Abstract
Background
IL-18 is a pro-inflammatory cytokine that has recently been demonstrated to be an important mediator of obstruction-induced renal tubulointerstitial fibrosis independent of TNF-α and TGF-β1 activity. We hypothesized that IL-18 stimulates a positive feedback loop during obstruction via the IL-18 receptor (IL-18R) to increase both IL-18 gene expression and protein production.
Methods
To study this, male C57BL6 IL-18R knockout (IL-18R-KO) and control (WT) mice were subjected to unilateral ureteral obstruction (UUO) vs. sham operation and sacrificed 1 week after surgery. Renal cortical tissue samples were harvested and analyzed for IL-18 protein levels (ELISA), and IL-18 and IL-18R gene expression (QPCR). The specific cellular localization of IL-18 and IL-18R expression during obstruction was assessed using dual labeling immunofluorescent staining (IFS).
Results
Renal IL-18R expression increased significantly in WT mice in response to obstruction, but remained at sham levels in IL-18R KO mice. Similarly, while IL-18 protein levels and gene expression were significantly increased in WT mice in response to obstruction, IL-18 levels and gene expression were significantly reduced during obstruction in IL-18R KO mice. Obstruction-induced IL-18 and IL-18R production localized predominantly to tubular epithelial cells (TEC) and to a lesser extent the renal interstitium.
Conclusion
These results demonstrate that IL-18 stimulates a positive feedback loop through the IL-18R during renal obstruction to stimulate IL-18 production and gene expression. The predominant cellular source of IL-18 production during renal obstruction appears to be tubular epithelial cells rather than infiltrating macrophage.
Keywords: Interleukin-18, Kidney, Obstruction, Localization
INTRODUCTION
Chronic renal obstruction results in progressive renal damage, and ultimately, an irreversible decline in renal function. The pathologic hallmarks of obstructive uropathy include tubular dilation, apoptotic tubular cell deletion, and progressive tubulointerstitial fibrosis.1 Interstitial fibrosis is a complex pathophysiological process involving inflammatory cell infiltration, fibroblast proliferation, and an imbalance in extracellular matrix (ECM) synthesis, deposition, and degradation51. In the kidney, interstitial fibrosis is characterized by de novo activation of a-SMA-positive myofibroblasts, the principal effector cells that are responsible for excess ECM deposition. Interstitial inflammatory cell infiltration occurs shortly after the onset of renal obstruction52, 53 and results in the release of a variety of cytokines and growth factors that stimulate ECM synthesis and fibroblast proliferation. Most notable among these is TGF-β1. TGF-β1 is a major regulator of fibrosis via stimulation of epithelial mesenchymal transition (EMT) and fibroblast proliferation60–66, extracellular matrix synthesis 58, 65, 67–70, and the simultaneous inhibition of collagenase and degradative matrix metalloproteinases. 65, 68, 69, 71 TNF-α also has a role in fibrotic renal injury, stimulating ECM accumulation, inhibition of ECM degradation, and the upregulation of a number of cytokines and transcription factors involved in tubulointerstitial fibrosis. 50, 72–75 While TGF-β1 and TNF-α are important mediators of renal fibrosis, we have recently demonstrated that IL-18 is an important mediator of obstruction-induced renal fibrosis, ECM deposition, and EMT independent of TNF-α or TGF-β1 activity. 9
Interleukin-18 (IL-18) is a pro-inflammatory cytokine that has been implicated in the pathogenesis of many inflammatory renal disease, including renal ischemia-reperfusion injury, allograft rejection, autoimmune disease, and obstructive uropathy.3–6 (add bani-hani ref) Urinary IL-18 levels have been shown to be a sensitive and early marker of renal tubular damage in patients with ischemic and post-transplantation ATN 7, and circulating IL-18 levels and renal IL-18 receptor (IL-18R) expression have been shown to be elevated in patients with chronic kidney disease.7, 8 We have previously observed that obstruction-induced IL-18 gene expression is dramatically reduced in the presence of IL-18BP, a circulating inhibitor of active IL-18. 9 This observation led us to hypothesize that IL-18 amplifies its own production and gene expression during renal obstruction by stimulating a positive feedback loop via the IL-18 receptor. To study this we examined renal cortical IL-18 production, IL-18 and IL-18R gene expression, and the cellular localization of IL-18 and IL-18R production in male C57B16 WT mice and IL-18R KO mice using a well established model of UUO.
