Abstract
Background and Aims:
The present study was designed and carried out aiming to evaluate the effects of local dexmedetomidine (Dex) on sedation rate and hemodynamic changes in candidate patients for fiberoptic nasotracheal intubation.
Material and Methods:
Candidate patients for fiberoptic nasotracheal intubation were randomly divided into three groups including intravenous (IV) Dex group, local Dex group, and control group. Local anesthesia using lidocaine was performed in all patients. After performing the intubation, propofol infusion was used to keep the patients on predetermined cerebral state index (CSI). Hemodynamic parameters, arterial blood O2 saturation (SpO2), and CSI were monitored in all patients before, during, and after the procedure. Coughing score, intubation score, and patient tolerance score during and after nasotracheal intubation were assessed. Propofol consumption was also measured.
Results:
A total of 95 patients with the mean age of 45.4 ± 6.7 years were evaluated (54.2% of females). Hemodynamic parameters and SpO2 were significantly different between the three groups (P < 0.001). The dose of propofol used for reaching proper CSI was significantly higher in the control group compared to IV and local Dex groups (P < 0.001). There is no significant statistical difference in propofol consumption between local and IV Dex groups. The number of patients who were cooperative during intubation was higher in local Dex group compared to IV Dex and control groups; however, the difference was not statistically significant.
Conclusion:
It is likely that using local Dex during fiberoptic bronchoscopy decreases sudden changes in hemodynamic values and decreases coughing and improves patient tolerance and intubation scores. Local Dex can be useful as IV form with the aim of propofol dose saving.
Keywords: Airway blocks, anesthesia, awake fiberoptic intubation, dexmedetomidine, local
Introduction
Managing difficult airway is one of the most challenging responsibilities of anesthesiologists. When an anesthesiologist encounters a difficult airway, fiberoptic intubation is a reliable technique for airway management. Use of airway blocks and providing proper sedation during fiberoptic intubation procedure will result in patient comfort, reduction in airway reflexes, and increase in cooperation and hemodynamic stability of the patient, as well as proper amnesia accompanied by airway preservation and spontaneous ventilation in the patient.[1,2,3] Numerous compounds have been used for the induction of conscious sedation during fiberoptic intubation.[4,5,6,7] Dexmedetomidine (Dex) is an α2-adrenergic agonist that has been used as a valuable drug during fiberoptic intubation. Proper sedation and analgesia without respiratory depression are among the advantages of prescribing intravenous (IV) Dex during fiberoptic intubation in patients.[8] Use of Dex adjuvant in local or neuraxial blocks for inducing analgesia or elongating the duration of sensorimotor blocks became worldwide in the past few years, yet few studies have evaluated the side effects of local use of this drug.[9,10,11,12,13] The positive effects of local Dex regarding pain management after surgery and desirable control of hemodynamic signals have been reported in some studies.[14,15,16] On the other hand, some studies have deemed its local use inefficient for some procedures such as epidural block.[17] Therefore, the present study was designed and carried out aiming to evaluate the effects of local Dex on sedation rate and hemodynamic changes in candidate patients for fiberoptic nasotracheal intubation.
Material and Methods
This randomized controlled trial was conducted in our university hospital from March 2013 to February 2015. Candidate patients for elective surgery with indications for nasotracheal intubation, aged between 18 and 65 years from both sexes with Class I and II American Society of Anesthesiologists, were considered in the study. Patients with a history of cardiac diseases, high blood pressure, consumption of blood pressure medication or agonist or antagonists of α-adrenergic receptors, those with liver and kidney diseases or history of addictive of psychotropic drug abuse or any psychiatric disease as well as pregnant women, those who had not fulfilled the fasting period (<8 h for solids and <3 h for transparent liquids), and emergency surgeries were excluded from the study. Patients with a baseline heart rate (HR) lower than 60 beats/min or atrio-ventricular block in electrocardiogram or history of allergy to α-receptor agonists and those with coagulation disorders were also excluded from the study.
