Abstract
In vivo biosensors are emerging as powerful tools in biomedical research and diagnostic medicine. Distinct from “labels” or “imaging”, in vivo biosensors are designed for continuous and long-term monitoring of target analytes in real biological systems and should be selective, sensitive, reversible and biocompatible. Due to the challenges associated with meeting all of the analytical requirements, we found relatively few reports of research groups demonstrating devices that meet the strict definition in vivo. However, we identified several case studies and a range of emerging materials likely to lead to significant developments in the field.
Keywords: sensor, biosensor, continuous, reversible, specificity, dynamic range, in vivo, implantable, nanoparticle, signal transduction
Graphic Abstract
In vivo biosensing has the potential to revolutionize health care by enabling personalized medicine. Imagine that the simple implantation of a sensor into a patient could transmit clinically relevant health information on a continuous basis. Through constant monitoring, an individual’s baseline health could be well understood, which could make small fluctuations from normal conditions meaningful indicators of impending disease. Another possibility includes continuous therapeutic drug monitoring which could take the guesswork out of dosing by offering an individualized report on the pharmacokinetics of a drug. There have been many examples of sensors that detect physiologically relevant analytes, with great potential for in vivo monitoring, but yet there are few examples of biosensors demonstrated in pre-clinical animal studies or approved for implantation in humans.
A strict definition of a biosensor describes a device that is comprised of at least a biologically-based component and a reporter that together detect a chemical or biomolecule.1–2 A commonly used definition is that of a chemical-based sensor that is used in a biological environment, although this is not a biosensor in the strictest sense.1–2 This relaxed description is currently most practical for an implantable sensor, due to the possibility of degradation of a biological component. In fact, it is often biological components, used as the recognition elements, that can limit the lifetime of implantable biosensors. These elements include receptors, antibodies, enzymes, and artificial receptors such as aptamers and surface-imprinted polymers.3 While an interesting area for future development, currently there are a limited number of these reporters used in vivo, due to inherent fragility, irreversible binding, nonselectivity, or difficulty in use. For in vivo use, transducers most commonly include but are not limited to optical reporters and electrodes. Both of these signal converters have their advantages and disadvantages. Namely, optical methods suffer from tissue scattering and absorption, whereas electrodes, for the most part, require an interface between the device and an external unit. Ideally, a biosensor should be reversible and in continuous equilibrium with its environment. The signal produced should be robust, free from drift over time, with minimal need for recalibration.
Despite the promise that in vivo biosensing offers, glucose monitoring remains the best example of a technology that has become clinical reality. Even then, the FDA approved continuous (implantable) sensors are worn externally with a cannula interfacing between the adipose tissue and the detection device. Although there has been extensive interest in implanting other types of sensors for other disease states, the stringent analytical requirements limit the feasibility for long term use. As previously reviewed for implantable nanoscale sensors4 a sensor for continuous monitoring needs to excel in all areas of analytical detection: dynamic range, sensitivity, specificity, reversibility, response time, and biocompatibility. Indeed, as detailed in a recent review,5 biocompatibility is a particularly important and often overlooked parameter for a sensing device. Poor biocompatibility can lead to an immune response from the user, as well as degradation in any one of the above mentioned analytical parameters.
No single technology or approach has been able to accomplish all the analytical requirements for in vivo sensing or biosensing. Often technologies show promise on the benchtop but do not translate to robust in vivo measurements, showing signs of signal drift or reduced performance. Herein, we will review what we consider to be the most promising recent technologies that have been developed in the laboratory setting and then demonstrated in animal models. We specifically are excluding glucose sensors and focus on other analytes that have shown potential, since an excellent recent review has already thoroughly examined progress in the field6. In addition, we will not cover the burgeoning field of genetic encoded sensors for in vivo use or the exciting field of wearable sensors, and instead refer readers to outstanding recent reviews.7–8 However, both of these fields have led to progress in materials for implantation that we will mention briefly. We will not limit the discussion to the strictest definition of a biosensor, due to the inherent fragility of the biological component for in vivo use. With these restrictions in mind, we will cover several technologies that have overcome some of the limitations of the field in unique ways. Each of these groups have demonstrated in vivo results, and have developed technologies to overcome tissue scattering, selectivity, reversibility, and/or limits of detection. In addition, we will discuss a range of new materials that could address key challenges in the field, ranging from the recent explosion of implantable sensors for neural detection (fueled by the BRAIN initiative), to new scaffolds and biological receptors.
CASE STUDIES OF TECHNOLOGIES FOR IN VIVO BIOSENSING
Surface-modified carbon nanotubes as sensitive and reversible biosensors
The Strano group has pioneered carbon nanotubes, specifically, single-walled carbon nanotubes (SWCNT), as a scaffold upon which sensors are built for chemical detection in vivo, taking the distinctive advantage of transducing optical signals in the near-infrared (NIR) window. In vivo optical sensors offer unique advantages compared with electrochemical counterparts in providing valuable spatial dynamics of targeted molecules. Many optical sensors have relied on the advantageous photophysical properties of fluorescent nanomaterials for signal transduction.9–11 In particular, SWCNTs are valuable candidates for in vivo biomedical applications, due to their superb biocompatibility, long fluorescence lifetime and stable fluorescence output. More importantly, the SWCNTs exhibit intrinsic fluorescence in the NIR region of the spectrum (900–1400 nm), and their optical properties are dependent on tube diameter and roll-up angle defined by the nanotube species/chirality.12 The Strano group was the first group to demonstrate the potential of SWCNT as the central protagonist in an in vivo biosensing platform.13 Based on the previous study that SWCNT fluorescence is sensitive to single-molecule nitric oxide (NO) absorption via SWCNT exciton quenching,14 Iverson et al. detected local NO both in the mouse liver following direct intravenous injection of SWNCTs, and subcutaneously following the implantation of alginate hydrogels containing SWCNTs. Coupled with spatialspectral imaging deconvolution algorithm they have demonstrated the ability of DNA-wrapped SWCNTs as implantable sensors to detect nitric oxide, produced in response to inflammation, with minimal decay in sensing capacity for more than 400 days. By carefully choosing the nanotube coating that balanced the need for both selectivity and biocompatibility in vivo, this sensing scheme also exhibits high binding specificity to target analyte to a pool of interfering molecules in the biological environment. They further systematically investigated different sensor parameters to improve performance for in vivo biomedical applications. By using hydrogel-SWCNT riboflavin sensor as a subcutaneous implant in a mouse model,15 Iverson et al. have identified the limit of optical detection depth to approximately 5mm, and confirmed the alginate as a suitable coating to improve sensor response and shelf life. The first pharmacokinetic model of an SWCNT-based insulin sensor was also developed to establish relations between the maximum sensor response, delay time and other parameters with sensor concentration, geometry, placement, etc.16 This study is of particular interest since insulin levels are generally more stable than glucose levels in diabetes monitoring and can provide complementary information to current continuous glucose monitoring (CGM) devices. Real-time insulin monitoring could also help assess insulin sensitivity for type I and II diabetes patients with insulin resistance.17–18 Several questions about long term health effects, low quantum yield and slow fluorescence recovery of SWCNT-based sensor remain to be addressed. It is also noted that SWCNT and multi-walled carbon nanotubes (MWCNT) have different physical properties and toxicity profiles, and different nanotube functionalization methods have biocompatibility implications, all of which have to be taken into account when translating in vitro sensing scheme to in vivo operation.
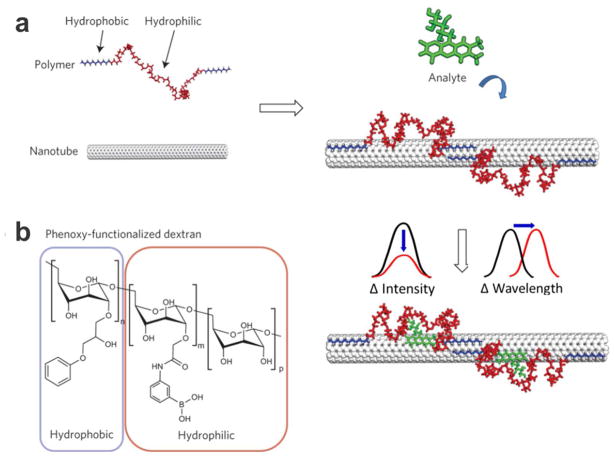
The potential of SWCNT as a functional building block for biosensors also spurred the development of novel molecular recognition motifs that can be integrated on carbon nanotube surfaces. One particular approach developed by Zhang et al. is termed “Corona Phase Molecular Recognition” (CoPhMoRe), 19 which consists of heteropolymers adsorbed onto nanoparticles (Figure 1). The nanoparticle-polymer interface enabled the formation of a new corona phase capable of recognizing small-molecule adsorbates with high selectivity based on conformational changes of the polymer at the particle surface. The polymer-particle complex effectively served as “synthetic antibody”, with analyte-binding events detectable as fluorescent changes from the polymer-conjugated nanoparticle. The polymer-nanoparticle interface thus has the potential to be the recognition moiety in designing new types of sensors and clinical assays.20 The Strano group has reported multiple sensors based on CoPhMoRe complexes, including riboflavin,19 dopamine21 and macromolecular targets such as fibrinogen.22 Bisker et al.23 and Ulissi et al.24 further proposed two mathematical models to describe the binding cavity generated from wrapping the polymer around a cylindrical substrate. These models can serve as the theoretical foundation for understanding CoPhMoRe mechanism and guiding rational polymer design, as an alternative to empirical library screening. Additionally, Salem et al. investigated chirality-dependent fluorescence modulation of DNA-wrapped SWCNTs for various molecular targets.25 They found that certain DNA sequences can generate distinctive CoPhMoRe phases dependent on SWCNT chirality and form a more compact packing to their respective chirality partners thus discouraging analyte absorption. Although CoPhMoRe has been exclusively studied in vitro, the powerful combination of SWCNT and CoPhMoRe has great potential as an in vivo spatiotemporal biosensor in biomedical applications.