MATERIALS AND METHODS
Animals
The animal protocol used in this study was reviewed and approved by the Indiana University Laboratory Animal Care and Use Committee. Male C57BL6 mice homozygous null for the IL-18R1 gene (The Jackson Laboratory, Bar Harbor, Maine) served as the IL-18 receptor knockout mice (IL-18R KO). These mice are healthy and have previously been confirmed to have absent IL-18R mRNA expression, a lack of IL-18 binding to splenocytes, and a loss of IL-18-induced NFκB DNA binding activity (Hoshino et al). Aged matched male C57BL6 mice served as wild type controls (WT). Mice weighing 25–30 grams (six animals per group) were anesthetized with inhaled isoflurane and the left ureter was ligated following isolation with 5-0 silk tie. Sham-operated mice underwent the same procedure without ureteral ligation. Seven days later mice were anesthetized and the left kidney harvested. The specimens were snap frozen in liquid nitrogen and the animals euthanized.
Tissue homogenization
A portion of each renal cortex was homogenized after the samples had been diluted in 10 volumes of homogenate buffer per gram of tissue (10 mM Hepes (pH 7.9), 10 mM KCL, 0.1 mM EGTA, 1 mM DTT, and Complete Protease Inhibitor tabs (Boehringer Mannheim, Indianapolis, IN)) using a vertishear tissue homogenizer. Renal homogenates were then centrifuged at 3000 g for 15 min, and the supernatants stored at −70 °C until the ELISA assays could be performed.
IL-18 ELISA
Renal homogenate and serum IL-18 levels were determined using an ELISA (mouse IL-18: MBL Int., Woburn, MA, USA). The ELISA was performed by adding 100 μl of each sample to wells in a 96-well plate of a commercially available ELISA kit and the assay performed according to the manufacturer’s instructions. All samples were tested in duplicate. The ELISA results were expressed as pg/mg protein for tissue and pg/ml for serum.
Real-time PCR
Total RNA was extracted from renal cortical tissue by homogenization in Trizol (Gibco BRL, Gaithersburg, MD, USA), then isolated by precipitation with chloroform and isopropanol. Total RNA (0.5 mg) was subjected to cDNA synthesis using iScript (Bio-Rad, Hercules, CA, USA). cDNA from each sample was analyzed for IL-18 (Mm00434226_m1) and IL-18 receptor (Mm00515180_m1) using a TaqMan gene expression assay (RT-PCR; Applied Biosystems, Foster City, CA, USA). FAM Dye/MGB-labeled probes for mouse β-actin (Applied Biosystems) served as endogenous controls.
Cellular Immunolocalization
Five micrometer renal sections were harvested from each sample, fixed in acetone for 10 minutes, rinsed in phosphate buffered solution (PBS) three times, and incubated for 20 minutes in blocking solution containing 10% goat or donkey serum. Slides were then incubated with goat anti-IL-18 (1:50; Santa Cruz Biotechnology; Santa Cruz, CA) or rabbit anti-IL-18R (1:50, Santa Cruz Biotechnology; Santa Cruz, CA) for 60 min at room temperature. Slides were then washed three times in PBS and incubated with a secondary antibody for 45 minutes at room temperature (donkey anti-goat Texas Red IgG 1:200 or donkey anti-rabbit Texas Red IgG 1:200 (Santa Cruz Biotechnology; Santa Cruz, CA)). The proximal renal tubules were then counterstained with fluorescein Lotus Tetragonolobus Lectin (2mg/ml; 1:200 in PBS with 1% Donkey Serum; Vector Laboratories) for 15 minutes, or the distal renal tubules were counterstained fluorescein Peanut Agglutinin for 15 minutes (5mg/ml; 1:500 in PBS with 1% Goat Serum; Vector Laboratories). The nuclei were then stained with bis-Benzimide (2μg/ml). To assess the specificity of the immunostaining, adjacent sections were incubated without primary antibody and processed using identical conditions. The slides were mounted and stored at −4° C. Digital photographs were taken (400X) using a fluorescent microscope (Leica DM IRB; Wetzlar, Germany).
