Abstract
Hearing loss is common in childhood cancer survivors exposed to platinum chemotherapy and/or cranial radiation and can severely impact quality of life. Early detection and appropriate management can mitigate academic, speech, language, social, and psychological morbidity resulting from hearing deficits. This review is targeted as a resource for providers involved in after-care of childhood cancers. The goal is to promote early identification of survivors at-risk for hearing loss, appropriate evaluation and interpretation of diagnostic tests, timely referral to an audiologist when indicated, and to increase knowledge of current therapeutic options.
Keywords: cancer survivor, hearing loss, ototoxicity, late effects
Introduction
Hearing loss (HL) is a common permanent sequela following therapy with platinum chemotherapy and/or cranial radiation in pediatric malignancies. Diagnoses routinely managed with platinum include neuroblastoma, hepatoblastoma, germ cell tumors, osteosarcoma, and certain brain tumors. Approximately 70% of children exposed to platinum agents develop HL, particularly when the cumulative cisplatin dose exceeds 400 mg/m2. [1–4] About one-half of affected patients have moderate to severe HL (grade 3 or 4 ototoxicity per the Common Terminology Criteria for Adverse Events, version 3 or Brock ototoxicity scales), necessitating hearing aids or other interventions. [2,3,5,6]
Hearing impairment has been associated with significant morbidity involving psychological, social, vocational, academic, and health-related outcomes. [7–10] Morbidity is greater when HL remains undetected and/or untreated, particularly for the developing child. [11–13] In addition, therapy-related ototoxicity can initially arise or progress years after completion of treatment. [1,5,14–18] Thus, at-risk populations need long-term follow-up of audiological function. The Children’s Oncology Group (COG) Late Effects Committee provides recommendations for surveillance of survivors at risk for HL after completion of therapy (http://www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf ; section 20: platinum-associated ototoxicity; sections 66 and 67: radiation-induced ototoxicity; Supplemental Figures 1 and 2). [19,20] In an effort to develop consistent guidelines for surveillance among survivors, the COG has recently initiated several collaborations with international organizations; the goal is to develop standard, internationally accepted guidelines for long-term follow-up for common toxicities observed in childhood cancer survivors.
During cancer-directed therapy, adherence to auditory monitoring is typically dictated by the treatment protocol. After completion of treatment, survivors may follow up with their primary care provider or pediatric oncology program. However, the survivor may not always receive recommended post-therapy screening. Reasons for inadequate audiological monitoring of at-risk survivors may include 1) loss to follow-up or 2) poor understanding of at-risk populations and lack of recommended surveillance by managing providers. An additional factor that may handicap healthcare providers is insufficient understanding of how to interpret audiological test results or management options.
This manuscript, written by the COG Long-Term Follow-Up Guidelines Task Force on Auditory Complications, targets healthcare providers who care for survivors of childhood, adolescent, and young-adult cancers. The objective is to provide education and a resource for 1) identifying survivors at risk for HL, 2) facilitating appropriate hearing screening, 3) understanding and interpreting commonly employed auditory tests, 4) implementing timely referrals to an audiologist, and 5) gaining a basic knowledge of HL management. The hope is that this knowledge will allow providers to not only identify and refer at-risk patients, but also empower providers to have a more meaningful conversation with patients about test results and management options.
Impact of HL
The functional impact of HL is affected by its severity and other factors. Speech and language development may be particularly affected when HL is acquired in early childhood [11–13]. The frequencies most important for understanding speech are 500–3,000 Hz. In young children, however, ability to hear frequencies between 4,000–8,000 Hz is also vital for proper language acquisition and speech development, since auditory and language processing is not yet mature and young children do not have the language base to “fill in the gaps” when portions of speech are not perceived. [21,22] Even mild or unilateral HL can delay academic, language, and social achievement in children. [9,10,23] Childhood survivors of neuroblastoma with high-frequency HL are twice more likely to have difficulties in reading, math, and/or attention, a greater need for special educational services, and an overall poorer quality of life compared to neuroblastoma survivors with normal hearing. [7] Similarly, significant HL (e.g., requiring the use of hearing aids) in survivors of childhood medulloblastoma is associated with declines in cognition and academic attainment. [8] Early detection and intervention of HL is critical in young children to help minimize the negative consequences of HL on speech, language, academic, and psychosocial outcomes [11–13].
In older children and adults, language acquisition may not be an issue, but they are still at risk for reduced educational achievement, social isolation, emotional difficulties, and poorer health-related quality of life when hearing is affected. [7,9] Furthermore, in the adolescent and adult populations, HL is associated with depression, underemployment and reduced earnings. [24–26] Adult survivors of childhood cancer with significant HL more often report a perceived negative impact on social functioning, not living independently, never marrying, and not graduating high school or being underemployed compared to survivors without significant HL. [27] The functional impact of mild, unilateral, and/or high-frequency HL may not be easily recognized by caregivers, teachers, significant others, or health care providers, further supporting the need for timely audiological evaluation and follow-up in at-risk survivors.
Categorization of HL
The human ear is functionally divided into 4 sections: the outer ear, middle ear, inner ear, and auditory nervous system. Conductive HL results from pathology of the outer and/or middle ear system (e.g., impaired Eustachian tube function with middle ear fluid accumulation). Middle ear disease may be transient or refractory (e.g., when radiation-associated mucosal damage results in persistent middle ear fluid or adhesive otitis). [28] Sensorineural HL results from pathology involving the cochlea and/or auditory nervous system. Platinum chemotherapy is ototoxic primarily to the cochlea; the cochlea is also the most sensitive structure in the auditory apparatus to radiation. The term mixed HL is used when there is a combination of conductive and sensorineural pathologies.