Figure 1.
Principle and application of CoPhMoRe. (a) A polymer with hydrophobic and hydrophilic sequences attached to the nanotube. The polymer adopts specific conformation to create a selective binding cavity for analyte of interest, inducing wavelength or intensity change in SWCNT fluorescence. (b). An example of a hydrophobic–hydrophilic alternating sequence. Reprinted with permission from Reference 19. Nat Nanotechnol 2013, 8 (12), 959–968. Copyright 2013, Macmillan Publishers Ltd.
Optode nanosensors for real-time monitoring
The Clark group has produced chemical-based nanosensors in addition to biological-based nanosensors for monitoring physiological analytes or therapeutic drugs. These implantable nanoparticle-based sensors excel in the analytical requirement of continuous monitoring, but are typically limited to skin-based applications due to their dependence on optical reporters. This technology has a modular platform containing sensing components for a range of analytes with significant progress made to translate in vivo sensors into practical use. The concept of optode-based sensors26 is derived from its electrochemical cousin, by substituting optical reporters for electrically active species. Here the recognition component of the sensor needs to induce a local protonation/deprotonation after an analyte binding event, for example an ionophore for ion detection, or certain enzymes for biomolecule detection. Other components include a pH sensitive molecule to translate the binding event into detectable signal, and additional charge balancing molecules, all of which are coencapsulated into a lipophilic polymeric matrix with an additional biocompatible surface coating such as PEG. The optode sensor platform also exhibits high selectivity over ions with the same charge and interfering biomolecules, comparable to bulk optode sensors in similar conditions.27 Dubach et al first demonstrated the potential of optode nanosensors to track changes in exogenous sodium concentrations in the subcutaneous area of the mouse skin with a whole animal imaging system.28 Decoupling the recognition component from the signal reporter increased the flexibility and modular nature of this sensing scheme, allowing rapid development of sensors for novel analytes, without the laborious process of designing and synthesizing new analyte-binding molecules. The sensor has sufficient resolving power to capture the rapid kinetics of histamine after systemic injection into small animals.29 The signal transducing mechanism was also broadened from fluorescence to other schemes such as phosphorescence in this modular, injectable sensing platform. Cash et al. demonstrated a phosphorescent oxygen nanosensor based on diamine oxidase to monitor the in vivo histamine dynamics and monitor real-time pharmacokinetics.30 This example also highlights the potential of the platform to widen the range of measurable analytes by incorporating an enzymatic recognition element in the sensor that is capable of measuring the effects of enzymatic activity.
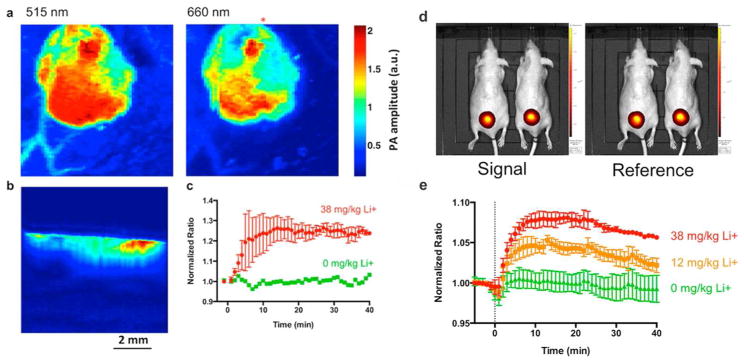
Personalized medicine could also benefit from the development of a robust modular sensing platform, providing physicians real-time feedback on the response to chronic disease treatment. One of the major obstacles for real-time drug monitoring is the need for tedious blood sampling, which could miss important events if sampling is infrequent and/or poorly calibrated. Cash et al. demonstrated, for the first time, an optode-based sensing solution for in vivo real-time tracking of therapeutic drug levels without blood sampling.31 They demonstrated that synthetic nanosensors can continuously track exogenous lithium levels in vivo with photoacoustic imaging (Figure 2). The robustness of the multimodal sensing platform was validated by comparing sensor responses between fluorescence and photoacoustic measurements. Similar lithium kinetics was obtained using both imaging modes, paving the way for applications of nanosensors implanted in the skin (outside the bloodstream). The ability for photoacoustic imaging to gain information in three dimensions also gave this sensing scheme a competitive edge by mapping the sensor responses in regions throughout the injection site, which is challenging for current fluorescence-based approaches. This 3D profiling will become more relevant for tracking larger molecules such as glucose or proteins since the physiological concentrations of analyte may differ substantially between the area closest to the bloodstream and the surface of the skin. The combination of photoacoustic imaging and optical nanosensors paves an experimental avenue to continuous in vivo tracking of a wide range of analytes.
Figure 2.
Photoacoustic nanosensors imaged in mouse model. Dual wavelength images from photoacoustic tomography (a) clearly show the entire sensor injection. A depth profile (b) taken below the red asterisk in (a) shows the nanosensor injection in the tissue. The nanosensors respond to systemic lithium administration (c) has a time to peak concentration of 14 min. The images of both wavelengths (d) demonstrate the excellent imaging quality. The lithium kinetics (e) obtained from fluorescence measurement is similar to that measured with photoacoustics in a dose-dependent manner. Reprinted with permission from Reference 31. ACS Nano 2015, 9 (2), 1692–1698. Copyright 2015, American Chemical Society.
One of the main challenges for injected nanosensors has always been off-site particle diffusion, as it severely restricts operating time in continuous monitoring applications. In order to mitigate sensor migration in vivo, gelling agents have been co-injected with nanosensors into the subcutaneous space in mice. Balaconis et al. assessed sensor performance with different gel encapsulation formulations and found that gels help improve nanosensor in vivo lifetime to over 1h without significantly altering the sensing capacity32. Balaconis et al. further improved the on-site residence time of the sensor by transforming the sensor geometry into a nanofiber by electrospinning the optode material, a scalable production technique often used for tissue engineering purposes.33 The nanofiber scaffold was shown to be more stable at the subdermal implantation site than spherical nanosensors, and the nanoscale geometry of the fiber retained a response time rapid enough for real-time monitoring. Although nanofibers have been employed as scaffold for detecting glucose34 and other analytes,35–37 this is the first time it has been used to monitor analytes in vivo. Overall, this fully reversible modular sensing platform has proven to be a viable candidate for continuous in vivo monitoring.
Semi-conducting polymers for multi-modal biosensing
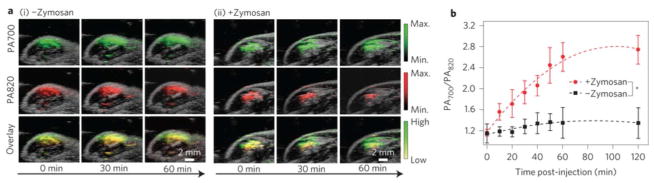
Rao and Gambhir et al. have demonstrated several probes to detect cancer, developing approaches for NIR fluorescent imaging, photoacoustic detection and concomitant deeper-tissue imaging. While many of these studies have focused on the design of highly sensitive/selective labels that allow early detection of tumors at the mm-length scale, they have also investigated several continuous biosensing approaches and made valuable contributions to early in vivo investigations. Pu et al. developed nanoparticle-based ROS sensors using a ratiometric photoacoustic output and demonstrated selective and real-time monitoring in a mouse model.38 The authors employed semi- conducting polymer nanoparticles with high NIR-absorption, which were originally designed for solar cell applications. The particles (40–50 nm) yielded strong fluorescence and photoacoustic signals both in gel phantoms and in vivo, with the photoacoustic signals stronger (on a mass basis) and more stable over time in comparison to SWCNTs and gold nanoparticles. Interestingly, upon systemic administration, the particles accumulated in the lymphatics, with the signal strength in the lymph nodes second only to that in the liver. Next, the authors incorporated a dye (IR775S) into the nanoparticles, which is sensitive to ROS-mediated oxidation and hence decreases both fluorescence intensity and photoacoustic amplitude in the presence of ROS. The photoacoustic signal from the nanoparticle itself was insensitive to ROS, hence a stable ratiometric signal could be generated. The authors then measured the ratiometric signal in a mouse model, injecting a ROS-stimulating drug (Zymosan) locally into the thigh, followed closely by injection of the nanoparticles at the same location (Figure 3). The authors demonstrated that they could monitor Zymosan-induced ROS generation over a 120-minute period to steady- state levels. While further investigation is required to evaluate reversibility, off-site diffusion, and biocompatibility, this is a promising platform for ROS sensing in vivo, either as stationary sensors, or sensors that accumulate in lymph nodes (which others have demonstrated are accessible with photoacoustic imaging in humans).39 The development and translation of photoaccoustic probes capable of in-depth monitoring could provide promising alternatives for continuous in vivo sensing applications.
Figure 3.
(a) Photoacoustic/ultrasound superimposed images of saline-treated (i) and zymosan-treated (ii) regions in the thigh of living mice (n = 3). Photoacoustic ROS sensor was injected 20 min after zymosan treatment. (b) Ratio of photoacoustic amplitude at 700 nm to that at 820 nm (PA700/PA820) as a function of time post-injection of ROS sensor. Reprinted with permission from Reference 38. Nat Nanotechnol 2014, 9 (3), 233–239. Copyright 2014, Macmillan Publishers Ltd.