Statistical Analysis
Data are presented as means ± SD. Differences at the 95% confidence intervals were considered significant. The experimental groups were compared using ANOVA with post hoc Bonferroni-Dunn (JMP Statistical Software version 5.0; Berkeley, CA).
RESULTS
IL-18 Receptor mRNA Expression (Real time PCR)
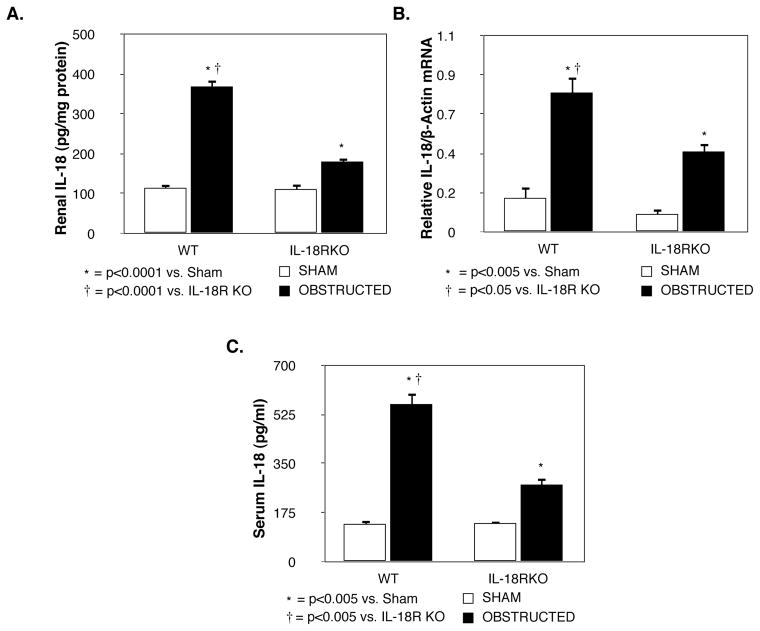
Renal IL-18R mRNA expression increased significantly in WT mice in response to obstruction (Figure 1). In contrast, IL-18R mRNA expression remained at sham levels in IL-18R KO mice exposed to the same degree of obstruction.
Figure 1. Renal cortical IL-18 receptor mRNA expression following UUO.