Sample size was calculated based on Type I error of α = 5% and power = 80% and minimum difference d = 30%, which was clinically significant in the pilot study. Patients were divided into three groups using random number table as follows: control group: patients whose airway block was done with the aid of lidocaine with a total dose of 5 mg/kg; IV Dex group: patients who received a single dose of 1 μg/kg body weight IV Dex during 10 min in addition to a total dose of 5 mg/kg local lidocaine when undergoing airway block; local Dex group: patients whose airway block was done using a combination of lidocaine with a total dose of 5 mg/kg and local Dex with a total dose of 1 μg/kg body weight.
Due to unknown effects of Dex on trachea, glossopharyngeal, superior laryngeal, and recurrent laryngeal nerves were blocked by local anesthetic injection and intranasal anesthesia using lidocaine in all groups. Immediately after blocking and local anesthetic injection, propofol infusion pump was injected with the initial dose of 30 mg/kg body weight to adjust the cerebral state index (CSI) to 65–75. The time of reaching 75 CSI was recorded for all patients. Propofol infusion continued up to sedation induction. Fiberoptic intubation was carried out by paying attention to CSI and symptoms of airway block. In all patients, fiberoptic intubation was done by an anesthesiologist with sufficient experience. The anesthesiologist in charge of intubation, patients, anesthesiology resident, and nurse were all blinded to the drug used, and the drug provided to the team was prepared by another anesthesiologist.
Four methods are used for evaluating airway block and intubation in patients:
Coughing score ranging from 1 to 4 (1 = no cough, 2 = mild cough, 3 = moderate cough, 4 = severe cough)
Intubation score based on vocal cord movements on a 1–4 scale. (1 = open, 2 = moving, 3 = closing, 4 = closed)
Patient tolerance score during nasotracheal intubation based on a 1–5 scale of patient comfort during fiberoptic intubation (1 = no reflex from the patient, 2 = mild grimace, 3 = significant grimace, 4 = verbal complaining, 5 = defensive posture with head or hands)
Patient tolerance score immediately after nasotracheal intubation on a 1–3 scale (1 = calm and cooperative, 2 = restless, 3 = complete resistance and in need of rapid general anesthesia).
Blood pressure, HR, electrocardiogram, arterial blood O2 saturation (SpO2), and CSI condition were monitored in all patients during and after airway blocking and then for every 2 min until general anesthesia. For blinding the procedure, the one who performed the local airway block was different than the one who assessed the mentioned outcomes. The assistant controlled drug infusion and condition of the patients and the nurse controlled and recorded the hemodynamic status of the patients and their SpO2.
All quantitative variables are reported as mean and standard deviation, and qualitative variables are listed as number (percentage). Kolmogorov–Smirnov test and Q-Q plot were used to assess normal distribution of data. To evaluate the difference between groups, ANOVA, Kruskal–Wallis test, Chi-square, and Fisher's exact test were used. Linear mixed model was used to evaluate the difference between the groups throughout the study. In all these evaluations, multiple comparisons were corrected by Bonferroni method. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. P < 0.05 was considered statistically significant.
The protocol of this study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran. Patients were informed about the study details and they signed a written consent. All authors declared their adherence to the Helsinki Declaration ethical principles for medical research involving human beings throughout the study. We followed the CONSORT statement guidelines during preparation and reporting of this randomized controlled trial. The methodology of this trial was registered on www.IRCT.IR under the registration number IRCT2015101224493N1.
Results
A total of 95 patients with the mean age of 45.4 ± 6.7 years were evaluated in this study, 52 (54.2%) of which were females. Table 1 shows baseline and demographic data of the studied patients, which demonstrates that the three groups were not significantly different regarding demographic characteristics such as age, sex, weight, and hemodynamic variables including systolic blood pressure (SBP) and diastolic blood pressure, HR, and SpO2.
Table 1.