Aptamer-modified electrodes for real-time monitoring
Plaxco and colleagues recently demonstrated real-time and continuous monitoring of therapeutic drugs in live animals using an electrochemical approach and a reversibly-binding, conformation-dependent aptamer.40 This example is distinct from established sensors used for continuous glucose monitoring because it is based on the engineered affinity of the aptamer for the target analyte, rather than the activity of an enzyme. Furthermore, using established SELEX approaches, high affinity aptamer sequences can be produced for a wide variety of targets, both for cell-bound and soluble analytes.41 They can also be further modified as required to improve sensor characteristics, including selectivity, kinetics, and long-term stability in biological fluids.42–43
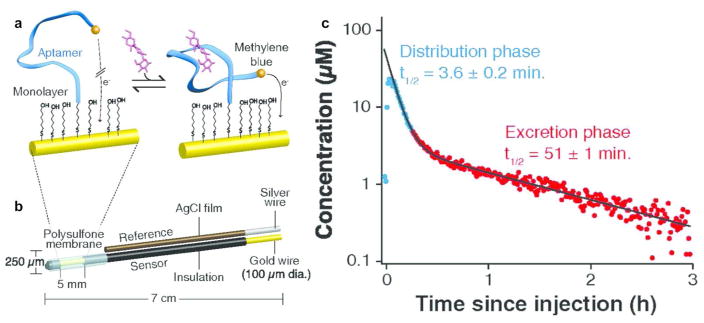
In 2013, Soh and Plaxco incorporated a doxorubicin-specific aptamer into a microfluidic device that could be interfaced directly with the blood flow from a live animal.44 The aptamer was tethered to a gold electrode via a 5′-thiol, and functionalized with methylene blue at the 3′ end, such that selective binding brought the methylene blue reporter closer to the electrode surface to facilitate current flow. This system showed excellent sensor characteristics, including submicromolar affinity, a dynamic range spanning the physiologically relevant range, and response times on the order of 45–100s. The selectivity was also demonstrated by showing that 1000-fold higher concentrations of related drugs did not result in conformational change of the aptamer. To reduce fouling at the electrode surface, the authors designed a “continuous diffusion filter” in which a buffer stream flows across the surface of the aptamer-modified electrode, parallel to blood flow, under laminar conditions. This provided a diffusion-limited barrier only allowing transport of small, highly diffusive, species (e.g. doxorubicin) to reach the electrode surface. With this system in place, the authors demonstrated that they could monitor doxorubicin levels in live rats in vivo and whole human blood in vitro, over 4.5hrs, including changes in response to drug spiking injections.
Recently, Plaxco’s group improved upon the initial design to produce an electrode that could be inserted directly into a catheter to monitor blood flow in the jugular vein.40 Rather than the continuous diffusion filter, the authors employed a biocompatible polysulfone membrane (0.2 μm pore size) to limit biofouling at the electrode surface (Figure 4). The authors then used the system to measure the real-time concentrations and pharmacokinetic curves associated with doxorubicin and several aminoglycoside antibiotics (intravenous or intra-muscular injection). The sensor discerned between interanimal variations and intra-animal variations (multiple injection cycles) in pharmacokinetic behavior, and could be used on awake and mobile animals.
Figure 4.
Aptamer-modified gold electrodes for long-term therapeutic drug monitoring in vivo. (a) Aptamers are immobilized onto electrode surfaces through gold-thiol chemistry. In the unbound state, the methylene blue reporter is a long way from the surface and so very little current is generated. In the bound state, the aptamer undergoes a conformational change and the reporter approaches the surface, generating increased current. (b) The electrode surface is coated in polysulfone to reduce biofouling from high molecular weight biomolecules and cells. (c) The sensor characteristics allow high-resolution monitoring of doxorubicin pharmacokinetics within an individual, potentially allowing for rapid changes in treatment regimen. Reprinted from Reference 40. Proceedings of the National Academy of Sciences of the United States of America 2017, 114 (4), 645–650. Copyright 2017, National Academy of Sciences.
The limitation in this system is the inability to monitor higher molecular weight species (proteins, cells, etc.) due to the need for a robust membrane to limit diffusion of charge-generating species to the electrode. However, this will be sufficient for many applications in therapeutic drug monitoring of “small molecule” drugs. The authors also noted some cross-reactivity of the aptamer with different drug analytes; while this should be less of a problem in the case of therapeutic drug monitoring (usually only single drug present from a structurally conserved class), it could be problematic in the case of monitoring endogenous biomarkers, for which further rounds of positive/negative selection in the SELEX process would be required.
Peptide-based sensors with signal detection in urine
The Bhatia group and colleagues developed a platform of protease activity sensors, comprised of nanoparticle-labeled peptides that are selectively cleaved by disease-specific proteases and detected in the urine, while the nanoparticles are cleared by the liver. Instead of an optical or electrochemical output, as is often used for detection, the peptide itself is the reporter. This approach enables the sensing chemistry to occur virtually anywhere in the body. Due to inherent limitations in deep-tissue optical imaging, it takes the clever step of removing the probe from the body for analysis. The capability of transducing the signal through noninvasive means is of great importance for in vivo biosensors to address point-of-care applications. To this end, urinary detection can serve as a natural conduit for transporting reporters of targeted biomolecule or biological activity of interest ex vivo for detailed analysis. The Bhatia group pioneered the use of in situ protease activity to track different features of human disease and evaluate pharmacological effects of drugs. In 2013, Kwong et al. built a sensing platform using protease-sensitive nanoparticles for tumor targeting, releasing mass-encoded reporters small enough for renal clearance and subsequent detection in urine using multiplexed mass spectrometry.45 This noninvasive approach has been successfully applied to mouse models of liver fibrosis and cancer, offering superb resolution and the benefit of early detection compared with clinically used blood biomarkers, opening up new possibilities for point-of-care diagnostics. The multiplexing approach can also profile disease-specific activity fingerprints to better discriminate disease with high specificity, which is challenging for single-biomarker assays.
The group recently advanced this platform further, by detecting the cleavage products in urine with a companion enzyme-linked immunosorbent assay (ELISA) or low-cost lateral flow immunoassay (LFA). This sensing scheme has been used in monitoring colorectal cancer and thrombosis in mouse models (Figure 5a).46–47 The low-cost LFA coupled with injectable synthetic biosensors that are readily accessible in body fluids provides a comprehensive solution for non-communicable disease diagnosis in resource-limited developing countries. This platform was further augmented by using orally administrated probiotic strains to generate urine products for detection of liver metastases in a rodent model without adverse health effects over a 1-year period.48 This study demonstrated that gene circuits could be delivered and colonized at diseased tissue sites selectively and safely by preprogrammed probiotics.
Figure 5.
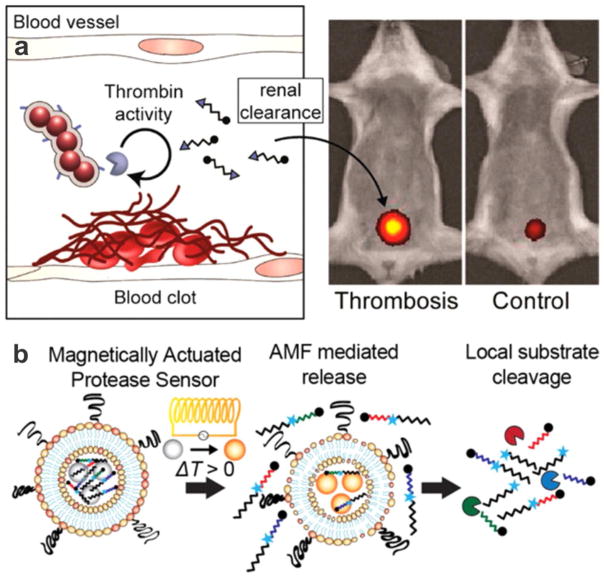
(a) Iron oxide nanoworms (NWs) modified with a thrombin-specific substrate coupled with a reporter. These constructs probe blood clots where the reporters are cleaved and released into urine by thrombin activities. In vivo image shows higher fluorescent signal to the bladders of thrombotic mice after thromboplastin administration. Reprinted with permission Reference 47. ACS Nano 2013, 7 (10), 9001–9009. Copyright 2013, American Chemical Society. (b) Magnetically actuated protease sensors. Thermosensitive liposomes were coloaded with magnetic nanoparticles and synthetic peptide substrate with near IR dye reporter. In the presence of alternating magnetic fields (AMF), heat dissipated by the MNPs melts the thermosensitive bilayer. This allows peptides to leak to the exterior, where they can respond to protease activity. Reprinted with permission from Reference 50. Nano Lett 2016, 16 (10), 6303–6310. Copyright 2016, American Chemical Society.
One of the challenges of the current protease activity sensors is that these probes are not immune to off-target activation. Hence, there is the possibility of generating false positive results. To address this, Dudani et al. recently developed a photocaged nanoscale construct to precisely control sensor activation both spatially and temporally.49 By conjugating photolabile protecting groups, off-site protease activity became minimal due to steric hindrance effects, but exposure of a disease site to UV light triggered the removal of the protecting groups, leading to site-specific protease activity. They applied this light-activated system to detect secreted protease activity at the primary tumor site in colorectal cancer xenografts. Another approach to realize remote sensor activation involved the use of alternating magnetic fields (AMFs), which offered sensor activation at deeper tissues in comparison with UV light activation (Figure 5b). Schuerle et al. took advantage of this property by designing protease substrates coated with thermosensitive-liposomes.50 The sensor was activated on-target by AMF-induced local temperature elevation via co-encapsulated magnetic nanoparticles. This model has been successfully applied to identify different protease substrate cleavage profiles in mouse models of human colorectal cancer. The ability to remotely control activity-based sensors will complement current tools that are vulnerable to off-target activation.