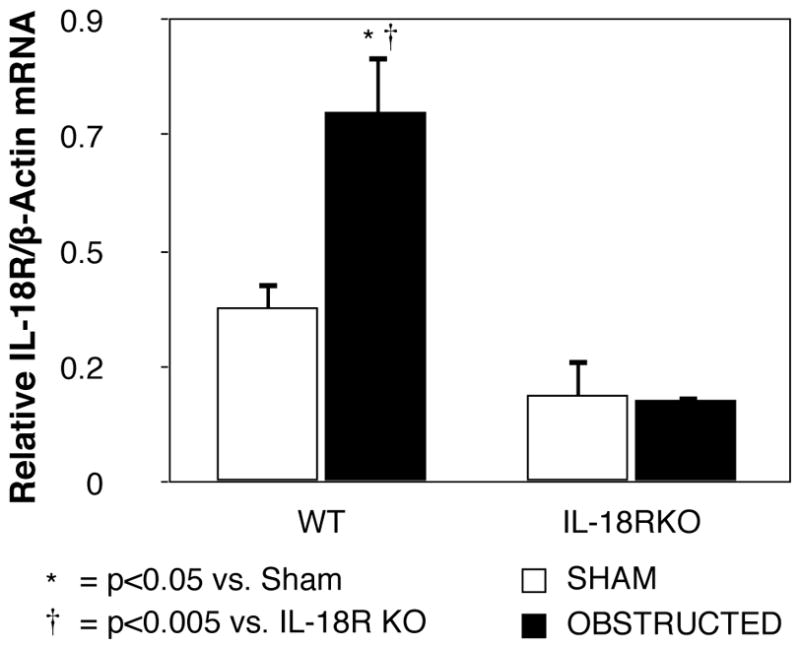
Quantitative IL-18 receptor (IL-18R) mRNA expression represented as a percentage of β-actin in WT and IL-18R KO animals exposed to sham operation or one week of UUO.
IL-18 Levels (ELISA) and mRNA Expression (Real time PCR)
Renal IL-18 protein production (Figure 2A) and gene expression (Figure 2B) remained low in sham treated animals, but increased significantly in both groups in response to obstruction. Obstruction-induced IL-18 protein production and mRNA expression; however, was significantly reduced in IL-18R KO mice as compared to WT mice. Similarly, while serum IL-18 levels were significantly increased in both groups in response to obstruction (Figure 2C), IL-18 levels were significantly reduced in IL-18RKO mice as compared to WT mice.
Figure 2. Renal cortical IL-18 production and quantitative mRNA expression following UUO.
A. Renal cortical IL-18 protein levels in WT and IL-18R KO animals exposed to sham operation or one week of UUO. B. Quantitative IL-18 mRNA expression represented as a percentage of