Abstract
We have previously shown that the tumor necrosis factor family member a proliferation-inducing ligand (APRIL) enhances intestinal tumor growth in various preclinical tumor models. Here, we have investigated whether APRIL serum levels at time of surgery predict survival in a large cohort of colorectal cancer (CRC) patients. We measured circulating APRIL levels in a cohort of CRC patients (n=432) using a novel validated monoclonal APRIL antibody (hAPRIL.133) in an enzyme-linked immunosorbent assay (ELISA) setup. APRIL levels were correlated with clinicopathological features and outcome. Overall survival was examined with Kaplan–Meier survival analysis, and Cox proportional hazards ratios were calculated. We observed that circulating APRIL levels were normally distributed among CRC patients. High APRIL expression correlated significantly with poor outcome measures, such as higher stage at presentation and development of lymphatic and distant metastases. Within the group of rectal cancer patients, higher circulating APRIL levels at time of surgery were correlated with poor survival (log-rank analysis P-value 0.008). Univariate Cox regression analysis for overall survival in rectal cancer patients showed that patients with elevated circulating APRIL levels had an increased risk of poor outcome (hazard ratio (HR) 1.79; 95% confidence interval (CI) 1.16–2.76; P-value 0.009). Multivariate analysis in rectal cancer patients showed that APRIL as a prognostic factor was dependent on stage of disease (HR 1.25; 95% CI 0.79–1.99; P-value 0.340), which was related to the fact that stage IV rectal cancer patients had significantly higher levels of APRIL. Our results revealed that APRIL serum levels at time of surgery were associated with features of advanced disease and prognosis in rectal cancer patients, which strengthens the previously reported preclinical observation of increased APRIL levels correlating with disease progression.
Introduction
A proliferation-inducing ligand (APRIL), also known as TNFSF13, is a member of the tumor necrosis factor family that is known to bind to two receptors, B-cell maturation antigen (BCMA) and transmembrane activator and CAML interactor.1, 2 In addition, APRIL can bind to heparan sulfate proteoglycans,3 which act as a scaffold for proper signaling.4 Several preclinical studies have shown that APRIL can have a tumor-promoting role in solid malignancies. For example, we have shown that systemic APRIL overexpression supports tumor outgrowth in several mouse models of intestinal cancer. Moreover, knockdown of APRIL in colon cancer cells, which endogenously express APRIL, inhibited in vitro and in vivo outgrowth.5 This is in agreement with studies in which BCMA-Fc has been used as a decoy receptor and resulted in reduced tumor outgrowth of colon carcinoma cell lines.6 It is likely that both tumor cell- and non-tumor cell-derived APRIL act together to support tumor growth and progression in these preclinical models.
Evidence also suggests a role for APRIL in tumor promotion in a clinical context. For instance, several studies measuring RNA levels reported overexpression of APRIL in solid tumors compared with non-malignant tissue.7, 8 Similarly, immunohistochemical analysis of a large panel of solid tumors detected an accumulation of APRIL in the majority of tumor tissues analyzed. Nonetheless, the primary source of APRIL has remained unclear. In the latter study, it was concluded that tumor-infiltrating neutrophils present in the stroma, rather than the tumor cells, constitute the main source of APRIL.9 The authors postulated that retention of APRIL in the lesion occurs by binding to heparan sulfate proteoglycans. This contrasts an immunohistochemical study by Petty et al.10 who detected APRIL expression in tumor cells in more than half of the 234 CRC samples tested. Irrespective of the exact source, circulating APRIL levels may thus represent a (surrogate) marker for tumor growth and potentially survival. In a more recent study, APRIL serum levels from patients suffering from CRC were suggested to have diagnostic value as they correlated to known biomarkers such as CEA and CA19-9.11 This prognostic relevance may not be restricted to solid malignancies. A retrospective study in chronic lymphocytic leukemia patients showed that increased levels of APRIL in serum correlated with increased risk of disease progression and lower overall patient survival.12 Similarly, elevated APRIL expression was observed in Hodgkin’s lymphoma and multiple myeloma.13, 14 A retrospective study in diffuse large B-cell lymphoma patients showed that elevated APRIL expression in lymphoma lesions correlated with a poor survival rate.15
In this study we used a new antibody against APRIL that is able to reliably detect HEK293 expressed human APRIL in the absence and presence of human serum. We used this antibody to design an enzyme-linked immunosorbent assay (ELISA) for the detection of APRIL in patient serum samples. We then used this newly developed strategy in CRC patients to analyze the relationship between APRIL serum levels and outcome in patients undergoing surgery for CRC.