Another challenge is that the in vivo lifetime of these activity-based sensors, before protease cleavage, is limited with a half-life of the nanoparticle-peptide conjugate of ~6 hrs.45 Recently, this was partially overcome by developing a sustained-release biosensing formulation. Dudani et al. modified sensors with nanoscale (~8 nm) poly (ethylene glycol) (PEG) as a low-cost, biocompatible lifetime extender, with minimal clearance by the reticuloendothelial system. PEG particles were employed in this study as sensor backbones to facilitate diffusion of particles delivered subcutaneously into the blood-stream in a sustained manner. They investigated the in vivo pharmacokinetic properties of these biosensors and formulated a mathematical model of sensor in vivo characteristics as a guideline for diagnostic applications. They found that the new platform could extend the utility of the sensor to more than a day in vivo as demonstrated in a thrombin sensor and matrix metalloproteinase (MMP) sensor for point-of-care detection in mouse models.51 By combining PEG scaffold with subcutaneous sensor delivery, the new controlled-release sensing plat-form could be integrated with established implants, therefore providing a valuable path for long-term facile monitoring of patients after discharge from hospital.
Since this approach relies on detection of signal reporters in the urine, it lacks the ability to monitor the protease activities in real time. Furthermore, the sensor functions in an irreversible manner, as the cleavage of the signaling motif is an irrevocable process; therefore it requires constant replenishment for long term monitoring applications. One can argue, however, the progression of the diseases targeted is slow enough to be captured within the time frame offered by this scheme. In terms of timing, the sensors have a half-life of ~ 6 hrs45 with strong liver uptake but negligible transfer to the urine. The peptides however are rapidly cleared through the kidneys into the urine, hence the response time of this sensor system is dependent on a combination of the protease cleavage kinetics and then the time that it takes to produce urine output. In the Bhatia group’s studies, the peptides were detectable in urine 30–60 minutes after initiation of thrombosis46–47 suggesting that the sensor response time is rapid enough for their diseases of interest, ranging from clot sensing through to early detection of solid tumors. Overall the versatility of urinary monitoring combined with the ability of remote activation of the sensor offers great potential in personalized diagnostics.
EMERGING MATERIALS FOR IN VIVO BIOSENSING
New materials are emerging which have the potential to address the current shortcomings of in vivo biosensors. While some of the particular examples discussed below may not yet constitute in vivo biosensors under our definitions, they open up new opportunities to address inherent challenges in this field, particularly in terms of biocompatibility, minimising offsite diffusion for long term operation, and opening up modes of signal transduction beyond fluorescence imaging.
Neural sensors and materials designed for soft tissue biocompatibility
Several recent studies have demonstrated the potential of in vivo biosensing using Magnetic Resonance Imaging (MRI) in neural tissues. Traditional neural approaches rely on implanted electrodes which are invasive, breach the blood-brain-barrier, and have mechanical properties (high modulus, stiffness) that are not compatible with soft biological material. Optical approaches have limited penetration depth through bone and tissue, therefore the development of approaches that combine non-optical signal transduction with non-invasive sensors would be highly significant.52 MRI is already in high clinical and research use, and has sufficient penetration depth to image internal regions of the body, albeit at lower resolution and sensitivity than optical imaging.53 Lee et al., demonstrated that a neurotransmitter (dopamine) could be monitored continuously in the rat brain. 54 They used a novel dopamine-specific engineered heme protein as the receptor (BM3h-97D) that modulates T1 contrast upon dopamine binding. Both spatial and temporal dopamine levels were recorded in response to electrical stimuli, and the MRI response correlated with electrochemical dopamine monitoring. However, to obtain continuous measurements, the authors relied on continuous injection and off-site diffusion of the sensor protein, which has limited potential for translation. Liu et al. addressed the issues of off-site diffusion and sensor lifetime by designing an MRI contrast agent based on biocompatible silicones embedded into an injectable polymer matrix.55 This oxygen-responsive sensor was stable, biocompatible, and yielded reversible oxygen monitoring for at least 4 weeks, whereas a comparable liquid siloxane injected directly into tissue had a half-life of just 35 hrs. The sensor yielded comparable results to pulse oximetry in terms of response time and kinetics. Interestingly, while the shape of the sensor was not consistent between animals, the data was reproducible because oxygen levels were calibrated to a bulk T1 measurement. Beyond the problem of off-site diffusion, another key problem in this space is that the analyte concentration measurement is dependent on the sensor concentration. However, several recent reports have been published on ratiometric MRI sensors, which could allow reversible monitoring independent of sensor concentration.56–58 Currently these approaches have only been demonstrated in vitro.
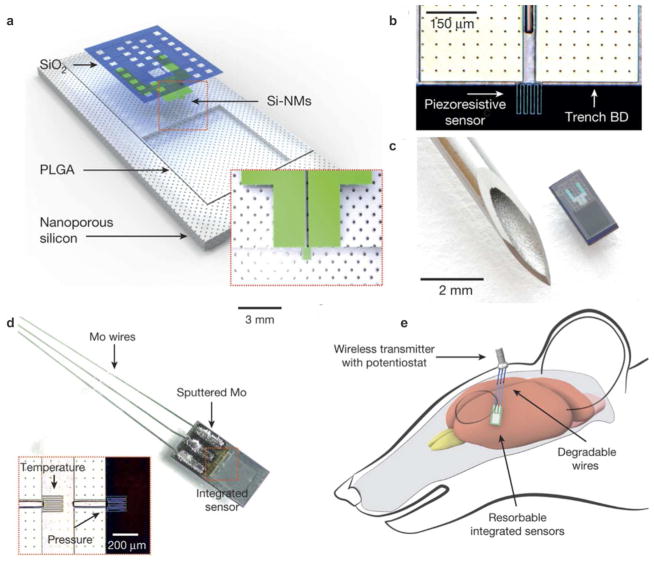
Beyond neural biosensors, a variety of new materials have recently been reported for accurate recording of neuronal activity in vivo, with significant improvements over traditional microelectrodes. While not yet fitting our definition for biosensing, their mechanical and biocompatibility profiles lend themselves to further development in this area. A range of organic and inorganic devices have been trialed in vivo, including bioresorbable silicon59–60, polymeric microthreads61 and microfibers62, carbon nanotubes63, semiconductor ZnO crystals64 and gold nanowires65. The materials were developed with the aim of improving long-term biocompatibility and recording capability while minimizing localized tissue damage at the sampling point. Rogers’ group have recently introduced “bioresorbable silicon” sensors (Figure 6), which at this point in time are designed to monitor temperature, flow, and pH based on electrical measurements. 59–60 These sensors degrade hydrolytically over time, with normal metabolism, and are designed to have wireless compatibility. Demonstrated both in vitro and in vivo in rat models, the material lasts for ~ 1 month before degrading, with negligible effect on local tissue.
Figure 6.
(a–c) Bioresorbable silicon pressure sensor, composed of a nanoporous silicon substrate coated with PLGA polymer. An air cavity in between these two layers allows membrane deflection in response to fluid pressure changes. The next layer comprises a silicon nanomembrane as a piezoresistive element to convert pressure change into an electrical signal, coated with a silica overlay. (d) Temperature sensors were also incorporated, based on the temperature-dependent resistance of the silicon nanomembrane elements located away from the air cavity. Dissolvable sputtered molybdenum coatings and molybdenum wires integrated the sensor to the (e) wireless transmitter system. Reprinted with permission from Reference 59. Nature 2016, 530 (7588), 71–76. Copyright 2016, Macmillan Publishers Limited.
Kotov and colleagues have also developed several materials61, 63, 65 that address the problem of how to monitor neural activity from the same neurons for long periods of time by developing materials that are significantly smaller than individual neurons. Initially, Kozai et al. fabricated an ultra-small and completely organic microelectrode (carbon fibers coated with parylene-N for electrical recording and polyethylene glycol for anti-fouling), with a subcellular cross-section61 and demonstrated neuronal activity modelling in a rat model. Importantly, while the electrodes produced were extremely small, they had the required stiffness/flexibility combination for standalone use. Based on the reduced size, the microelectrodes induced less bleeding and disruption of the blood-brain-barrier in comparison to a traditional silicon electrode. Furthermore, they showed improved tissue response after 2-week implantation in comparison to the silicon electrode, indicated by higher levels of neural cells (astrocytes, microglia, endothelial cells) adjacent to the surface, which were histologically similar to unperturbed nearby tissue. Zhang et al. moved onto carbon nanotubes, which have excellent electrical, mechanical and chemical properties for neural interface applications, but previously had only been fabricated into electrodes on the order of ~200 μm. Here they fabricated flexible electrode arrays with sensing regions on the order of single neurons and demonstrated a new implantation procedure for soft devices into brain tissue. While preliminary neural activity monitoring was performed in rat brains, these devices are yet to undergo rigorous stimulus-response testing in vivo. Most recently, Kang et al. produced super-flexible, single-crystal gold nanowire probes with a diameter of ~100 nm. When loaded onto tungsten-tips, these electrodes were easily inserted into brain tissue with minimal tissue disruption, and used for short-term recording in mouse brains following response to different stimuli. Further investigations on the effects of micromotions of the nanowire in the brain, and the biocompatibility of the devices are required for further development.