β-actin in WT and IL-18R KO animals exposed to sham operation or one week of UUO. C. Serum IL-18 levels in WT and IL-18R KO animals exposed to sham operation or one week of UUO.
IL-18 & IL-18 Receptor Localization
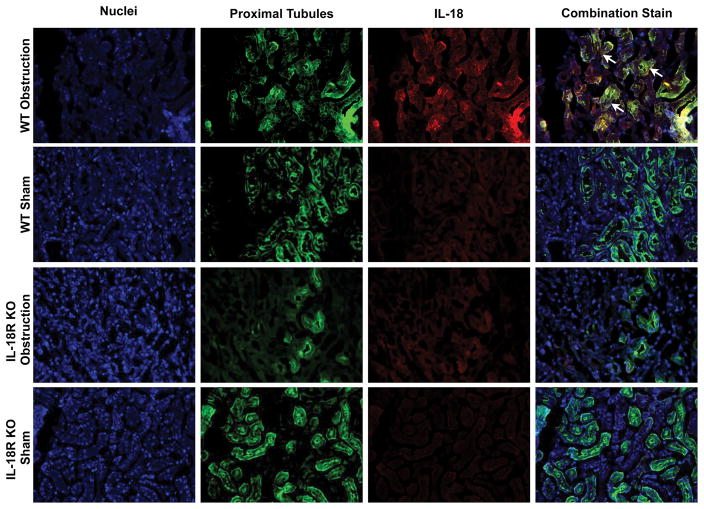
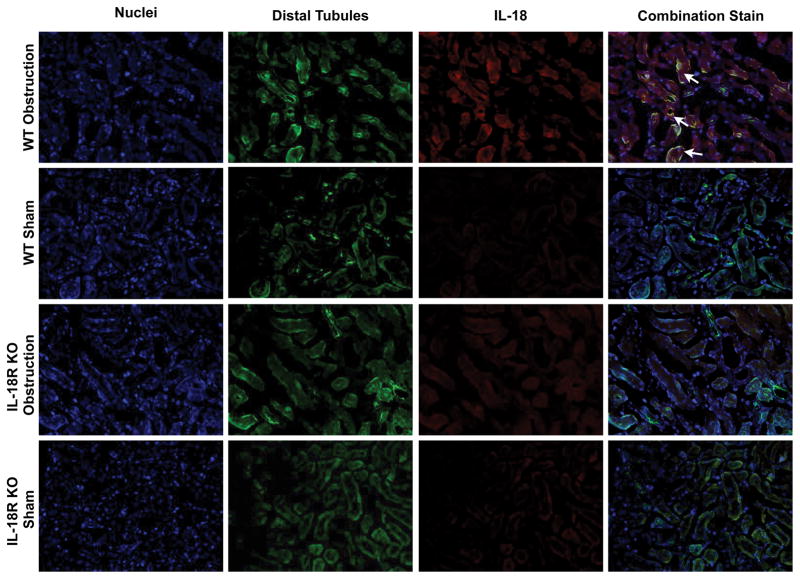
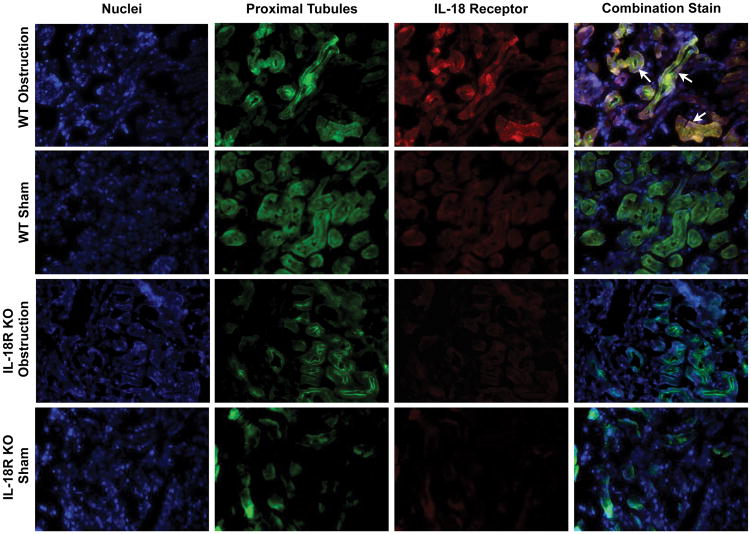
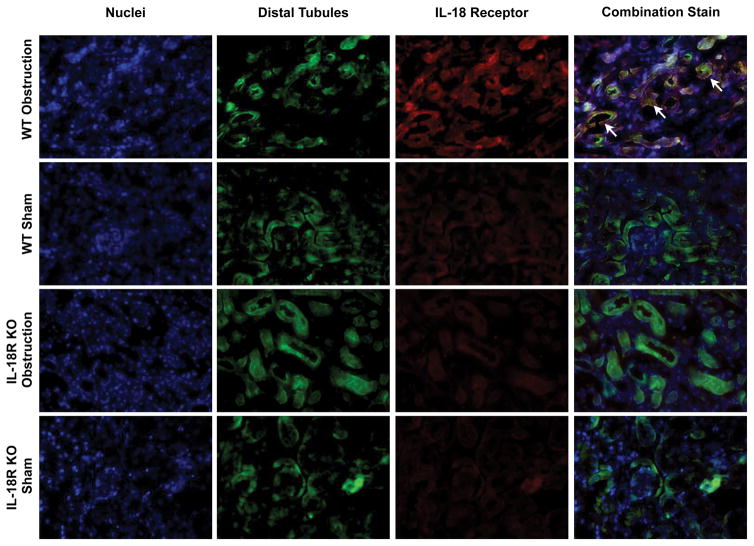
The cellular localization of IL-18 and IL-18R was determined using dual labeling immunofluorescent staining. Results demonstrate a significant increase in IL-18 (Figure 3) and IL-18R (Figure 4) staining in the kidneys of WT mice in response to obstruction. As expected IL-18R KO mice exhibited minimal IL-18R staining in both sham and obstructed kidneys. IL-18 and IL-18R production localized predominantly to tubular epithelial cells (TEC), with a uniform distribution between proximal and distal tubules (Figures 3 and 4), and to a much lesser extent the renal interstitium. The degree of IL-18 staining was significantly less in IL-18R KO in response to obstruction as compared to WT mice.