Ototoxic agents
Radiation-associated HL
Clinical studies show that a threshold cochlear dose exceeding 30 gray (Gy) can result in HL from radiation alone. [14,29] Reported incidence of HL following isolated exposure to cranial radiation above 30–35 Gy is low at 10–14%. [14,30] However, higher radiation doses [14] or exposure to concomitant cisplatin significantly increases the occurrence as well as severity of HL. [30,31] Radiation exposure can also affect function of the outer or middle ear. Hence, radiation-associated HL can be conductive, sensorineural or of mixed type. [1,16] The frequency of impairment from radiation-associated sensorineural HL is variable and can affect low, mid, or high frequencies although higher frequencies tend to be preferentially affected. [14,32] Deficits can occur months to years following treatment and may be progressive with time. [14,16] Hence, children exposed to ≥30 Gy of cochlear radiation require long-term audiological follow-up. [20] (Supplemental Figure 2)
Radiation delivery techniques with improved conformity result in lower scatter outside of the target zone. [33–35] In patients with medulloblastoma, 3D intensity-modulated and proton beam radiation therapy resulted in lower dosimetry to the cochlea when compared to conventional radiation techniques. [33,35] While long-term audiological outcomes data are awaited, these newer techniques show promise for decreased incidence and severity of permanent HL in children with brain tumors.
Platinum-associated HL
Ototoxicity is a common dose-limiting adverse outcome associated with platinum agents. [1,2,16] Ototoxicity results specifically from degeneration of cochlear inner and outer hair cells, which once damaged, cannot regenerate. [16] Of the two ototoxic platinum agents, cisplatin and carboplatin, cisplatin is more ototoxic in standard dosing regimens. [1,16,36,37] Carboplatin has also been implicated in auditory damage, but in a specific settings. [6,38–40] Although infants treated with traditional dose carboplatin may also be at risk for developing HL, [40] this agent is more widely implicated in ototoxicity following its use in myeloablative regimens (e.g., in treatment of patients with neuroblastoma, particularly when carboplatin exposure occurs in the setting of prior cisplatin exposure). [6,38,39]
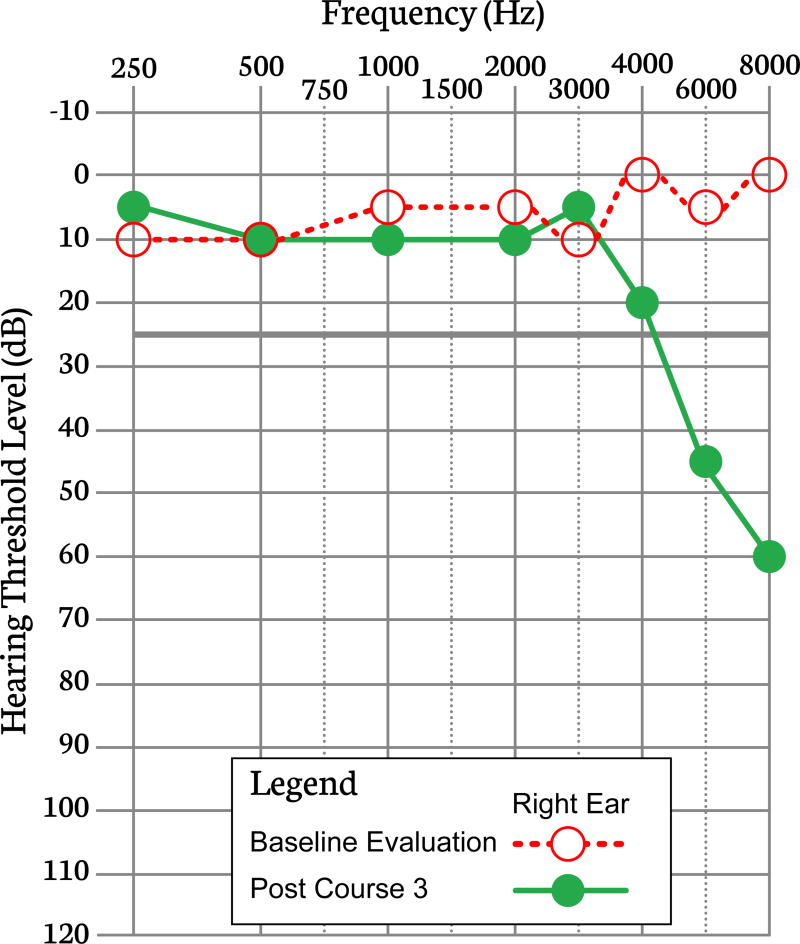
Hearing loss from platinum exposure is typically bilateral, sensorineural, and permanent. [16] Overt HL may be preceded by tinnitus and/or difficulty hearing in the presence of background noise. The deficit first develops in the higher frequencies (>4,000 Hz), but with increasing cumulative exposure can progress to involve lower frequencies (500–4,000 Hz) which are most significant for understanding speech (Figure 1). Some reports also suggest that HL can be progressive after completion of treatment, and at times this worsening can occur years later. [5,14,15,17,18,41] One study concluded that only patients who have some degree of HL at the end of therapy are at risk for ongoing deterioration after cessation of therapy. [17] At present, there is insufficient evidence to make strict recommendations on duration of surveillance; however, audiology organizations, such as the American Speech-Language-Hearing Association and the American Academy of Audiology, recommend that any individual with HL be evaluated annually. In addition to monitoring hearing sensitivity, these visits allow evaluation of hearing technology, review of the patient’s current communication needs/challenges, and an opportunity to counsel about hearing protection/preservation.
Figure 1.
This figure illustrates a decrease in hearing thresholds from baseline for the high-frequency range 4000–8000 Hz commonly observed following cisplatin therapy (thresholds from only one ear are displayed).
Risk factors for developing HL
Cumulative cisplatin exposure exceeding 400 mg/m2 and younger age are the most significant risk factors for ototoxicity. [1,2,16,38,42] Cisplatin therapy combined with cranial radiation (as used in management of certain brain tumors) is associated with higher incidence and severity of HL as compared to single modality exposure. [1,2,16,30,31,43–45] Concomitant administration of other ototoxic agents, such as loop diuretics or aminoglycosides, and presence of renal impairment, can potentially amplify platinum toxicity. [1,16]
Methods for evaluating hearing
Regardless of a patient’s age, medical condition, or developmental status, a comprehensive evaluation of hearing is possible. Audiologic assessments employ a battery of tests that include behavioral evaluation, physiologic assessment, and electrophysiological measurement of the auditory system as indicated (Table 1).