Although we will not be reviewing the sensors developed in this area, the field of optogenetics has significantly driven the recent development of interesting devices that combine the desired mechanical properties for neural interfacing with electrical recording and transmission of light. In the most recent developments, individual sensing units have been combined in microelectrode arrays, enabling spatio-temporal control over light delivery and electrical recording. Canales et al. used scalable thermal drawing processes (TPD) to produce optical fibers that combined optical stimulation, electrical recording of responses, and also delivery of drugs through microfluidic channels.62 Distinct from traditional electrodes, their fibers were highly bendable and flexible, with stiffness sufficient to allow for micromotion related to physiological processes including heartbeat and respiration. After verifying each function independently in the brains of live mice, they observed a significantly reduced tissue response over the first 3 days in comparison to a microwire electrode, but then comparable responses from 1–12 wks. Lee et al. took a different approach and designed an intracortical ZnO-based microprobe array.64 ZnO has a unique combination of optical transparency, impendence and biocompatibility, and is hence potentially well-suited to the application. Uniquely, both light activation and recording occur in the same ZnO pillars without spatial separation, hence combining these pillars into a 4-by-4 array allowed the authors to investigate high-resolution optogenetic activity.
Hydrogel materials enabling improved sensor performance in vivo
Outside of neural tissue, several researchers have also investigated the inclusion of biosensors into hydrogels to monitor the “cargo” and to address the issue of off-site diffusion. Chan et al., designed a system to monitor the viability of hydrogel-encapsulated therapeutic cells following in vivo insertion.66 Here the authors synthesized ~350 μm microcapsules containing a mixture of the therapeutic cells and pH-responsive L-arginine liposomes. When the pH inside the alginate-coated capsules becomes more acidic, it led to reduced proton exchange rate between the L-arginine NH-groups and surrounding water molecules, detectable by MRI (using “chemical exchange saturation transfer” or CEST). The authors showed that when using immunosuppressive drugs to protect the cells during implantation, the cells remained viable for longer (maintained pH), and this correlated with bioluminescence data generated in vivo over 14 days.
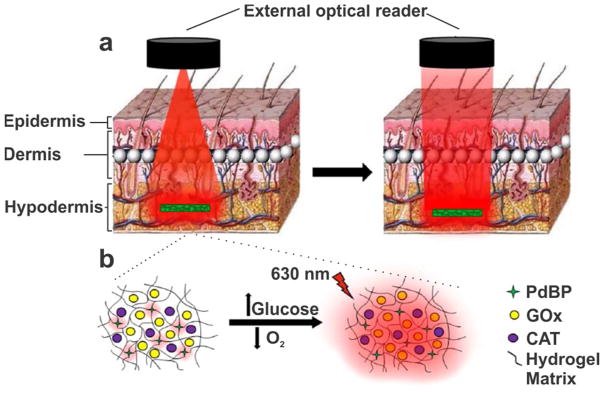
In another example, McShane’s group have investigated key issues related to transport phenomena, sensor characteristics, long-term stability and bio-fouling in polymeric implants, both in vitro and in vivo. Recently, Roberts et al. designed a group of hydrogels based on a combination of polyacrylamide (PAM) and poly(2-hydroxyethyl methacrylate) (polyHEMA), containing encapsulated glucose oxidase (GOx) and an oxygen-sensitive phosphor.67 The inclusion of PAM into the matrix increased the diffusivity of glucose through the material, and increased the analytical sensitivity of the sensor, but shifted the dynamic range below the clinically relevant window. Furthermore the sensor characteristics of the polyHEMA gels were relatively unaffected by serum exposure, whereas the PAM-co-polyHEMA materials showed decreased sensitivity as a result of bio-fouling. Unruh et al. then implanted their sensors into pigs with the aim of realizing fully implantable glucose sensors without the need for percutaneous wire probes.68 The authors demonstrated that tuning the composition of their polyHEMA-based hydrogels varied the diffusion of oxygen and glucose through the material, in turn tuning the response time of the reporter chemistry without changing the composition of the sensor itself. The authors successfully tracked glucose levels in comparison to blood glucose over a hyperglycemia/euglycemia/hypoglycemia cycle, and further studies will investigate the long-term stability and biocompatibility of these sensors (Figure 7). This important work high-lights the importance of the materials used for implantation on the performance of the sensor itself.
Figure 7.
(a) Hydrogel system as a fully-implantable, optically active glucose sensor. The excitation source ((i); light-emitting diode, l = 630 nm) and detector ((ii); photo-multiplier tube) were housed in a 1.5-in diameter “puck”. (b) The hydrogel sensor was composed of a poly(HEMA)-co-PAM hydrogel matrix containing encapsulated glucose oxidase (GOx), catalase (CAT), and an oxygen-sensitive benzoporphyrin phosphor (PdBP). In the presence of glucose, GOx catalyzes the oxidation of b-D-glucose to D-glucono-1,5-lactone, reducing the oxygen to hydrogen peroxide. Catalase is included as a peroxide scavenger, to increase the lifetime of GOx. Reprinted with permission from Reference 68. J Diabetes Sci Technol 2015, 9 (5), 985–992. Copyright 2015, SAGE Publications.
Emerging materials for new scaffolds and receptors for in vivo biosensing
Polymeric scaffolds have a long history of use as biocompatible excipients based on their synthetic versatility, with a range of FDA-approved polymer-drug conjugates and nanoparticles on the market.69 Indeed the majority of in vivo biosensors to date involve a polymer component, including several of the case studies discussed in the earlier section; here we highlight some recent developments. Polymer-dye conjugates are commonly employed in the area of imaging contrast agents, however Zheng et al recently demonstrated ratiometric oxygen sensing using a simple PVP-dye conjugate in a mouse tumor xenograft model.70 While PVP (polyvinyl pyrrolidone) can be used to extend the blood half-life of poorly soluble drugs, in this case the PVP was conjugated to an iridium (III) complex whose peak phosphorescent intensity varies inversely to the oxygen concentration; hence the fluorescence is “switched on” in hypoxic tissue. This lead to highly specific tumor labelling over 1–120 hours after systemic administration. In another example, Sowers et al synthesised a “branched-bottlebrush” polymer which showed both 1H-MRI contrast enhancement (organic nitroxide groups) and fluorescence increase (Cy-5.5) upon exposure to ascorbate both in vitro and in vivo in mice.71 Intriguingly, the dual-modality behaviour is dependent on the redox state of the polymer; hence no fluorescence increase was observed in the absence of nitroxide groups. Approximately 30% of the injected systemic dose was still circulating after 3 days, suggesting reasonably good biodistribution and half-life.
A wide variety of inorganic and hybrid materials have also been reported for sensing, imaging, and multi-modal applications, yet relatively few have progressed to in vivo studies.9, 72–73 Silica-based materials offer a number of advantages including low toxicity, size tunability, facile chemistry, brightness and photostability (with dyes encapsulated). Brabury, Weisner, and colleagues, have shown that ultra-fluorescent silica nanospheres are biocompatible in humans and could be used to identify cancerous lesions in patients.74 In a clinical trial, Phillips et al. evaluated the biodistribution properties and also monitored the metabolic profiles of study subjects, with renal clearance over several days. Other groups have used silica shells to provide biocompatibility and dispersibility properties for nanomedicines also in human trials.75 Given these studies are highly positive in terms of biocompatibility, this may be an interesting material to pursue for future in vivo biosensing applications. Indeed, several recent studies have been published demonstrating reversible sensing of pH76–79 among other examples reviewed elsewhere,9, 72 hence translating these sensors into animal studies could yield some highly interesting developments. Another emerging inorganic material is porous silicon, which has been recently shown by Tong et al. to be biocompatibility in subcutaneous tissues.80 This material can be fabricated using standard and scalable microfabrication procedures, with facile surface chemistry, and has been used to develop a range of interesting assays using near-infrared and infrared light for signal transduction.81–82
The emergence of novel synthetic and biological receptors is another recent development, potentially opening up opportunities beyond traditional small molecule and ion sensing. “Nanozymes” – synthetic molecules or nanoparticles possessing intrinsic catalytic activity – have recently been applied to real-time glucose monitoring in the rat brain.83 Although in this study a microdialysis approach was employed, the authors combined hemin (peroxidase mimic) and glucose oxidase inside a metal organic framework (MOF) and demonstrated that the proximity of the two enzyme systems allowed sensing of glucose with fast kinetics. The combination of multiple enzyme systems into a single sensor for real-time sensing, with protection from the MOF scaffold, could lend itself to further developments. In terms of biological receptors, the use of “directed evolution” library approaches (e.g. phage, SELEX, etc.)84 have been employed to discover, engineer, and optimize a range of binders to specific targets without the need for individual assay development which is the case historically for enzymatic activity sensors. The difficulty in applying them in vivo is in designing the positive/negative selection of clones in an environment that closely resembles that in vivo. Furthermore, to date, few directed evolution approaches have been used successfully to design reversible probes, perhaps due to the low “hit” rate for selecting high quality responsive binders.85. Jasanoff’s BM3h family86 (including the MRI-based dopamine sensor discussed earlier) is one seemingly successful example of applying a directed evolution approach to develop a receptor, however the reversibility in a non-diffusing scaffold is yet to be demonstrated. Aptamers, on the other hand, could be an alternative library platform to address these issues, based on their simple structures and ability to be engineered in terms of binding affinity, kinetics, and long-term stability in biological environments.
In conclusion, significant progress has been made both in the demonstration of novel biosensors in vivo, but also in the scientific pipeline that will lead to breakthroughs in this field. Further research is required in the areas of novel scaffold materials, novel biological receptors, and the design of novel biosensing concepts that can utilize existing and emerging biomedical imaging modalities. Further innovations in developing stable biological receptors for long-term monitoring will open doors for sensing new analytes. Given the wealth of knowledge and the cautionary tales surrounding the field of implantable/injectable materials (including nanomedicines, sensors, etc.), we have the opportunity to investigate issues of biocompatibility, tissue response, cellular uptake, biodistribution and clearance, without necessarily “re-inventing the wheel”. Although there was a surprisingly narrow number of groups meeting the analytical requirements to take biosensing technologies in vivo, we see this field as fertile ground for further fundamental and translational research.