Figure 3. Dual labelled immunofluorescent staining for IL-18 and tubular epithelial cells following UUO.
A. Photographs (magnification 400X) depicting renal cortical IL-18 production (red) and proximal tubular staining (Lotus Tetragonolobus Lectin; green) in WT and IL-18R KO animals exposed to sham operation or one week of UUO. B. Photographs (magnification 400X) depicting renal cortical IL-18 production (red) and distal tubular staining (Peanut Agglutinin; green) in WT and IL-18R KO animals exposed to sham operation or one week of UUO. White arrows indicate IL-18 staining overlying tubules.
Figure 4. Dual labelled immunofluorescent staining for IL-18 receptor and tubular epithelial cells following UUO.
A. Photographs (magnification 400X) depicting renal cortical IL-18R production (red) and proximal tubular staining (Lotus Tetragonolobus Lectin; green) in WT and IL-18R KO animals exposed to sham operation or one week of UUO. B. Photographs (magnification 400X) depicting renal cortical IL-18R production (red) and distal tubular staining (Peanut Agglutinin; green) in WT and IL-18R KO animals exposed to sham operation or one week of UUO. White arrows indicate IL-18R staining overlying tubules.
DISCUSSION
IL-18 is a recently discovered pro-inflammatory cytokine that is structurally and functionally related to the IL-1 family. While IL-18 has been demonstrated to be a critical mediator of obstruction-induced renal fibrosis, the mechanism by which IL-18 exerts its injurious effects during obstructive uropathy remains unknown. We have previously demonstrated that IL-18 binding protein, a potent inhibitor of mature IL-18, significantly attenuates renal IL-18 mRNA expression in response to one or two weeks of obstruction.9 We therefore hypothesized that an IL-18 amplification loop exists, whereby IL-18 stimulates its own production during renal obstruction through IL-18 receptor signaling. Since these signaling changes are likely to occur early in the course of obstruction-induced renal injury, a one week time point was selected for our experiments. This is the first study to demonstrate that IL-18 and IL-18 receptor production localize primarily to the tubular epithelial cells during renal obstruction, and further, that IL-18 stimulates a positive feedback loop through the IL-18 receptor during obstruction, which in turn, amplifies IL-18 gene expression and production.
IL-18 binds to its specific receptor, IL-18R, resulting in the recruitment of MyD88 to the cytosolic Toll/IL-1R (TIR) domain of the IL-18R itself. This, in turn, activates a signaling cascade leading to the activation of NFκB, p38MAPK, and AP-1.10, 11 Through this mechanism, IL-18 can induce the production of other inflammatory cytokines, such as IL-1β, TNF-α, and IL-6.12, 13 Our data reveal that renal cortical IL-18 protein production and gene expression are significantly increased in response to obstruction, a finding that is supported by our previous observations.9 In addition, our data demonstrate that serum levels of IL-18 and renal cortical IL-18 receptor mRNA expression increase significantly in response to obstruction. Liang et al have reported similar findings in humans in which IL-18 receptor expression was found to be significantly increased in patients with chronic kidney disease.14 Interestingly, both IL-18 protein and serum levels, and IL-18 gene expression were dramatically reduced in IL-18R KO mice subjected to obstruction, suggesting that intact IL-18 receptor signaling is critical to downstream IL-18 gene transcription and protein production. This provides evidence of a positive feedback loop whereby IL-18 is capable of amplifying its own production and gene transcription during obstruction via IL-18 receptor signaling. The increase in IL-18 receptor expression observed during obstruction would serve to further augment this response. Our findings in IL-18 binding protein (IL-18BP) transgenic mice provide further evidence of a positive feedback loop for IL-18 production, as we have previously demonstrated that IL-18 neutralization with circulating IL-18BP results in significantly decreased IL-18 mRNA levels during renal obstruction.9 Finally, additional evidence of a positive feedback loop is provided by Fortin et al, who recently demonstrated that exogenous IL-18 can induce IL-18 gene expression in human neutrophils in vitro, and that this response is under the control of NF-KB. 15 Cumulatively, these studies suggest that IL-18 exhibits autocrine properties during inflammatory signaling.