Table I.
Summary of basic panel of audiometric tests used in evaluation of survivors. An appropriate audiology clinic should ideally offer the tests listed below.
| Core tests | ||
|---|---|---|
| Test | Function | Comments |
| PTA*¥ | Evaluates nature (conductive vs. sensorineural), frequency (Hz), and severity (decibel) of hearing loss |
|
| Speech Audiometry*¥ | Evaluates functional hearing (speech awareness and comprehension) |
|
| Tympanometry¥¥ | Evaluates middle ear function |
|
| OAE¥¥ | Evaluates cochlear function (cochlear outer hair cells) across many frequencies |
|
| Alternative test | ||
| ABR*¥¥ | Evaluates auditory neurological pathway from VIIIth cranial nerve to brainstem, which can be used to estimate peripheral hearing sensitivity |
|
Abbreviations: PTA, pure tone audiometry; OAE, otoacoustic emissions; ABR, auditory brainstem response.
Symbols:
Pediatric specific test material/equipment and/or expertise may be required;
Behavioral test (requires active patient participation);
Active patient participation not required, but patient should be able to stay still as movement may degrade results
Pure tone audiometry
Behavioral assessments, which require active participation of the individual, include pure tone audiometry and speech audiometry. Pure tone audiometry is the test of choice to monitor patients for ototoxicity both during treatment and also for late-onset or progressive HL in survivors. While pure tone audiometry can measure hearing at frequencies from 250–20,000 Hz, hearing thresholds are routinely measured from 250–8,000 Hz, the frequency range most relevant for speech perception and recognition.
Auditory thresholds (softest sound intensity level at which a tone is detected) are measured by asking the patient to provide a behavioral response, such as pressing a button or raising a hand, when the patient detects the tone. The behavioral method used to measure pure tone thresholds varies depending on patient age and development. Children aged 24 months to 5 or 6 years are usually evaluated with conditioned play audiometry; the child is taught to perform an action (such as placing a ring on a peg, placing an object in a container) whenever a tone is heard. Children between ages 7–8 months and 24–30 months are evaluated with visual reinforcement audiometry (e.g. responding to sounds with a head turn toward a reinforcing toy that will light up, dance, or make similar movements).
For air conduction measurements, tones are transmitted to the ear via earphones or headphones; these allow individual ears to be independently tested. Sound-field speakers can be used when a child will not tolerate earphones/headphones. However, sound-field testing does not evaluate hearing of each ear separately, and hence does not reliably detect asymmetrical or unilateral HL. Also, sound-field testing is often limited to testing 500–4,000 Hz and may miss ototoxicity at higher frequencies. Overall, results of air conduction analysis reflect hearing sensitivity of the entire auditory system.
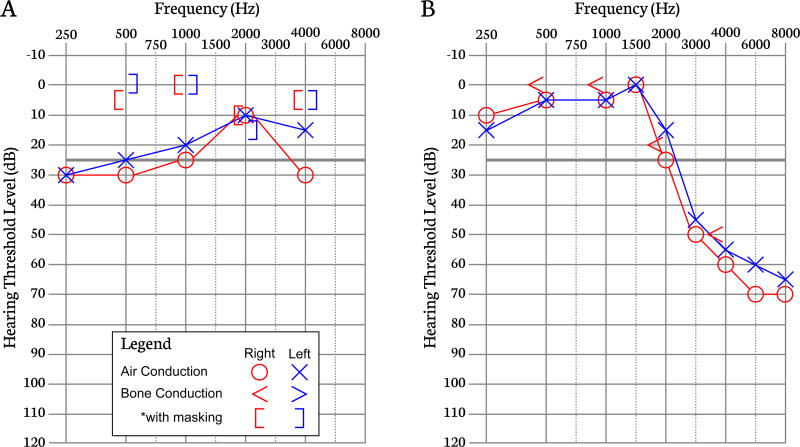
Bone conduction hearing thresholds are measured with a bone conduction oscillator placed on the mastoid process or forehead. This method evaluates hearing sensitivity of the inner ear and auditory nervous system (or sensorineural system) only, bypassing the outer and middle ear systems. Comparison of air and bone conduction thresholds determines the type of HL (sensorineural vs. conductive) (Figure 2A and 2B).
Figure 2.
Figure 2A. Audiogram illustrating a bilateral conductive hearing loss, which is diagnosed when air conduction thresholds are >10 dBHL worse than bone conduction thresholds and bone conduction thresholds fall within the normal hearing range but air conduction thresholds do not. In cases of conductive hearing losses, the etiology of the hearing loss lies in the outer or middle ear while the inner ear remains intact. *As bone conduction is perceived in both ears, masking noise is presented to the non-test ear to evaluate bone conduction only in the test ear. Figure 2B. Audiogram demonstrating a bilateral high-frequency sensorineural hearing loss. In the absence of a conductive impairment, the difference between air and bone conduction thresholds is <10 dBHL. This audiogram suggests damage to the inner ear in the presence of a normally functioning outer and middle ear.
Speech audiometry
Speech audiometry assesses an individual’s ability to hear and understand speech and is usually measured by asking the patient to verbally repeat words and/or sentences. Young children may point to a picture or object that represents the target word. Speech audiometry is most commonly performed in a quiet environment, which may underestimate the patient’s speech understanding in real life situations. For example, patients with high-frequency HL complain of disproportionate hearing difficulty in noisy environments. [46,47] Hence, incorporating speech-in-noise testing into the audiological test battery provides a better functional evaluation of a patient’s ability to perceive and discriminate speech in a more realistic environment.
Physiologic assessments
Physiologic assessments such as tympanometry and otoacoustic emissions (OAEs) evaluate the physiologic function of the auditory system. These tests do not require active participation by the patient. Tympanometry is an objective measurement of outer and middle ear function and assists in determining if a patient has a conductive pathology, (e.g., middle ear fluid). Tympanometry is performed by placing a probe into the ear canal and measuring changes in the transmission and reflection of sound throughout the middle ear system. Conductive pathology can complicate interpretation of audiological results that rely on air conduction responses such as pure tone audiometry, speech audiometry, and OAEs. Hence, tympanometry adds vital information for the audiologist.