Acknowledgments
Funding Sources
SRC acknowledges funding support from the Australian Research Council (CE170100059) and HAC acknowledges funding support from the National Institutes of Health (R01NS081641).
The authors gratefully acknowledge feedback/editing support from Mr Daniel Bobo, Mr Jiaul Islam, and Ms Julia Walker.
ABBREVIATIONS
- PVP
polyvinylpyrrolidone
- DNA
deoxyribonucleic acid
- SELEX
Systematic evolution of ligands by exponential enrichment
- MOF
metal-organic framework
- FDA
federal drug administration (USA)
- PAM
polyacrylamide
- poly(HEMA)
poly(2-hydroxyethyl methacrylate)
- CEST
chemical exchange saturation transfer
- AMF
alternating magnetic field
- PEG
polyethylene glycol
- MRI
magnetic resonance imaging
Footnotes
VOCABULARY
Near infrared (NIR); a region of the visible spectrum >700 nm, in which minimal tissue absorption/scattering effects facilitate high-resolution fluorescent imaging. Carbon nanotubes (CNTs); graphite sheets wrapped into cylinders, which can be single wall CNTs (SWCNTs; from a single graphite sheet), or multi-wall CNTs (MWCNTs; multiple sheets wrapped into concentric cylinders). Off-site diffusion; the process by which sensors diffuse away from the target location, potentially biasing the signal output. Reversibility; the ability of the reporter to bind and release the target analyte, in order to measure analyte dynamics. Ratiometric signal; the practice of normalizing the analyte-sensitive signal to an analyte-insensitive signal to eliminate effects of signal drift, environmental variation, and variations in sensor concentration in the imaging region.
Author Contributions
The manuscript was written through contributions of all authors
References
- 1.Borisov SM, Wolfbeis OS. Optical Biosensors. Chem Rev. 2008;108(2):423–461. doi: 10.1021/cr068105t. [DOI] [PubMed] [Google Scholar]
- 2.Wolfbeis OS. Fiber-Optic Chemical Sensors and Biosensors. Anal Chem. 2008;80(12):4269–4283. doi: 10.1021/ac800473b. [DOI] [PubMed] [Google Scholar]
- 3.Eersels K, Lieberzeit P, Wagner P. A Review on Synthetic Receptors for Bioparticle Detection Created by Surface-Imprinting Techniques - from Principles to Applications. ACS Sensors. 2016;1(10):1171–1187. [Google Scholar]
- 4.Ruckh TT, Clark HA. Implantable Nanosensors: Toward Continuous Physiologic Monitoring. Anal Chem. 2014;86(3):1314–1323. doi: 10.1021/ac402688k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soto R, Hall JR, Brown MD, Taylor JB, Schoenfisch MH. In Vivo Chemical Sensors: Role of Biocompatibility on Performance and Utility. Anal Chem. 2016;89(1):276–299. doi: 10.1021/acs.analchem.6b04251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkowska-Nery E, Kundys M, Jeleń PS, Jönsson-Niedziółka M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal Chem. 2016;88(23):11271–11282. doi: 10.1021/acs.analchem.6b03151. [DOI] [PubMed] [Google Scholar]
- 7.Bandodkar AJ, Jeerapan I, Wang J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sensors. 2016;1(5):464–482. [Google Scholar]
- 8.Lin MZ, Schnitzer MJ. Genetically Encoded Indicators of Neuronal Activity. Nat Neurosci. 2016;19(9):1142–1153. doi: 10.1038/nn.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfbeis OS. An Overview of Nanoparticles Commonly Used in Fluorescent Bioimaging. Chem Soc Rev. 2015;44(14):4743–4768. doi: 10.1039/c4cs00392f. [DOI] [PubMed] [Google Scholar]
- 10.Howes PD, Chandrawati R, Stevens MM. Colloidal Nanoparticles as Advanced Biological Sensors. Science. 2014;346(6205):53. doi: 10.1126/science.1247390. [DOI] [PubMed] [Google Scholar]
- 11.Tagit O, Hildebrandt N. Fluorescence Sensing of Circulating Diagnostic Biomarkers Using Molecular Probes and Nanoparticles. ACS Sensors. 2017;2(1):31–45. doi: 10.1021/acssensors.6b00625. [DOI] [PubMed] [Google Scholar]
- 12.Bachilo SM, Strano MS, Kittrell C, Hauge RH, Smalley RE, Weisman RB. Structure-Assigned Optical Spectra of Single-Walled Carbon Nanotubes. Science. 2002;298(5602):2361–2366. doi: 10.1126/science.1078727. [DOI] [PubMed] [Google Scholar]
- 13.Iverson NM, Barone PW, Shandell M, Trudel LJ, Sen S, Sen F, Ivanov V, Atolia E, Farias E, McNicholas TP, Reuel N, Parry NMA, Wogan GN, Strano MS. In Vivo Biosensing Via Tissue-Localizable near-Infrared-Fluorescent Single-Walled Carbon Nanotubes. Nat Nanotechnol. 2013;8(11):873–880. doi: 10.1038/nnano.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JQ, Boghossian AA, Barone PW, Rwei A, Kim JH, Lin DH, Heller DA, Hilmer AJ, Nair N, Reuel NF, Strano MS. Single Molecule Detection of Nitric Oxide Enabled by D(at)(15) DNA Adsorbed to near Infrared Fluorescent Single-Walled Carbon Nanotubes. J Am Chem Soc. 2011;133(3):567–581. doi: 10.1021/ja1084942. [DOI] [PubMed] [Google Scholar]
- 15.Iverson NM, Bisker G, Farias E, Ivanov V, Ahn J, Wogan GN, Strano MS. Quantitative Tissue Spectroscopy of near Infrared Fluorescent Nanosensor Implants. J Biomed Nanotechnol. 2016;12(5):1035–1047. doi: 10.1166/jbn.2016.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisker G, Iverson NM, Ahn J, Strano MS. A Pharmacokinetic Model of a Tissue Implantable Insulin Sensor. Adv Healthc Mater. 2015;4(1):87–97. doi: 10.1002/adhm.201400264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahn SE. The Relative Contributions of Insulin Resistance and Beta-Cell Dysfunction to the Pathophysiology of Type 2 Diabetes. Diabetologia. 2003;46(1):3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 18.Donga E, van Dijk M, Hoogma RPLM, Corssmit EPM, Romijn JA. Insulin Resistance in Multiple Tissues in Patients with Type 1 Diabetes Mellitus on Long-Term Continuous Subcutaneous Insulin Infusion Therapy. Diabetes-Metab Res. 2013;29(1):33–38. doi: 10.1002/dmrr.2343. [DOI] [PubMed] [Google Scholar]
- 19.Zhang JQ, Landry MP, Barone PW, Kim JH, Lin SC, Ulissi ZW, Lin DH, Mu B, Boghossian AA, Hilmer AJ, Rwei A, Hinckley AC, Kruss S, Shandell MA, Nair N, Blake S, Sen F, Sen S, Croy RG, Li DY, Yum K, Ahn JH, Jin H, Heller DA, Essigmann JM, Blankschtein D, Strano MS. Molecular Recognition Using Corona Phase Complexes Made of Synthetic Polymers Adsorbed on Carbon Nanotubes. Nat Nanotechnol. 2013;8(12):959–968. doi: 10.1038/nnano.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landry MP, Kruss S, Nelson JT, Bisker G, Iverson NM, Reuel NF, Strano MS. Experimental Tools to Study Molecular Recognition within the Nanoparticle Corona. Sensors-Basel. 2014;14(9):16196–16211. doi: 10.3390/s140916196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruss S, Landry MP, Vander Ende E, Lima BMA, Reuel NF, Zhang JQ, Nelson J, Mu B, Hilmer A, Strano MS. Neurotransmitter Detection Using Corona Phase Molecular Recognition on Fluorescent Single-Walled Carbon Nanotube Sensors. J Am Chem Soc. 2014;136(2):713–724. doi: 10.1021/ja410433b. [DOI] [PubMed] [Google Scholar]
- 22.Bisker G, Dong J, Park HD, Iverson NM, Ahn J, Nelson JT, Landry MP, Kruss S, Strano MS. Protein-Targeted Corona Phase Molecular Recognition. Nat Commun. 2016;7:10241. doi: 10.1038/ncomms10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bisker G, Ahn J, Kruss S, Ulissi ZW, Salem DP, Strano MS. A Mathematical Formulation and Solution of the Cophmore Inverse Problem for Helically Wrapping Polymer Corona Phases on Cylindrical Substrates. J Phys Chem C. 2015;119(24):13876–13886. [Google Scholar]
- 24.Ulissi ZW, Zhang JQ, Sresht V, Blankschtein D, Strano MS. 2d Equation-of-State Model for Corona Phase Molecular Recognition on Single-Walled Carbon Nanotube and Graphene Surfaces. Langmuir. 2015;31(1):628–636. doi: 10.1021/la503899e. [DOI] [PubMed] [Google Scholar]
- 25.Salem DP, Landry MP, Bisker G, Ahn J, Kruss S, Strano MS. Chirality Dependent Corona Phase Molecular Recognition of DNA-Wrapped Carbon Nanotubes. Carbon. 2016;97:147–153. [Google Scholar]
- 26.Mistlberger G, Crespo GA, Bakker E. Ionophore-Based Optical Sensors. Annu Rev Anal Chem. 2014;7:483–512. doi: 10.1146/annurev-anchem-071213-020307. [DOI] [PubMed] [Google Scholar]
- 27.Xie XJ, Bakker E. Ion Selective Optodes: From the Bulk to the Nanoscale. Anal Bioanal Chem. 2015;407(14):3899–3910. doi: 10.1007/s00216-014-8413-4. [DOI] [PubMed] [Google Scholar]