While macrophage are a well known immunologic source of IL-18, 16 accumulating evidence suggests that renal tubular epithelial cells are an important source of IL-18 production during fibrotic renal injury.9,14 Our immunofluorescent studies reveal that both IL-18 and IL-18 receptor expression localize primarily to the renal tubular cells during obstruction, and to a lesser extent, the renal interstitium. The distribution of renal tubular staining for IL-18 and IL-18R was not appreciably different for the proximal and distal tubules, and obstruction-induced IL-18 staining was noted to be significantly greater in WT mice as compared to IL-18R KO mice, providing additional evidence of a positive feedback loop for IL-18 production. These findings suggest that tubular cells, not infiltrating macrophage, are the predominant source of IL-18 production during renal obstruction. Support for this observation is evidenced in a study by He et al 17, who demonstrated that renal IL-18 production was not diminished in macrophage depleted mice in response to ischemia, and further, that the adoptive transfer of peritoneal macrophages with inhibited IL-18 function did not reverse the functional protection afforded by macrophage depletion. This observation led the authors to suggest that tubular cells, not macrophages, are the primary source of increased IL-18 in ischemic renal injury.17 Indeed, Liang et al have demonstrated that HK-2 tubular epithelial cells express IL-18 mRNA in response to lipopolysaccharide stimulation in vitro,14 and Gauer et al have demonstrated that IL-18 is expressed in the intercalated cells of the distal convoluted tubules in human kidneys using immunohistochemistry, dual labeled immunofluorescent detection, and in situ hybridization for IL-18 mRNA.18
CONCLUSIONS
Renal obstruction stimulates a signaling cascade that results in IL-18 production and subsequent renal injury. This study demonstrates that IL-18 and IL-18 receptor expression are primarily localized to the tubular epithelial cells, not the infiltrating macrophage, during obstruction. The study also reveals that IL-18 stimulates a positive feedback loop through the IL-18 receptor during obstruction, which in turn, amplifies IL-18 gene expression and production. This response may be further augmented by the observed increase in IL-18 receptor expression that occurs during obstruction. As the mechanisms of IL-18’s signaling interactions become more clearly defined, therapeutic strategies aimed at ameliorating obstruction-induced renal injury may be realized.
Acknowledgments
This research was supported by NIH grant DK08165 (KKM).