Baseline characteristics of participants
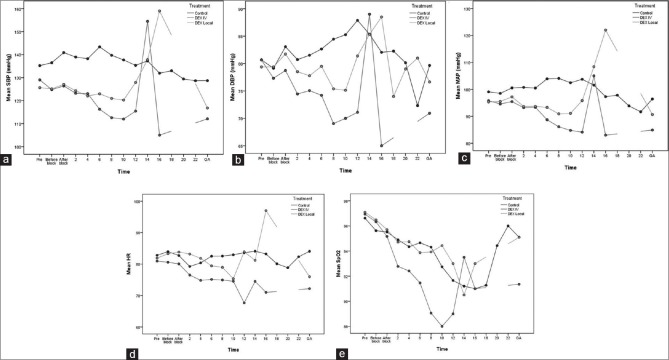
Evaluation of SBP showed that, after adjustment for baseline blood pressure, there is a significant difference between the groups during the study (P < 0.001). In addition, after multi-purpose analysis with Bonferroni test, SBP was significantly higher in the control group compared to IV Dex (difference = 12.4, 95% confidence interval [CI]: 8.9–15.9, P < 0.001) and local Dex (difference = 8.1, 95% CI: 4.7–11.5, P < 0.001) groups. The difference between local and IV Dex groups was also significant (difference = 4.2, 95% CI: 0.8–7.7, P = 0.017) [Figure 1a].
Figure 1.
Linear Mixed model showed the difference between the groups regarding the mean systolic blood pressure (a), diastolic blood pressure (b), mean arterial pressure (c), heart rate (d) and SpO2 (e) after adjustment for the baseline values during the study periods
Assessing diastolic blood pressure revealed that, after adjustment for baseline blood pressure, diastolic blood pressure is significantly different between groups (P < 0.001). In addition, after multi-purpose analysis with Bonferroni test, diastolic blood pressure in the control group was significantly higher than IV Dex group (difference = 6, 95% CI: 4–9, P < 0.001). However, the difference between control and local Dex groups was not significant (difference = 2.3, 95% CI: −0.1–4.6, P = 0.053). There was also a significant difference between local and IV Dex groups regarding diastolic blood pressure (difference = 4, 95% CI: 2–7, P = 0.017) [Figure 1b].
Mean arterial pressure (MAP) evaluation showed that, after adjustment for baseline blood pressure, MAP is significantly different between groups (P < 0.001). In addition, after multi-purpose analysis with Bonferroni test, MAP was significantly higher in the control group compared to IV Dex (difference = 7.3, 95% CI: 4.4–10.0, P < 0.001) and local Dex (difference = 4.7, 95% CI: 2.0–7.4, P = 0.001) groups. However, local and IV Dex groups were not different in this regard (difference = 2.5, 95% CI: −0.3–5.3, P = 0.063) [Figure 1c].
HR assessment revealed a significant difference between groups over the course of the study after adjustment for baseline HR (P < 0.001). In addition, after multi-purpose analysis with Bonferroni test, HR in the control group was significantly higher than IV Dex group (difference = 4.0, 95% CI: 2.0–6.0, P < 0.001). However, the difference between control and local Dex groups was not significant (difference = 0.9, 95% CI: −1.1–2.8, P = 0.382). There was also a significant difference between local and IV Dex groups regarding HR (difference = 3.1, 95% CI: 1.1–5.1, P = 0.002), and in IV Dex group, HR was significantly lower than local Dex group [Figure 1d].
Evaluation of SpO2 revealed that, after adjustment for baseline SpO2, SpO2 is significantly different between groups (P < 0.001). In addition, after multi-purpose analysis with Bonferroni test, SpO2 in the control group was significantly higher than IV Dex group (difference = 1.4, 95% CI: 0.8–2.0, P < 0.001). However, the difference between control and local Dex groups was not significant (difference = −0.1, 95% CI: −0.7–0.25, P = 0.723). SpO2 of IV Dex group was significantly lower than local Dex group (difference = 1.5, 95% CI: 0.9–2.1, P < 0.001) [Figure 1e].
Comparison between the three groups showed that the dose of propofol used for reaching a 65–75 CSI before sedation induction was significantly higher in the control group compared to IV and local Dex groups (P < 0.001) [Figure 2]. There was no significant statistical difference in propofol consumption between local and IV Dex groups.
Figure 2.