The OAE measurement specifically evaluates cochlear outer hair cell function. When sound stimulates outer hair cells, these cells elongate and contract, producing vibrations that are reflected back as sound to the middle and outer ear; this can be measured with a small probe inserted into the ear canal. OAEs are only generated by healthy cochlear outer hair cells. Hence, they are typically present at frequencies where hearing thresholds are within the normal to near-normal hearing range. Two types of evoked OAEs are used clinically, distortion product OAEs and transient evoked OAEs. Distortion product OAEs are capable of measuring a higher frequency range and are more sensitive to early ototoxic changes compared to transient evoked OAEs. [48]
Since OAEs rely on transmission of sounds from the cochlea back to a probe in the outer ear, OAEs cannot be reliably measured in the presence of middle ear fluid or cerumen impaction because these interfere with the detection of cochlear emissions. The OAE measurement requires the patient to be in a relatively quiet state, but the patient does not have to actively participate. Unlike pure tone audiometry, OAEs cannot fully determine or estimate severity of HL. On the other hand, abnormalities in OAEs may be evident even before HL is detected by pure tone audiometry. [49,50] In survivors, OAEs may be used to cross-check behavioral test results or as a screening tool when pure tone audiometry is not feasible.
Electrophysiological assessments such as the auditory brainstem response (ABR) assess neurologic function of the auditory pathway from the VIIIth cranial nerve to the lower brainstem in response to sound and, thus, can provide an estimation of peripheral hearing sensitivity. These techniques are utilized when behavioral testing is not possible due to young age, development, cooperation, or medical condition. To perform the procedure, electrodes are applied to the head and sounds are presented through earphones placed in the ear canals. The ABR response is a series of waveforms, representing functioning at the sequential anatomy of the auditory pathway. ABR also reflects the function of the middle ear and cochlea because the auditory signal passes through these systems first. Thus, air and bone conduction ABR can determine the nature of HL. ABR is also used to identify pathologies of the auditory nerve and/or auditory brainstem pathways. ABR testing can be conducted during natural sleep in infants, or with sedation because movement degrades the measurement of responses from the auditory system.
Although audiologic evaluations are fairly standardized, clinical audiologists can tailor each assessment based on an individual patient’s characteristics, audiologic history, and test results. The technique used for behavioral evaluation is selected based on the patient’s age and developmental stage. Physiologic and electrophysiologic evaluations can augment behavioral audiometry and are useful when complete behavioral testing is not possible.
Management of HL
When a patient has significant HL, using technology to improve hearing can be beneficial. Although hearing aids cannot restore hearing to normal, they can enhance hearing and speech comprehension by amplifying and modifying external sounds. Results from a nationwide survey of hearing-impaired individuals revealed significant improvement in social, emotional, psychological, and physical function among individuals who wore hearing aids compared to those who did not. [51]
The main objective of audiological intervention is to improve the individual’s ability to hear and recognize speech in a variety of real life situations. Hence, intervention(s) should aim to optimize comprehension of soft speech, distant speech, and speech in background noise as well as when listening to music and talking on the phone.
Because hearing deficits can vary from patient to patient, hearing aids can be tailored to target an individual’s specific HL needs. For instance, hearing aids for patients with exclusively high-frequency HL are programmed to provide amplification to high frequencies and not to the low frequencies where the patient hears normally and amplification would be counterproductive. In addition, hearing aids come in a variety of shapes, sizes, external visibility and colors to meet individual aesthetic and lifestyle needs. An overview of different types of hearing aids is provided in Table II.
Table II.
Hearing Aids: devices worn inside or outside the ear that electroacoustically modify and amplify sound for the hearing impaired. They are available in different styles and sizes depending on hearing loss severity and patient preference.
| Type | Description | Pros | Cons | Appropriate for |
|---|---|---|---|---|
| Lyric™ | Fits completely inside the ear canal and is inserted by a hearing professional |
|
|
Adults with mild to moderately-severe hearing loss |
| Invisible in-the-canal (IIC) | Fits completely in the canal |
|
|
Older teens and adults with mild to moderate hearing loss |
| Completely-in-the-canal (CIC) | Fits inside the ear canal, not as deeply as the IIC. |
|
|
Older teens and adults with mild to moderate hearing loss |
| In-the-canal (ITC) | A little larger than the CIC style, fitting partially in the ear canal but not as deeply as the CIC |
|
|
Older teens and adults with mild to moderate hearing loss |
| In-the-ear (ITE) | The largest of the ITC/ITE hearing aids, the full-shell style fills the bowl area of the outer ear |
|
|
Older teens and adults with mild to severe hearing loss |
| Behind-the-ear (BTE) | The largest of the hearing aid models. The hearing aid fits behind the ear and is coupled to a custom made earmold that is placed in the ear |
|
|
All ages and almost all types and severity of hearing loss |
| Mini or open-fit BTE | Typically a smaller BTE, although some larger BTEs can be modified to accommodate an open-fit style. The open-fit BTE is coupled to a slim tube and small dome that sits in the ear canal. |
|
|
Older children, teens and adults with mild to moderate hearing loss |
| Receiver-in-canal (RIC) | The smallest BTE available. The RIC consists of a tiny BTE hearing aid coupled to a wire with a small dome covering the receiver that sits in the ear canal. |
|
|
Teens and adults with mild to moderate hearing loss |
Extra features such as a telecoil, wireless connectivity, FM compatibility, and water resistance vary depending on the style of hearing aid. These extra features are typically unavailable in the smaller, more discreet hearing aid styles due to small size.
