentanil on perioperative haemodynamics, hypnosis and sedation, and postoperative pain control. J Int Med Res. 2011;39:1890–9. doi: 10.1177/147323001103900533. [DOI] [PubMed] [Google Scholar]
- 3.Awad H, Walker CM, Shaikh M, Dimitrova GT, Abaza R, O’Hara J. Anesthetic considerations for robotic prostatectomy: A review of the literature. J Clin Anesth. 2012;24:494–504. doi: 10.1016/j.jclinane.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology. 2006;104:570–87. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: Effect of age, gender, and race. Clin Pharmacol Ther. 2003;74:102–12. doi: 10.1016/S0009-9236(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 6.Trabulsi EJ, Patel J, Viscusi ER, Gomella LG, Lallas CD. Preemptive multimodal pain regimen reduces opioid analgesia for patients undergoing robotic-assisted laparoscopic radical prostatectomy. Urology. 2010;76:1122–4. doi: 10.1016/j.urology.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 7.Feld JM, Laurito CE, Beckerman M, Vincent J, Hoffman WE. Non-opioid analgesia improves pain relief and decreases sedation after gastric bypass surgery. Can J Anaesth. 2003;50:336–41. doi: 10.1007/BF03021029. [DOI] [PubMed] [Google Scholar]
- 8.Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth. 2006;18:24–8. doi: 10.1016/j.jclinane.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Salman N, Uzun S, Coskun F, Salman MA, Salman AE, Aypar U. Dexmedetomidine as a substitute for remifentanil in ambulatory gynecologic laparoscopic surgery. Saudi Med J. 2009;30:77–81. [PubMed] [Google Scholar]
- 10.Ziemann-Gimmel P, Goldfarb AA, Koppman J, Marema RT. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br J Anaesth. 2014;112:906–11. doi: 10.1093/bja/aet551. [DOI] [PubMed] [Google Scholar]
- 11.Bakan M, Umutoglu T, Topuz U, Uysal H, Bayram M, Kadioglu H, et al. Opioid-free total intravenous anesthesia with propofol, dexmedetomidine and lidocaine infusions for laparoscopic cholecystectomy: A prospective, randomized, double-blinded study. Rev Bras Anestesiol. 2015;65:191–9. doi: 10.1016/j.bjane.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Zechmeister JR, Pua TL, Boyd LR, Blank SV, Curtin JP, Pothuri B. A prospective comparison of postoperative pain and quality of life in robotic assisted vs conventional laparoscopic gynecologic surgery. Am J Obstet Gynecol. 2015;212:194.e1–7. doi: 10.1016/j.ajog.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Aliyev S, Karabulut K, Agcaoglu O, Wolf K, Mitchell J, Siperstein A, et al. Robotic versus laparoscopic adrenalectomy for pheochromocytoma. Ann Surg Oncol. 2013;20:4190–4. doi: 10.1245/s10434-013-3134-z. [DOI] [PubMed] [Google Scholar]
- 14.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Penttilä J, Helminen A, Anttila M, Hinkka S, Scheinin H. Cardiovascular and parasympathetic effects of dexmedetomidine in healthy subjects. Can J Physiol Pharmacol. 2004;82:359–62. doi: 10.1139/y04-028. [DOI] [PubMed] [Google Scholar]
- 16.Aghamohammadi H, Mehrabi S, Mohammad Ali Beigi F. Prevention of bradycardia by atropine sulfate during urological laparoscopic surgery: A randomized controlled trial. Urol J. 2009;6:92–5. [PubMed] [Google Scholar]
- 17.Vilo S, Rautiainen P, Kaisti K, Aantaa R, Scheinin M, Manner T, et al. Pharmacokinetics of intravenous dexmedetomidine in children under 11 yr of age. Br J Anaesth. 2008;100:697–700. doi: 10.1093/bja/aen070. [DOI] [PubMed] [Google Scholar]
- 18.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani N, Kida K, Shoji K, Yasui Y, Masaki E. Recovery profiles from dexmedetomidine as a general anesthetic adjuvant in patients undergoing lower abdominal surgery. Anesth Analg. 2008;107:1871–4. doi: 10.1213/ane.0b013e3181887fcc. [DOI] [PubMed] [Google Scholar]
- 20.Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53:646–52. doi: 10.1007/BF03021622. [DOI] [PubMed] [Google Scholar]