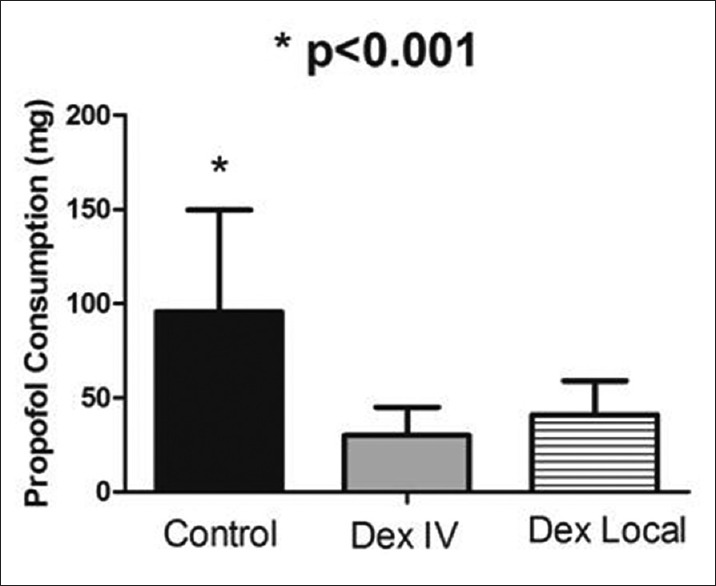
Propofol consumption to achieve cerebral state index: 65–75
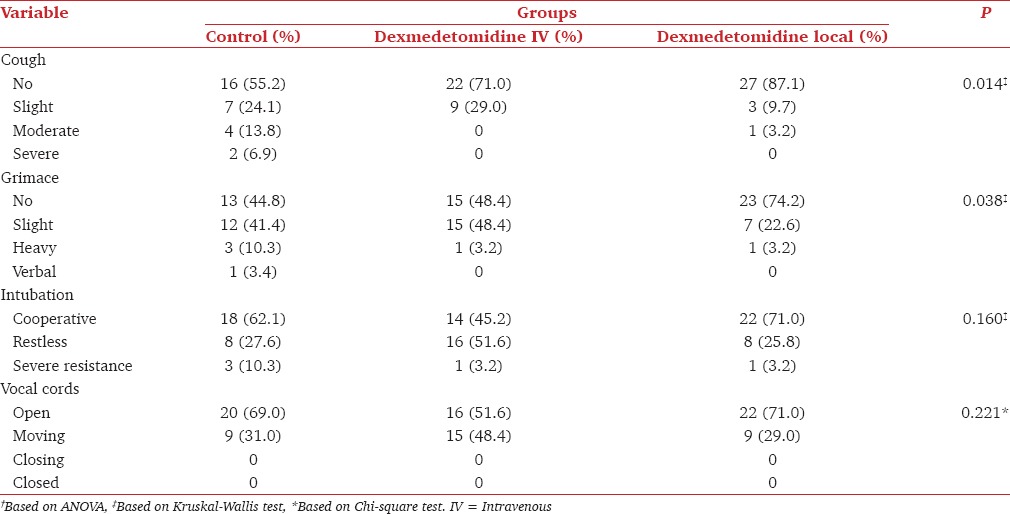
The number of patients who did not cough during fiberoptic intubation was significantly higher in local Dex group compared to both IV Dex and control groups (P = 0.014). The number of patients who did not show grimace was significantly higher in local Dex group in comparison with IV Dex and control groups (P = 0.038). The number of patients who were cooperative during intubation was higher in local Dex group compared to IV Dex and control groups; however, the difference was not statistically significant. The number of patients whose vocal cords were open during intubation was also higher in local Dex group compared to IV Dex and control groups, but the difference was not statistically significant [Table 2].
Table 2.
Scoring for evaluating airway block and intubation in patients
Discussion
Findings of the present study showed that during awake intubation under fiberoptic bronchoscopy, local Dex is more efficient in controlling hemodynamics and improving the quality of intubation compared to local lidocaine alone (control group).