- 28.Dubach JM, Lim E, Zhang N, Francis KP, Clark H. In Vivo Sodium Concentration Continuously Monitored with Fluorescent Sensors. Integr Biol-Uk. 2011;3(2):142–148. doi: 10.1039/c0ib00020e. [DOI] [PubMed] [Google Scholar]
- 29.Cash KJ, Clark HA. In Vivo Histamine Optical Nanosensors. Sensors-Basel. 2012;12(9):11922–11932. doi: 10.3390/s120911922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cash KJ, Clark HA. Phosphorescent Nanosensors for in Vivo Tracking of Histamine Levels. Anal Chem. 2013;85(13):6312–6318. doi: 10.1021/ac400575u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cash KJ, Li CY, Xia J, Wang LHV, Clark HA. Optical Drug Monitoring: Photoacoustic Imaging of Nanosensors to Monitor Therapeutic Lithium in Vivo. ACS Nano. 2015;9(2):1692–1698. doi: 10.1021/nn5064858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balaconis MK, Clark HA. Gel Encapsulation of Glucose Nanosensors for Prolonged in vivo Lifetime. J Diabetes Sci Technol. 2013;7(1):53–61. doi: 10.1177/193229681300700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balaconis MK, Luo Y, Clark HA. Glucose-Sensitive Nanofiber Scaffolds with an Improved Sensing Design for Physiological Conditions. Analyst. 2015;140(3):716–723. doi: 10.1039/c4an01775g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou CS, Shi YL, Ding XD, Li M, Luo JJ, Lu ZY, Xiao D. Development of a Fast and Sensitive Glucose Biosensor Using Iridium Complex-Doped Electrospun Optical Fibrous Membrane. Anal Chem. 2013;85(2):1171–1176. doi: 10.1021/ac303107d. [DOI] [PubMed] [Google Scholar]
- 35.Kacmaz S, Ertekin K, Suslu A, Ergun Y, Celik E, Cocen U. Sub-Nanomolar Sensing of Ionic Mercury with Polymeric Electrospun Nanofibers. Mater Chem Phys. 2012;133(1):547–552. [Google Scholar]
- 36.Kacmaz S, Ertekin K, Suslu A, Ozdemir M, Ergun Y, Celik E, Cocen U. Emission Based Sub-Nanomolar Silver Sensing with Electrospun Nanofibers. Sens Actuator B-Chem. 2011;153(1):205–213. [Google Scholar]
- 37.Yang YF, Wang HM, Su K, Long YY, Peng Z, Li N, Liu F. A Facile and Sensitive Fluorescent Sensor Using Electrospun Nanofibrous Film for Nitroaromatic Explosive Detection. J Mater Chem. 2011;21(32):11895–11900. [Google Scholar]
- 38.Pu KY, Shuhendler AJ, Jokerst JV, Mei JG, Gambhir SS, Bao ZN, Rao JH. Semiconducting Polymer Nanoparticles as Photoacoustic Molecular Imaging Probes in Living Mice. Nat Nanotechnol. 2014;9(3):233–239. doi: 10.1038/nnano.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoffels I, Morscher S, Helfrich I, Hillen U, Lehy J, Burton NC, Sardella TCP, Claussen J, Poeppel TD, Bachmann HS, Roesch A, Griewank K, Schadendorf D, Gunzer M, Klode J. Metastatic Status of Sentinel Lymph Nodes in Melanoma Determined Noninvasively with Multispectral Optoacoustic Imaging. Sci Transl Med. 2015;7(317):199. doi: 10.1126/scitranslmed.aad1278. [DOI] [PubMed] [Google Scholar]
- 40.Arroyo-Currás N, Somerson J, Vieir PA, Ploense KL, Kippin TE, Plaxco KW. Real-Time Measurement of Small Molecules Directly in Awake, Ambulatory Animals. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(4):645–650. doi: 10.1073/pnas.1613458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng HM, Liu H, Kuai HL, Peng RZ, Mo LT, Zhang XB. Aptamer-Integrated DNA Nanostructures for Biosensing, Bioimaging and Cancer Therapy. Chemical Society Reviews. 2016;45(9):2583–2602. doi: 10.1039/c5cs00645g. [DOI] [PubMed] [Google Scholar]
- 42.Yang L, Qing ZH, Liu CH, Tang Q, Li JS, Yang S, Zheng J, Yang RH, Tan WH. Direct Fluorescent Detection of Blood Potassium by Ion-Selective Formation of Intermolecular G-Quadruplex and Ligand Binding. Analytical Chemistry. 2016;88(18):9285–9292. doi: 10.1021/acs.analchem.6b02667. [DOI] [PubMed] [Google Scholar]
- 43.Shi H, He XX, Cui WS, Wang KM, Deng K, Li D, Xu FZ. Locked Nucleic Acid/DNA Chimeric Aptamer Probe for Tumor Diagnosis with Improved Serum Stability and Extended Imaging Window in vivo. Analytica Chimica Acta. 2014;812:138–144. doi: 10.1016/j.aca.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson BS, Hoggarth DA, Maliniak D, Ploense K, White RJ, Woodward N, Hsieh K, Bonham AJ, Eisenstein M, Kippin TE, Plaxco KW, Soh HT. Real-Time, Aptamer-Based Tracking of Circulating Therapeutic Agents in Living Animals. Sci Transl Med. 2013;5(213):165. doi: 10.1126/scitranslmed.3007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwong GA, von Maltzahn G, Murugappan G, Abudayyeh O, Mo S, Papayannopoulos IA, Sverdlov DY, Liu SB, Warren AD, Popov Y, Schuppan D, Bhatia SN. Mass-Encoded Synthetic Biomarkers for Multiplexed Urinary Monitoring of Disease. Nat Biotechnol. 2013;31(1):63–70. doi: 10.1038/nbt.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warren AD, Kwong GA, Wood DK, Lin KY, Bhatia SN. Point-of-Care Diagnostics for Noncommunicable Diseases Using Synthetic Urinary Biomarkers and Paper Microfluidics. P Natl Acad Sci USA. 2014;111(10):3671–3676. doi: 10.1073/pnas.1314651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin KY, Kwong GA, Warren AD, Wood DK, Bhatia SN. Nanoparticles That Sense Thrombin Activity as Synthetic Urinary Biomarkers of Thrombosis. ACS Nano. 2013;7(10):9001–9009. doi: 10.1021/nn403550c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danino T, Prindle A, Kwong GA, Skalak M, Li H, Allen K, Hasty J, Bhatia SN. Programmable Probiotics for Detection of Cancer in Urine. Sci Transl Med. 2015;7(289):1–11. doi: 10.1126/scitranslmed.aaa3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dudani JS, Jain PK, Kwong GA, Stevens KR, Bhatia SN. Photoactivated Spatiotemporally-Responsive Nanosensors of in Vivo Protease Activity. ACS Nano. 2015;9(12):11708–11717. doi: 10.1021/acsnano.5b05946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuerle S, Dudani JS, Christiansen MG, Anikeeva P, Bhatia SN. Magnetically Actuated Protease Sensors for in Vivo Tumor Profiling. Nano Lett. 2016;16(10):6303–6310. doi: 10.1021/acs.nanolett.6b02670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dudani JS, Buss CG, Akana RTK, Kwong GA, Bhatia SN. Sustained-Release Synthetic Biomarkers for Monitoring Thrombosis and Inflammation Using Point-of-Care Compatible Readouts. Adv Funct Mater. 2016;26(17):2919–2928. doi: 10.1002/adfm.201505142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong JW, Shin G, Il Park S, Yu KJ, Xu LZ, Rogers JA. Soft Materials in Neuroengineering for Hard Problems in Neuroscience. Neuron. 2015;86(1):175–186. doi: 10.1016/j.neuron.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 53.Wang LHV, Hu S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science. 2012;335(6075):1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee T, Cai LX, Lelyveld VS, Hai A, Jasanoff A. Molecular-Level Functional Magnetic Resonance Imaging of Dopaminergic Signaling. Science. 2014;344(6183):533–535. doi: 10.1126/science.1249380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu VH, Vassiliou CC, Imaad SM, Cima MJ. Solid Mri Contrast Agents for Long–Term, Quantitative in vivo Oxygen Sensing. P Natl Acad Sci USA. 2014;111(18):6588–6593. doi: 10.1073/pnas.1400015111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunduz S, Savic T, Pohmann R, Logothetis NK, Scheffler K, Angelovski G. Ratiometric Method for Rapid Monitoring of Biological Processes Using Bioresponsive Mri Contrast Agents. Acs Sensors. 2016;1(5):483–487. doi: 10.1021/acssensors.6b00011. [DOI] [PubMed] [Google Scholar]
- 57.Okada S, Mizukami S, Sakata T, Matsumura Y, Yoshioka Y, Kikuchi K. Ratiometric Mri Sensors Based on Core-Shell Nanoparticles for Quantitative Ph Imaging. Advanced Materials. 2014;26(19):2989–2992. doi: 10.1002/adma.201305018. [DOI] [PubMed] [Google Scholar]
- 58.Oukhatar F, Meme S, Meme W, Szeremeta F, Logothetis NK, Angelovski G, Toth E. Mri Sensing of Neurotransmitters with a Crown Ether Appended Gd3+ Complex. Acs Chemical Neuroscience. 2015;6(2):219–225. doi: 10.1021/cn500289y. [DOI] [PubMed] [Google Scholar]