Cochlear implants are an option for patients with bilateral severe to profound sensorineural HL not correctable by hearing aids. They can be inserted either unilaterally or bilaterally as outpatient surgeries. Cochlear implant surgeries are more commonly performed for, and have become standard of care for children born with congenital deafness, with approximately 8,000 cochlear implants performed per year in the United States. The hybrid cochlear implant was recently approved by the Federal Drug Administration (FDA) in 2014 for patients aged ≥18 years. The hybrid implant can potentially restore hearing and speech perception for patients with normal/near normal low-frequency hearing who have severe to profound mid- to high-frequency HL, and do not benefit from conventional hearing aids. Implantable devices are reviewed in Table III. Overall, these devices manipulate sound differently than hearing aids (e.g., a cochlear implant changes acoustic energy into electrical pulses to directly stimulate neural pathways) and require surgery for placement that places the patient at additional risk. They are regulated and approved by the FDA for certain age groups and severities of HL.
Table III.
Implantable Devices: partially or totally implantable hearing devices, typically recommended for individuals with extreme or atypical hearing loss who cannot wear or benefit from conventional hearing aids.
| Type | Description | Pros | Cons | Appropriate for |
|---|---|---|---|---|
| Cochlear implant | An electronic hearing device that consists of an electrode array inserted into the cochlea to directly stimulate neural pathways. The external component consists of a microphone, sound processor and transmitter, which communicates with the internal device by a magnet positioned behind the ear. |
|
|
Children and adults diagnosed with severe to profound deafness who do not benefit from conventional hearing aids. |
| Hybrid Cochlear Implant | An amplification system combining acoustic amplification via a hearing aid worn behind the ear with electrical amplification delivered via a cochlear implant. |
|
|
FDA approved (2014) for adults aged ≥18 years with normal to moderate low- frequency hearing loss and severe to profound mid to high-frequency hearing loss who do not benefit from conventional hearing aid use. |
| Osseo-integrated cochlear stimulators (bone conduction hearing devices) | A hearing aid system consisting of either: 1) a titanium fixture implanted into the skull behind the ear. A percutaneous abutment connects the sound processor to the titanium fixture, or 2) a sound processor held in placed by an implanted magnet (abutment free) Sound travels through the bone to stimulate the cochlea, bypassing the outer and middle ear spaces. |
|
|
Surgical implant is approved for age ≥5 years. Children <5 years may wear the bone oscillating sound processor with a soft headband. Appropriate for those with conductive and mixed hearing losses as well as single-sided deafness |
| Middle ear implant | An implantable device that stimulates the middle ear structures directly. Some devices are partially implanted while others are completely implanted (invisible). |
|
|
Currently, only two devices are FDA approved for people 18 years and older. Appropriate for people with moderate to severe sensorineural hearing loss who cannot wear or do not benefit from conventional hearing aids. Future applications may apply to those with conductive loss as well. |
| Auditory brainstem implant | A prosthetic hearing device that directly stimulates neurons on the brainstem bypassing the cochlea and auditory nerve. | Potential to restore some functional hearing and speech perception for individuals diagnosed with neural deafness. |
|
FDA approved for adults and most recently for children enrolled in clinical trials (as of Jan. 2013) diagnosed with profound hearing loss secondary to cranial nerve VIII (auditory nerve) insult. |
Although hearing aids and implantable hearing devices provide significant benefit to hearing-impaired individuals, they do not always work well in every situation, particularly in noisy environments such as meetings, restaurants, workplace, and classrooms. A student’s ability to hear and understand what is being taught in the classroom is critical for learning. However, poor acoustics (e.g., background noise, reverberation) are commonplace in this setting, which can negatively impact a child’s understanding of speech. Classroom accommodations and modifications can help survivors with HL perform better in the learning environment. Common classroom accommodations include communication and/or teaching strategies specific to the student’s needs, preferential classroom seating, reduction of extraneous noise, and use of assistive listening technology such as frequency-modulation (FM) or induction loop systems.
Assistive listening devices, such as FM systems and audio streamers, can reduce the negative effects of distance, reverberation, and background noise in difficult listening environments. Most assistive listening devices transmit the desired signal (e.g., speech) wirelessly to hearing aids or cochlear implants and, thereby, increase the intensity level of speech relative to background noise (signal-to-noise ratio or SNR) to maximize speech intelligibility. Research indicates that adults and children with HL require a 4–12 dB and >15 dB higher SNR, respectively, to achieve the same level of understanding as normal hearing listeners. [52] Thus, in specific circumstances, assistive listening technology is recommended in addition to hearing aid use to enhance speech intelligibility and quality of life (Table IV).
Table IV.
Assistive listening devices: devices used by hearing impaired individuals to improve hearing ability in difficult listening environments (e.g., background noise) and/or for safety precautions.
| Type | Description |
|---|---|
| FM System | This system transmits audio signals via radio waves. The speaker wears a transmitter/microphone that transmits the signal wirelessly to earphones/hearing aids attached to a receiver. This system is worn to improve audibility in difficult listening situations (e.g., classrooms, restaurants, meetings). |
| Audio streamers | These systems wirelessly connect hearing aids to TVs, MP3 players, computers, and Bluetooth-enabled phones and devices. Some are also compatible with FM systems. The signal from the connected device (TV, computer, phone, etc.) is sent wirelessly and directly to the hearing aids. |
| Contralateral Routing of Signal (CROS) | A behind-the-ear hearing aid designed specifically for patients diagnosed with single-sided deafness. The CROS is fit on the poor ear, which transmits sound to a hearing aid worn on the better hearing ear. This device helps patients with single-sided deafness better localize sound and understand speech in noisy environments. |
| Telecommunication | A variety of options are available to help the hearing impaired use the telephone such as alerting lights, amplified phones, telecoil circuitry, and text telephone (TTY). |
| Infrared systems | This system uses an invisible light beam that transmits sound from the speaker to earphones or a neck loop (if hearing aids have a telecoil option). |
| Induction loop systems | An induction loop wire is installed in the periphery of a room and connects to a microphone worn by the speaker. The signal from the microphone generates a current in the loop wire, which creates an electromagnetic signal that can be received by the telecoil inside a hearing aid. These systems are most common in large group areas such as classrooms, churches, performing arts centers, airports, etc. but can be purchased for individual use. |
| Alerting systems | Systems that use flashing lights, loud sounds, or vibrations to alert the person of environmental sounds (e.g., telephone, alarms, doorbell, baby crying). |
Under the Individuals with Disabilities Education Act, [53] the federal government provides state funding for services to children (ages 3–21 years) with HL in the educational setting. This funding scope includes provision of assistive devices used in the classroom (e.g., FM systems) and audiological services including assessment and selection and fitting of assistive technologies. Also, under federal law, an Individualized Educational Program (IEP) is developed for students who qualify for special educational services. Accommodations, services, and supplementary aids within the scope of an IEP for hearing-impaired students may include assistive listening devices, preferential seating, note-takers, extended test time, shortened assignments, a sign language interpreter, speech therapy, and similar assistance.