Results
Development and characterization of hAPRIL.133-based ELISA
We derived monoclonal APRIL antibodies by immunizing mice with APRIL-encoding plasmids. We selected several candidates based on the ability to bind recombinant APRIL when bound to BCMA-Fc (Supplementary Figure 1a). We used a collection of candidate anti-APRIL monoclonal antibodies (mAbs) to setup an ELISA. We coated 96-wells plates with BCMA-Fc and detected APRIL with the newly derived anti-APRIL mAbs followed by an horseradish peroxidase-labeled anti-mouse IgG (Figure 1a). We tested the mAbs for their capacity to detect APRIL in serum of chronic lymphocytic leukemia patients. We observed that all candidates detect APRIL to a similar extent, thus we continued with one of them, hAPRIL.133, for further development (Supplementary Figure 1b). The hAPRIL.133 ELISA specifically recognized APRIL as shown by detection of APRIL in supernatant from APRIL-transfected HEK293T cells. The sensitivity of this ELISA was 0.06 ng/ml. Note that APRIL was reliably detected irrespective of whether it was serially diluted in phosphate buffered saline–bovine serum albumin (PBS–BSA) or in human serum (Figure 1b). We compared this new ELISA setup to other ELISAs and found that it provided the most reliable method to detect APRIL in human serum (Supplementary Figure 2).
Figure 1.
hAPRIL.133 reliably detects human APRIL in the presence of human serum. (a) Schematic representation of the ELISA setup. We coated 96-wells plates with BCMA-Fc, and detected recombinant APRIL with the newly derived anti-APRIL mAbs followed by an horseradish peroxidase-labeled anti-mouse IgG. (b) The selected hAPRIL.133 mAb detects APRIL reliably independent of whether it was serially diluted in PBS–BSA or in human serum. Two standard curves were generated by serial dilutions of human APRIL in PBS+fetal calf serum 10%+human serum 20% (PBS–fetal calf serum–HS) or in PBS+BSA 1% (PBS–BSA).
APRIL correlates with measures of poor outcome in colorectal cancer patients
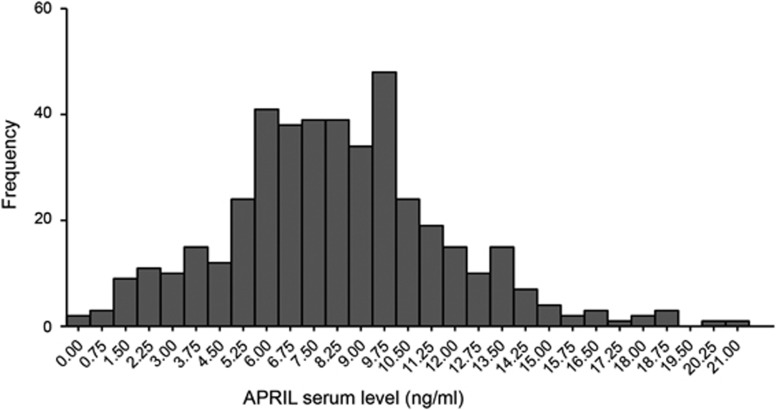
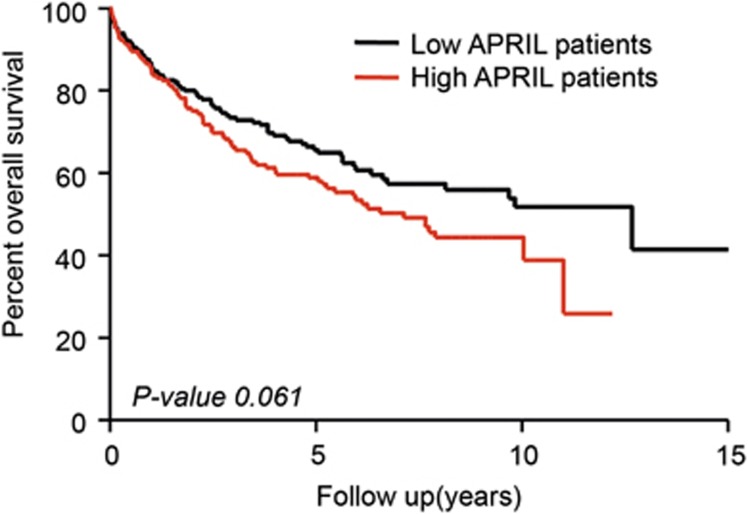
We next used this ELISA to test APRIL serum levels in a cohort of 432 colorectal cancer patients (patient characteristics in Table 1). APRIL levels displayed a Gaussian distribution throughout the CRC patient cohort with a mean serum level of 8.24 ng/ml (± 3.50 s.d.) (Figure 2). On the basis of this mean serum level, we divided the patients into two groups with either high or low APRIL serum levels. Circulating APRIL levels did not significantly correlate with factors such as age, gender and grade. In contrast, elevated APRIL significantly correlated with poor outcome measures such as higher stage, and increased risk of lymphatic and distant metastases (χ2 test) (Table 2). The impact on prognosis was analyzed by the cumulative overall survival (Kaplan–Meier curve). Patients with elevated circulating APRIL levels showed a trend towards a reduced survival rate, however this did not reach significance (P-value 0.061) (Figure 3).