References
- 1.Diamond JR, Ricardo SD, Klahr S. Mechanisms of interstitial fibrosis in obstructive nephropathy. Semin Nephrol. 1998;18:594–602. [PubMed] [Google Scholar]
- 2.Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7:2495–2508. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- 3.Daemen MA, van’t Veer C, Wolfs TG, et al. Ischemia/reperfusion-induced IFN-gamma up-regulation: involvement of IL-12 and IL-18. J Immunol. 1999;162:5506–5510. [PubMed] [Google Scholar]
- 4.Striz I, Krasna E, Honsova E, et al. Interleukin 18 (IL-18) upregulation in acute rejection of kidney allograft. Immunol Lett. 2005;99:30–35. doi: 10.1016/j.imlet.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Faust J, Menke J, Kriegsmann J, et al. Correlation of renal tubular epithelial cell-derived interleukin-18 up-regulation with disease activity in MRLFaslpr mice with autoimmune lupus nephritis. Arthritis Rheum. 2002;46:3083–3095. doi: 10.1002/art.10563. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27:98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16:3046. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 8.Chiang CK, Hsu SP, Pai MF, Peng YS, Ho TI, Liu SH, Hung KY, Tsai TJ, Hsieh BS. Plasma interleukin-18 levels in chronic renal failure and continuous ambulatory peritoneal dialysis. Blood Purif. 2005;23:144–8. doi: 10.1159/000083620. [DOI] [PubMed] [Google Scholar]
- 9.Bani-Hani AH, Leslie JA, Asanuma H, Dinarello CA, Campbell MT, Meldrum DR, Zhang H, Hile K, Meldrum KK. IL-18 neutralization ameliorates obstruction-induced epithelial-mesenchymal transition and renal fibrosis. Kidney Int. 2009;76:500–11. doi: 10.1038/ki.2009.216. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino K, Tsutsui H, Kawai T, Takeda K, Nakanishi K, Takeda Y, Akira S. Cutting edge: generation of IL-18 receptor-deficient mice: evidence for IL-1 receptor-related protein as an essential IL-18 binding receptor. J Immunol. 1999;162:5041–4. [PubMed] [Google Scholar]
- 11.Bombardieri M, McInnes IB, Pitzalis C. Interleukin-18 as a potential therapeutic target in chronic autoimmune/inflammatory conditions. Expert Opin Biol Ther. 2007;7:31–40. doi: 10.1517/14712598.7.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Nakahira M, Ahn HJ, Park WR, Gao P, Tomura M, Park CS, Hamaoka T, Ohta T, Kurimoto M, Fujiwara H. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J Immunol. 2002;168:1146–1153. doi: 10.4049/jimmunol.168.3.1146. [DOI] [PubMed] [Google Scholar]
- 13.Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, Field M, Foulis A, Liew FY, McInnes IB. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamura M, Kawashima M, Taniai M, Yamauchi H, Tanimoto T, Kurimoto M, Morita Y, Ohmoto Y, Makino H. Interferon-gamma-inducing activity of interleukin-18 in the joint with rheumatoid arthritis. Arthritis Rheum. 2001;44:275–85. doi: 10.1002/1529-0131(200102)44:2<275::AID-ANR44>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 15.Liang D, Liu HF, Yao CW, Liu HY, Huang-Fu CM, Chen XW, Du SH, Chen XW. Effects of interleukin 18 on injury and activation of human proximal tubular epithelial cells. Nephrology. 2007;12:53–61. doi: 10.1111/j.1440-1797.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- 16.Fortin CF, Ear T, McDonald PP. Autocrine role of endogenous interleukin-18 on inflammatory cytokine generation by human neutrophils. FASEB J. 2009;23:194–203. doi: 10.1096/fj.08-110213. [DOI] [PubMed] [Google Scholar]
- 17.Obregon C, Dreher D, Kok M, Cochand L, Kiama GS, Nicod LP. Human alveolar macrophages infected by virulent bacteria expressing SipB are a major source of active interleukin-18. Infect Immun. 2003;71:4382. doi: 10.1128/IAI.71.8.4382-4388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Z, Dursun B, Oh DJ, Lu L, Faubel S, Edelstein CL. Macrophages are not the source of injurious interleukin-18 in ischemic acute kidney injury in mice. Am J Physiol Renal Physiol. 2009;296:F535–42. doi: 10.1152/ajprenal.90634.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauer S, Sichler O, Obermüller N, Holzmann Y, Kiss E, Sobkowiak E, Pfeilschifter J, Geiger H, Mühl H, Hauser IA. IL-18 is expressed in the intercalated cell of human kidney. Kidney Int. 2007;72:1081–7. doi: 10.1038/sj.ki.5002473. [DOI] [PubMed] [Google Scholar]