Local and IV Dex groups preserve hemodynamic values better than control group. Local Dex like IV Dex could result in propofol dose saving during the procedure. As far as we know, there is no similar clinical trial in literature that assessed the efficacy of local Dex in same situation. However, the findings are compatible with previous studies that all just assessed the IV form of Dex.[18,19] Mean HR and diastolic blood pressure in IV Dex group were lower than the other two groups, which is obviously due to bradycardia caused by systemic Dex effect. Although Dex can stabilize hemodynamics, in patients with hypovolemia and heart block, it should be used cautiously.[20]
IV Dex injection for facilitating fiberoptic intubation was studied frequently, and most of researches stated the ease of patient care and patient satisfaction during and after the procedures.[21,22] Moreover, it could be said that due to low systemic uptake, the effect of local Dex is different from that of IV Dex. In fact, local Dex with more powerful local anesthesia and low systemic uptake not only stabilizes hemodynamics better but also improves the quality of intubation. Unlike IV Dex, local Dex does not have a hypnotic effect, does not decrease O2 saturation during fiberoptic bronchoscopy, and enables awake intubation with beneficial local effects.[14] Local Dex was used and studied in different procedures such as septoplasty, third molar surgery, and peripheral nerve blockade, and usually reported as a proper option with less adverse effects than IV administration of this agent.[14,23,24]
The superiority of local Dex in decreasing the required propofol dose needed for sedating the patients in comparison with control group was another interesting finding of the present study, which had also been noted in some previous studies for IV Dex.[5] This study also revealed that local Dex improves intubation score and decreases coughing compared to control and IV Dex groups. Most of the previous studies have shown a better quality of awake intubation under fiberoptic bronchoscopy after IV Dex administration, but this study is the first one to evaluate local Dex in this procedure.[21,22,25,26]
Conclusion
It is likely that using local Dex during fiberoptic bronchoscopy decreases sudden changes in hemodynamic values and decreases coughing and improves patient tolerance and intubation scores. Local Dex can be as useful as IV form with the aim of propofol dose saving. These effects of local Dex take place without causing side effects such as decreasing SpO2 and bradycardia.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. It should be mentioned that this study was a part of Dr. Parisa Sezari's thesis as an anesthesiology resident at Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Kundra P, Kutralam S, Ravishankar M. Local anaesthesia for awake fibreoptic nasotracheal intubation. Acta Anaesthesiol Scand. 2000;44:511–6. doi: 10.1034/j.1399-6576.2000.00503.x. [DOI] [PubMed] [Google Scholar]
- 2.Goksu S, Arik H, Demiryurek S, Mumbuc S, Oner U, Demiryurek AT. Effects of dexmedetomidine infusion in patients undergoing functional endoscopic sinus surgery under local anaesthesia. Eur J Anaesthesiol. 2008;25:22–8. doi: 10.1017/S0265021507001317. [DOI] [PubMed] [Google Scholar]
- 3.Alimohammadi H, Baratloo A, Abdalvand A, Rouhipour A, Safari S. Effects of pain relief on arterial blood o2 saturation. Trauma Mon. 2014;19:e14034. doi: 10.5812/traumamon.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta K, Jain M, Gupta PK, Rastogi B, Saxena SK, Manngo A. Dexmedetomidine premedication for fiberoptic intubation in patients of temporomandibular joint ankylosis: A randomized clinical trial. Saudi J Anaesth. 2012;6:219–23. doi: 10.4103/1658-354X.101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knolle E, Oehmke MJ, Gustorff B, Hellwagner K, Kress HG. Target-controlled infusion of propofol for fibreoptic intubation. Eur J Anaesthesiol. 2003;20:565–9. doi: 10.1017/s0265021503000905. [DOI] [PubMed] [Google Scholar]
- 6.Rai MR, Parry TM, Dombrovskis A, Warner OJ. Remifentanil target-controlled infusion vs. propofol target-controlled infusion for conscious sedation for awake fibreoptic intubation: A double-blinded randomized controlled trial. Br J Anaesth. 2008;100:125–30. doi: 10.1093/bja/aem279. [DOI] [PubMed] [Google Scholar]