Survivors with HL should also be counselled about environmental risks that may further worsen their hearing. A particular employment or recreation related hazard is significant exposure to loud noise. Noise-induced HL is caused by damage to the inner ear structures from exposure to excessively loud or repetitive loud noise such as from working tools, loud music, fireworks, guns, etc. However, this ototoxic environmental hazard is nearly preventable. Avoidance or limited exposure to loud noise and/or use of hearing protection devices such as earplugs, earmuffs, and semi-inserts are recommended to attenuate loud noise and protect residual hearing, particularly in patient populations with pre-existing HL.
Conclusion
In summary, childhood cancer survivors exposed to platinum-containing chemotherapies or radiation to the auditory apparatus are at risk for permanent sensorineural HL and, to a lesser extent, conductive HL. These adverse effects can present early or even years after treatment and progress with time. Failure to diagnose and adequately address HL can have adverse consequences on language acquisition, speech development, and socioeconomic domains. Conversely, timely diagnosis and appropriate interventions can significantly improve speech understanding, language development, academic performance, and social interaction in the survivor. Hence, identification of at-risk populations, appropriate screening, and timely referral to an audiologist are critical to the appropriate care of at-risk survivors of childhood and adolescent cancers.
Supplementary Material
Supplemental Figure 1. Chemotherapy exposure-based screening recommendations from the Children’s Oncology Group Long-term Follow-up guidelines. (Reproduced with permission from the Children’s Oncology Group Long-term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Version 4.0. Arcadia, CA: Children’s Oncology Group; 2014)
Supplemental Figure 2. Radiation exposure-based screening recommendations from the Children’s Oncology Group Long-term Follow-up guidelines. (Reproduced with permission from the Children’s Oncology Group Long-term Follow-up Guidelines for Survivors of Childhood, Adole scent, and Young Adult Cancers. Version 4.0. Arcadia, CA: Children’s Oncology Group; 2014)
Acknowledgments
Funding Source: Children’s Oncology Group National Clinical Trials Network Operations Grant (U10CA180886-Adamson)
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Abbreviations
- ABR
Auditory Brainstem Response
- dB
Decibels
- COG
Children’s Oncology Group
- FDA
Federal Drug Administration
- FM
Frequency Modulation
- Gy
Gray
- HL
Hearing Loss
- IEP
Individualized Educational Program
- OAEs
Otoacoustic Emissions
- SNR
Signal-To-Noise Ratio
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Grewal S, Merchant T, Reymond R, McInerney M, Hodge C, Shearer P. Auditory late effects of childhood cancer therapy: a report from the Children's Oncology Group. Pediatrics. 2010;125(4):e938–950. doi: 10.1542/peds.2009-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(34):8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 3.Landier W, Knight K, Wong FL, Lee J, Thomas O, Kim H, Kreissman SG, Schmidt ML, Chen L, London WB, Gurney JG, Bhatia S. Ototoxicity in children with high-risk neuroblastoma: prevalence, risk factors, and concordance of grading scales--a report from the Children's Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(6):527–534. doi: 10.1200/JCO.2013.51.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bass JK, Huang J, Onar-Thomas A, Chang KW, Bhagat SP, Chintagumpala M, Bartels U, Gururangan S, Hassall T, Heath JA, McCowage G, Cohn RJ, Fisher MJ, Robinson G, Broniscer A, Gajjar A, Gurney JG. Concordance between the chang and the International Society of Pediatric Oncology (SIOP) ototoxicity grading scales in patients treated with cisplatin for medulloblastoma. Pediatr Blood Cancer. 2014;61(4):601–605. doi: 10.1002/pbc.24830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N, Hartmann O. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26(10):649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 6.Parsons SK, Neault MW, Lehmann LE, Brennan LL, Eickhoff CE, Kretschmar CS, Diller LR. Severe ototoxicity following carboplatin-containing conditioning regimen for autologous marrow transplantation for neuroblastoma. Bone Marrow Transplant. 1998;22(7):669–674. doi: 10.1038/sj.bmt.1701391. [DOI] [PubMed] [Google Scholar]
- 7.Gurney JG, Tersak JM, Ness KK, Landier W, Matthay KK, Schmidt ML, Children's Oncology G Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children's Oncology Group. Pediatrics. 2007;120(5):e1229–1236. doi: 10.1542/peds.2007-0178. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber JE, Gurney JG, Palmer SL, Bass JK, Wang M, Chen S, Zhang H, Swain M, Chapieski ML, Bonner MJ, Mabbott DJ, Knight SJ, Armstrong CL, Boyle R, Gajjar A. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol. 2014;16(8):1129–1136. doi: 10.1093/neuonc/nou006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19(5):339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Davis JM, Elfenbein J, Schum R, Bentler RA. Effects of mild and moderate hearing impairments on language, educational, and psychosocial behavior of children. J Speech Hear Disord. 1986;51(1):53–62. doi: 10.1044/jshd.5101.53. [DOI] [PubMed] [Google Scholar]
- 11.Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998;102(5):1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]