- 59.Kang SK, Murphy RKJ, Hwang SW, Lee SM, Harburg DV, Krueger NA, Shin JH, Gamble P, Cheng HY, Yu S, Liu ZJ, McCall JG, Stephen M, Ying HZ, Kim J, Park G, Webb RC, Lee CH, Chung SJ, Wie DS, Gujar AD, Vemulapalli B, Kim AH, Lee KM, Cheng JJ, Huang YG, Lee SH, Braun PV, Ray WZ, Rogers JA. Bioresorbable Silicon Electronic Sensors for the Brain. Nature. 2016;530(7588):71–76. doi: 10.1038/nature16492. [DOI] [PubMed] [Google Scholar]
- 60.Yu KJ, Kuzum D, Hwang SW, Kim BH, Juul H, Kim NH, Won SM, Chiang K, Trumpis M, Richardson AG, Cheng HY, Fang H, Thompson M, Bink H, Talos D, Seo KJ, Lee HN, Kang SK, Kim JH, Lee JY, Huang YG, Jensen FE, Dichter MA, Lucas TH, Viventi J, Litt B, Rogers JA. Bioresorbable Silicon Electronics for Transient Spatiotemporal Mapping of Electrical Activity from the Cerebral Cortex. Nat Mater. 2016;15(7):782–791. doi: 10.1038/nmat4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kozai TDY, Langhals NB, Patel PR, Deng XP, Zhang HN, Smith KL, Lahann J, Kotov NA, Kipke DR. Ultrasmall Implantable Composite Microelectrodes with Bioactive Surfaces for Chronic Neural Interfaces. Nature Materials. 2012;11(12):1065–1073. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Canales A, Jia XT, Froriep UP, Koppes RA, Tringides CM, Selvidge J, Lu C, Hou C, Wei L, Fink Y, Anikeeva P. Multifunctional Fibers for Simultaneous Optical, Electrical and Chemical Interrogation of Neural Circuits in vivo. Nat Biotechnol. 2015;33(3):277–284. doi: 10.1038/nbt.3093. [DOI] [PubMed] [Google Scholar]
- 63.Zhang HN, Patel PR, Xie ZX, Swanson SD, Wang XD, Kotov NA. Tissue-Compliant Neural Implants from Microfabricated Carbon Nanotube Multilayer Composite. ACS Nano. 2013;7(9):7619–7629. doi: 10.1021/nn402074y. [DOI] [PubMed] [Google Scholar]
- 64.Lee J, Ozden I, Song YK, Nurmikko AV. Transparent Intracortical Microprobe Array for Simultaneous Spatiotemporal Optical Stimulation and Multichannel Electrical Recording. Nat Methods. 2015;12(12):1157–1162. doi: 10.1038/nmeth.3620. [DOI] [PubMed] [Google Scholar]
- 65.Kang M, Jung S, Zhang HN, Kang T, Kang H, Yoo Y, Hong JP, Ahn JP, Kwak J, Jeon D, Kotov NA, Kim B. Subcellular Neural Probes from Single-Crystal Gold Nanowires. Acs Nano. 2014;8(8):8182–8189. doi: 10.1021/nn5024522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan KWY, Liu GS, Song XL, Kim H, Yu T, Arifin DR, Gilad AA, Hanes J, Walczak P, van Zijl PCM, Bulte JWM, McMahon MT. Mri-Detectable Ph Nanosensors Incorporated into Hydrogels for in vivo Sensing of Transplanted-Cell Viability. Nature Materials. 2013;12(3):268–275. doi: 10.1038/nmat3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts JR, Park J, Helton K, Wisniewski N, McShane MJ. Biofouling of Polymer Hydrogel Materials and Its Effect on Diffusion and Enzyme-Based Luminescent Glucose Sensor Functional Characteristics. J Diabetes Sci Technol. 2012;6(6):1267–1275. doi: 10.1177/193229681200600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Unruh RM, Roberts JR, Nichols SP, Gamsey S, Wisniewski NA, McShane MJ. Preclinical Evaluation of Poly(Hema-Co-Acrylamide) Hydrogels Encapsulating Glucose Oxidase and Palladium Benzoporphyrin as Fully Implantable Glucose Sensors. J Diabetes Sci Technol. 2015;9(5):985–992. doi: 10.1177/1932296815590439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-Based Medicines: A Review of Fda-Approved Materials and Clinical Trials to Date. Pharmaceutical Research. 2016;33(10):2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 70.Zheng XC, Wang X, Mao H, Wu W, Liu BR, Jiang XQ. Hypoxia-Specific Ultrasensitive Detection of Tumours and Cancer Cells in vivo. Nat Commun. 2015;6:5834. doi: 10.1038/ncomms6834. [DOI] [PubMed] [Google Scholar]
- 71.Sowers MA, McCombs JR, Wang Y, Paletta JT, Morton SW, Dreaden EC, Boska MD, Ottaviani MF, Hammond PT, Rajca A, Johnson JA. Silica-Responsive Branched-Bottlebrush Polymers for in vivo Mri and Fluorescence Imaging. Nat Commun. 2014;5:5460. doi: 10.1038/ncomms6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Q, Li Y, Su XG. Silica-Nanobead-Based Sensors for Analytical and Bioanalytical Applications. Trac-Trends in Analytical Chemistry. 2015;74:130–145. [Google Scholar]
- 73.Wang KM, He XX, Yang XH, Shi H. Functionalized Silica Nanoparticles: A Platform for Fluorescence Imaging at the Cell and Small Animal Levels. Accounts of Chemical Research. 2013;46(7):1367–1376. doi: 10.1021/ar3001525. [DOI] [PubMed] [Google Scholar]
- 74.Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye YP, Humm J, Gonen M, Kalaigian H, Schoder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS. Clinical Translation of an Ultrasmall Inorganic Optical-PET Imaging Nanoparticle Probe. Sci Transl Med. 2014;6(260):149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anselmo AC, Mitragotri S. A Review of Clinical Translation of Inorganic Nanoparticles. Aaps Journal. 2015;17(5):1041–1054. doi: 10.1208/s12248-015-9780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang FL, Raval Y, Tzeng TRJ, Anker JN. X-Ray Excited Luminescence Chemical Imaging of Bacterial Growth on Surfaces Implanted in Tissue. Advanced Healthcare Materials. 2015;4(6):903–910. doi: 10.1002/adhm.201400685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang FL, Raval Y, Chen HY, Tzeng TRJ, DesJardins JD, Anker JN. Development of Luminescent Ph Sensor Films for Monitoring Bacterial Growth through Tissue. Advanced Healthcare Materials. 2014;3(2):197–204. doi: 10.1002/adhm.201300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang FL, Widejko RG, Yang ZQ, Nguyen KT, Chen HY, Fernando LP, Christensen KA, Anker JN. Surface-Enhanced Raman Scattering Detection of Ph with Silica-Encapsulated 4-Mercaptobenzoic Acid-Functionalized Silver Nanoparticles. Analytical Chemistry. 2012;84(18):8013–8019. doi: 10.1021/ac3018179. [DOI] [PubMed] [Google Scholar]
- 79.Lapresta-Fernandez A, Doussineau T, Moro AJ, Dutz S, Steiniger F, Mohr GJ. Magnetic Core-Shell Fluorescent Ph Ratiometric Nanosensor Using a Stober Coating Method. Analytica Chimica Acta. 2011;707(1):164–170. doi: 10.1016/j.aca.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 80.Tong WY, Sweetman MJ, Marzouk ER, Fraser C, Kuchel T, Voelcker NH. Towards a Subcutaneous Optical Biosensor Based on Thermally Hydrocarbonised Porous Silicon. Biomaterials. 2016;74:217–230. doi: 10.1016/j.biomaterials.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 81.Jenie SNA, Plush SE, Voelcker NH. Recent Advances on Luminescent Enhancement-Based Porous Silicon Biosensors. Pharmaceutical Research. 2016;33(10):2314–2336. doi: 10.1007/s11095-016-1889-1. [DOI] [PubMed] [Google Scholar]
- 82.Gupta B, Zhu Y, Guan B, Reece PJ, Gooding JJ. Functionalised Porous Silicon as a Biosensor: Emphasis on Monitoring Cells in vivo and in vitro. Analyst. 2013;138(13):3593–3615. doi: 10.1039/c3an00081h. [DOI] [PubMed] [Google Scholar]
- 83.Cheng HJ, Zhang L, He J, Guo WJ, Zhou ZY, Zhang XJ, Nie SM, Wei H. Integrated Nanozymes with Nanoscale Proximity for in Vivo Neurochemical Monitoring in Living Brains. Anal Chem. 2016;88(10):5489–5497. doi: 10.1021/acs.analchem.6b00975. [DOI] [PubMed] [Google Scholar]
- 84.Williams RM, Sooter LJ. In Vitro Selection of Cancer Cell-Specific Molecular Recognition Elements from Amino Acid Libraries. J Immunol Res. 2015;2015:186586. doi: 10.1155/2015/186586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Picciotto S, Dickson PM, Traxlmayr MW, Marques BS, Socher E, Zhao SX, Cheung S, Kiefer JD, Wand AJ, Griffith LG, Imperiali B, Wittrup KD. Design Principles for Sucessful Biosensors: Specific Fluorophore/Analyte Binding and Minimization of Fluorophore/Scaffold Interactions. Journal of Molecular Biology. 2016;428(20):4228–4241. doi: 10.1016/j.jmb.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shapiro MG, Westmeyer GG, Romero PA, Szablowski JO, Kuster B, Shah A, Otey CR, Langer R, Arnold FH, Jasanoff A. Directed Evolution of a Magnetic Resonance Imaging Contrast Agent for Noninvasive Imaging of Dopamine. Nat Biotechnol. 2010;28(3):264–270. doi: 10.1038/nbt.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]