- 7.Stamenkovic DM, Hassid M. Dexmedetomidine for fiberoptic intubation of a patient with severe mental retardation and atlantoaxial instability. Acta Anaesthesiol Scand. 2006;50:1314–5. doi: 10.1111/j.1399-6576.2006.01157.x. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 9.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, Aggarwal R, Gupta P. Dexmedetomidine prolongs the effect of bupivacaine in supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2014;30:36–40. doi: 10.4103/0970-9185.125701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115:958–62. doi: 10.1213/ANE.0b013e318265bab7. [DOI] [PubMed] [Google Scholar]
- 12.Elhakim M, Abdelhamid D, Abdelfattach H, Magdy H, Elsayed A, Elshafei M. Effect of epidural dexmedetomidine on intraoperative awareness and post-operative pain after one-lung ventilation. Acta Anaesthesiol Scand. 2010;54:703–9. doi: 10.1111/j.1399-6576.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- 13.Abdalla MI, Al Mansouri F, Bener A. Dexmedetomidine during local anesthesia. J Anesth. 2006;20:54–6. doi: 10.1007/s00540-005-0351-z. [DOI] [PubMed] [Google Scholar]
- 14.Cheung CW, Ng KF, Choi WS, Chiu WK, Ying CL, Irwin MG. Evaluation of the analgesic efficacy of local dexmedetomidine application. Clin J Pain. 2011;27:377–82. doi: 10.1097/AJP.0b013e318208c8c5. [DOI] [PubMed] [Google Scholar]
- 15.Cheung CW, Ng KF, Liu J, Yuen MY, Ho MH, Irwin MG. Analgesic and sedative effects of intranasal dexmedetomidine in third molar surgery under local anaesthesia. Br J Anaesth. 2011;107:430–7. doi: 10.1093/bja/aer164. [DOI] [PubMed] [Google Scholar]
- 16.Paul S, Bhattacharjee DP, Ghosh S, Dawn S, Chatterjee N. Efficacy of intra-articular dexmedetomidine for postoperative analgesia in arthroscopic knee surgery. Ceylon Med J. 2010;55:111–5. doi: 10.4038/cmj.v55i4.2627. [DOI] [PubMed] [Google Scholar]
- 17.Konakci S, Adanir T, Yilmaz G, Rezanko T. The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol. 2008;25:403–9. doi: 10.1017/S0265021507003079. [DOI] [PubMed] [Google Scholar]
- 18.Mondal S, Ghosh S, Bhattacharya S, Choudhury B, Mallick S, Prasad A. Comparison between dexmedetomidine and fentanyl on intubation conditions during awake fiberoptic bronchoscopy: A randomized double-blind prospective study. J Anaesthesiol Clin Pharmacol. 2015;31:212–6. doi: 10.4103/0970-9185.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harsha MS, Vishal P. A comparative study of fentanyl with midazolam versus dexmedetomidine for the attenuation of hemodynamic response during laryngoscopy and intubation. Indian J Appl Res. 2016;6:361–4. [Google Scholar]
- 20.Piao G, Wu J. Systematic assessment of dexmedetomidine as an anesthetic agent: A meta-analysis of randomized controlled trials. Arch Med Sci. 2014;10:19–24. doi: 10.5114/aoms.2014.40730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu R, Liu JX, Jiang H. Dexmedetomidine versus remifentanil sedation during awake fiberoptic nasotracheal intubation: A double-blinded randomized controlled trial. J Anesth. 2013;27:211–7. doi: 10.1007/s00540-012-1499-y. [DOI] [PubMed] [Google Scholar]
- 22.Madhere M, Vangura D, Saidov A. Dexmedetomidine as sole agent for awake fiberoptic intubation in a patient with local anesthetic allergy. J Anesth. 2011;25:592–4. doi: 10.1007/s00540-011-1154-z. [DOI] [PubMed] [Google Scholar]
- 23.Dogan R, Erbek S, Gonencer HH, Erbek HS, Isbilen C, Arslan G. Comparison of local anaesthesia with dexmedetomidine sedation and general anaesthesia during septoplasty. Eur J Anaesthesiol. 2010;27:960–964. doi: 10.1097/EJA.0b013e32833a45c4. [DOI] [PubMed] [Google Scholar]
- 24.Brummett CM, Williams BA. Additives to local anesthetics for peripheral nerve blockade. Int Anesthesiol Clin. 2011;49:104–16. doi: 10.1097/AIA.0b013e31820e4a49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu HH, Zhou T, Wei JQ, Ma WH. Comparison between remifentanil and dexmedetomidine for sedation during modified awake fiberoptic intubation. Exp Ther Med. 2015;9:1259–64. doi: 10.3892/etm.2015.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avitsian R, Lin J, Lotto M, Ebrahim Z. Dexmedetomidine and awake fiberoptic intubation for possible cervical spine myelopathy: A clinical series. J Neurosurg Anesthesiol. 2005;17:97–9. doi: 10.1097/01.ana.0000161268.01279.ba. [DOI] [PubMed] [Google Scholar]