- 12.Yoshinaga-Itano C. Benefits of early intervention for children with hearing loss. Otolaryngologic clinics of North America. 1999;32(6):1089–1102. doi: 10.1016/s0030-6665(05)70196-1. [DOI] [PubMed] [Google Scholar]
- 13.Downs MP, Yoshinaga-Itano C. The efficacy of early identification and intervention for children with hearing impairment. Pediatric clinics of North America. 1999;46(1):79–87. doi: 10.1016/s0031-3955(05)70082-1. [DOI] [PubMed] [Google Scholar]
- 14.Hua C, Bass JK, Khan R, Kun LE, Merchant TE. Hearing loss after radiotherapy for pediatric brain tumors: effect of cochlear dose. International journal of radiation oncology, biology, physics. 2008;72(3):892–899. doi: 10.1016/j.ijrobp.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 15.Kolinsky DC, Hayashi SS, Karzon R, Mao J, Hayashi RJ. Late onset hearing loss: a significant complication of cancer survivors treated with Cisplatin containing chemotherapy regimens. J Pediatr Hematol Oncol. 2010;32(2):119–123. doi: 10.1097/MPH.0b013e3181cb8593. [DOI] [PubMed] [Google Scholar]
- 16.Landier W, Merchant T. Adverse Effects of Cancer Treatment on Hearing. In: Schwartz CL, Hobbie W, Constine LS, et al., editors. Survivors of Childhood and Adolescent Cancer. 2. Heidelberg: Springer; 2005. pp. 109–124. [Google Scholar]
- 17.Weissenstein A, Deuster D, Knief A, Zehnhoff-Dinnesen AA, Schmidt CM. Progressive hearing loss after completion of cisplatin chemotherapy is common and more pronounced in children without spontaneous otoacoustic emissions before chemotherapy. Int J Pediatr Otorhinolaryngol. 2012;76(1):131–136. doi: 10.1016/j.ijporl.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Yasui N, Adachi N, Kato M, Koh K, Asanuma S, Sakata H, Hanada R. Cisplatin-induced hearing loss: the need for a long-term evaluating system. J Pediatr Hematol Oncol. 2014;36(4):e241–245. doi: 10.1097/MPH.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 19.Group CsO. Long-term follow up guidelines for survivors of childhood, adolescent, and young adult cancers. [Accessed 2015 February 20];2006 Feb 20; < http://www.survivorshipguidelines.org/>.
- 20.Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, Darling J, Armstrong FD, Blatt J, Constine LS, Freeman CR, Friedman DL, Green DM, Marina N, Meadows AT, Neglia JP, Oeffinger KC, Robison LL, Ruccione KS, Sklar CA, Hudson MM. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(24):4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 21.Liberman PH, Schultz C, Goffi-Gomez MV, Lopes LF. Speech recognition and frequency of hearing loss in patients treated for cancer in childhood. Pediatr Blood Cancer. 2013;60(10):1709–1713. doi: 10.1002/pbc.24560. [DOI] [PubMed] [Google Scholar]
- 22.Stelmachowicz PG, Pittman AL, Hoover BM, Lewis DE, Moeller MP. The importance of high-frequency audibility in the speech and language development of children with hearing loss. Arch Otolaryngol Head Neck Surg. 2004;130(5):556–562. doi: 10.1001/archotol.130.5.556. [DOI] [PubMed] [Google Scholar]
- 23.Bess FH, Tharpe AM. Unilateral hearing impairment in children. Pediatrics. 1984;74(2):206–216. [PubMed] [Google Scholar]
- 24.Emmett SD, Francis HW. The socioeconomic impact of hearing loss in U.S. adults. Otol Neurotol. 2015;36(3):545–550. doi: 10.1097/MAO.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fellinger J, Holzinger D, Beitel C, Laucht M, Goldberg DP. The impact of language skills on mental health in teenagers with hearing impairments. Acta Psychiatr Scand. 2009;120(2):153–159. doi: 10.1111/j.1600-0447.2009.01350.x. [DOI] [PubMed] [Google Scholar]
- 26.Moeller MP. Current state of knowledge: psychosocial development in children with hearing impairment. Ear Hear. 2007;28(6):729–739. doi: 10.1097/AUD.0b013e318157f033. [DOI] [PubMed] [Google Scholar]
- 27.Brinkman TM, Bass JK, Li Z, Ness KK, Gajjar A, Pappo AS, Armstrong GT, Merchant TE, Srivastava DK, Robison LL, Hudson MM, Gurney JG. Treatment-induced hearing loss and adult social outcomes in survivors of childhood CNS and non-CNS solid tumors: Results from the St. Jude Lifetime Cohort Study. Cancer. 2015;121(22):4053–4061. doi: 10.1002/cncr.29604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jereczek-Fossa BA, Zarowski A, Milani F, Orecchia R. Radiotherapy-induced ear toxicity. Cancer treatment reviews. 2003;29(5):417–430. doi: 10.1016/s0305-7372(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 29.Merchant TE, Gould CJ, Xiong X, Robbins N, Zhu J, Pritchard DL, Khan R, Heideman RL, Krasin MJ, Kun LE. Early neuro-otologic effects of three-dimensional irradiation in children with primary brain tumors. International journal of radiation oncology, biology, physics. 2004;58(4):1194–1207. doi: 10.1016/j.ijrobp.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Kortmann RD, Kuhl J, Timmermann B, Mittler U, Urban C, Budach V, Richter E, Willich N, Flentje M, Berthold F, Slavc I, Wolff J, Meisner C, Wiestler O, Sorensen N, Warmuth-Metz M, Bamberg M. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT '91. International journal of radiation oncology, biology, physics. 2000;46(2):269–279. doi: 10.1016/s0360-3016(99)00369-7. [DOI] [PubMed] [Google Scholar]
- 31.Schell MJ, McHaney VA, Green AA, Kun LE, Hayes FA, Horowitz M, Meyer WH. Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1989;7(6):754–760. doi: 10.1200/JCO.1989.7.6.754. [DOI] [PubMed] [Google Scholar]
- 32.Fong RS, Beste DJ, Murray KJ. Pediatric sensorineural hearing loss after temporal bone radiation. Am J Otol. 1995;16(6):793–796. [PubMed] [Google Scholar]
- 33.Huang E, Teh BS, Strother DR, Davis QG, Chiu JK, Lu HH, Carpenter LS, Mai WY, Chintagumpala MM, South M, Grant WH, 3rd, Butler EB, Woo SY. Intensity-modulated radiation therapy for pediatric medulloblastoma: early report on the reduction of ototoxicity. International journal of radiation oncology, biology, physics. 2002;52(3):599–605. doi: 10.1016/s0360-3016(01)02641-4. [DOI] [PubMed] [Google Scholar]
- 34.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(22):3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moeller BJ, Chintagumpala M, Philip JJ, Grosshans DR, McAleer MF, Woo SY, Gidley PW, Vats TS, Mahajan A. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiat Oncol. 2011;6:58. doi: 10.1186/1748-717X-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert MP, Shields C, Meadows AT. A retrospective review of hearing in children with retinoblastoma treated with carboplatin-based chemotherapy. Pediatr Blood Cancer. 2008;50(2):223–226. doi: 10.1002/pbc.21155. [DOI] [PubMed] [Google Scholar]
- 37.Smits C, Swen SJ, Theo Goverts S, Moll AC, Imhof SM, Schouten-van Meeteren AY. Assessment of hearing in very young children receiving carboplatin for retinoblastoma. Eur J Cancer. 2006;42(4):492–500. doi: 10.1016/j.ejca.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Kushner BH, Budnick A, Kramer K, Modak S, Cheung NK. Ototoxicity from high-dose use of platinum compounds in patients with neuroblastoma. Cancer. 2006;107(2):417–422. doi: 10.1002/cncr.22004. [DOI] [PubMed] [Google Scholar]
- 39.Dean JB, Hayashi SS, Albert CM, King AA, Karzon R, Hayashi RJ. Hearing loss in pediatric oncology patients receiving carboplatin-containing regimens. J Pediatr Hematol Oncol. 2008;30(2):130–134. doi: 10.1097/MPH.0b013e31815d1d83. [DOI] [PubMed] [Google Scholar]
- 40.Jehanne M, Lumbroso-Le Rouic L, Savignoni A, Aerts I, Mercier G, Bours D, Desjardins L, Doz F. Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatr Blood Cancer. 2009;52(5):637–643. doi: 10.1002/pbc.21898. [DOI] [PubMed] [Google Scholar]
- 41.Al-Khatib T, Cohen N, Carret AS, Daniel S. Cisplatinum ototoxicity in children, long-term follow up. Int J Pediatr Otorhinolaryngol. 2010;74(8):913–919. doi: 10.1016/j.ijporl.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40(16):2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Low WK, Toh ST, Wee J, Fook-Chong SM, Wang DY. Sensorineural hearing loss after radiotherapy and chemoradiotherapy: a single, blinded, randomized study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(12):1904–1909. doi: 10.1200/JCO.2005.05.0096. [DOI] [PubMed] [Google Scholar]
- 44.Walker DA, Pillow J, Waters KD, Keir E. Enhanced cis-platinum ototoxicity in children with brain tumours who have received simultaneous or prior cranial irradiation. Med Pediatr Oncol. 1989;17(1):48–52. doi: 10.1002/mpo.2950170110. [DOI] [PubMed] [Google Scholar]
- 45.Miettinen S, Laurikainen E, Johansson R, Minn H, Laurell G, Salmi TT. Radiotherapy enhanced ototoxicity of cisplatin in children. Acta Otolaryngol Suppl. 1997;529:90–94. doi: 10.3109/00016489709124092. [DOI] [PubMed] [Google Scholar]
- 46.Einarsson EJ, Petersen H, Wiebe T, Fransson PA, Magnusson M, Moell C. Severe difficulties with word recognition in noise after platinum chemotherapy in childhood, and improvements with open-fitting hearing-aids. Int J Audiol. 2011;50(10):642–651. doi: 10.3109/14992027.2011.585667. [DOI] [PubMed] [Google Scholar]
- 47.Vermiglio AJ, Soli SD, Freed DJ, Fisher LM. The relationship between high-frequency pure-tone hearing loss, hearing in noise test (HINT) thresholds, and the articulation index. J Am Acad Audiol. 2012;23(10):779–788. doi: 10.3766/jaaa.23.10.4. [DOI] [PubMed] [Google Scholar]
- 48.Campbell KC, Durrant J. Audiologic monitoring for ototoxicity. Otolaryngologic clinics of North America. 1993;26(5):903–914. [PubMed] [Google Scholar]
- 49.Coradini PP, Cigana L, Selistre SG, Rosito LS, Brunetto AL. Ototoxicity from cisplatin therapy in childhood cancer. J Pediatr Hematol Oncol. 2007;29(6):355–360. doi: 10.1097/MPH.0b013e318059c220. [DOI] [PubMed] [Google Scholar]
- 50.Knight KR, Kraemer DF, Winter C, Neuwelt EA. Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(10):1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- 51.Kochkin SR, CMA Quantifying the obvious: The impact of hearing instruments on quality of life. Hearing Review. 2000:8–34. [Google Scholar]
- 52.Crandell CC, Smaldino JJ. Classroom acoustics for children with normal hearing and with hearing impairment. Lang Speech Hear Serv Sch. 2000;31:362–370. doi: 10.1044/0161-1461.3104.362. [DOI] [PubMed] [Google Scholar]
- 53.1997. Individuals with Disability Education Act Amendments of 1997 [IDEA] [Accessed 2015];1997 < http://ideaedgov/>.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Chemotherapy exposure-based screening recommendations from the Children’s Oncology Group Long-term Follow-up guidelines. (Reproduced with permission from the Children’s Oncology Group Long-term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Version 4.0. Arcadia, CA: Children’s Oncology Group; 2014)
Supplemental Figure 2. Radiation exposure-based screening recommendations from the Children’s Oncology Group Long-term Follow-up guidelines. (Reproduced with permission from the Children’s Oncology Group Long-term Follow-up Guidelines for Survivors of Childhood, Adole scent, and Young Adult Cancers. Version 4.0. Arcadia, CA: Children’s Oncology Group; 2014)