Table 1. Patient characteristics of the colorectal cancer cohort.
| Factor | Case number |
|---|---|
| Overall | 432 |
| Organ | |
| Colon | 221 |
| Rectum | 209 |
| Both | 2 |
| Gender | |
| Male | 237 |
| Female | 195 |
| Age | |
| <67 | 206 |
| ⩾67 | 226 |
| Stage | |
| I | 117 |
| II | 106 |
| III | 106 |
| IV | 95 |
| Unknown | 8 |
| Grade | |
| G1 | 16 |
| G2 | 328 |
| G3 | 76 |
| Unknown | 12 |
| Lymphatic metastasis | |
| Negative | 224 |
| Positive | 203 |
| Unknown | 5 |
| Distant metastasis | |
| Negative | 329 |
| Positive | 95 |
| Unknown | 8 |
| Adjuvant treatment | |
| Chemotherapy | 98 |
| Immunotherapie | 3 |
| Radiochemotherapy | 43 |
| Radiation | 9 |
| None | 234 |
| Unknown | 45 |
| Neoadjuvant treatment | |
| Chemotheraphy | 1 |
| Radiochemotherapy | 52 |
| None | 359 |
| Unknown | 20 |
Figure 2.
Histogram showing the distribution of APRIL levels in the serum of colorectal cancer patients (n=432). The mean level is 8.24 ng/ml (± 3.50 s.d.).
Table 2. APRIL serum levels associate to features of advanced disease in colorectal cancer patients.
| Factor | Case number |
APRIL serum levels |
P-value | |
|---|---|---|---|---|
| Low | High | |||
| Overall | 432 | |||
| Gender | 0.805 | |||
| Male | 237 | 128 | 109 | |
| Female | 195 | 103 | 92 | |
| Age | 0.349 | |||
| <67 | 206 | 115 | 91 | |
| ⩾67 | 226 | 116 | 110 | |
| Organ | ||||
| Colon | 221 | 106 | 115 | 0.015 |
| Rectum | 209 | 125 | 84 | |
| Both | 2 | 0 | 2 | |
| Stage | 0.013 | |||
| I | 117 | 71 | 46 | |
| II | 106 | 60 | 46 | |
| III | 106 | 54 | 52 | |
| IV | 95 | 39 | 56 | |
| Unknown | 8 | 7 | 1 | |
| Grade | ||||
| G1 | 16 | 7 | 9 | 0.224 |
| G2 | 328 | 179 | 147 | |
| G3 | 76 | 35 | 41 | |
| Unknown | 12 | 10 | 4 | |
| Lymphatic metastasis | 0.004 | |||
| Negative | 224 | 132 | 92 | |
| Positive | 203 | 94 | 109 | |
| Unknown | 5 | 5 | 0 | |
| Distant metastasis | 0.005 | |||
| Negative | 329 | 185 | 144 | |
| Positive | 95 | 39 | 56 | |
| Unknown | 8 | 7 | 1 | |
APRIL serum levels and clinicopathological parameters APRIL levels were divided into high and low using the mean value as a cutoff. APRIL levels were not associated with parameters such as gender, age and grade (data not shown), whereas APRIL levels differ significantly for stage, lymphatic and distant metastasis (all χ2 test, except χ2 test by trend was performed for analysis of stage).
Figure 3.
Kaplan–Meier curve shows a trend towards reduced survival in patients with high APRIL serum levels. Full cohort of colorectal cancer patients (n=432) was divided for high and low APRIL level patients by the mean expression (Log-rank test).
APRIL has a significant effect on overall survival in rectal cancer patients
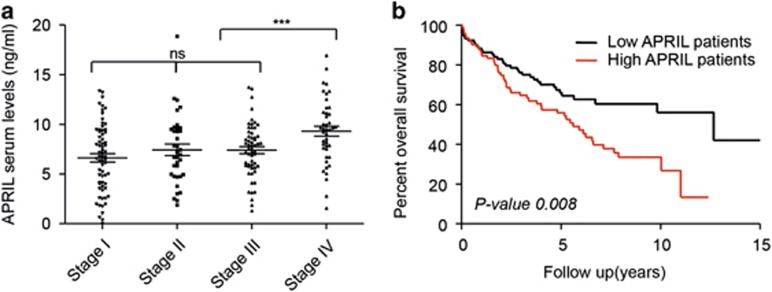
We next analyzed colon and rectal cancer patients separately. We observed that the correlation of APRIL serum levels with poor outcome measures in the entire cohort of colorectal cancer patients was mostly determined by the group of rectal cancer patients (Table 3, Supplementary Table 1, Supplementary Figure 3). Within the group of rectal cancer patients, circulating APRIL levels at time of surgery increased towards higher stages (mean stage I=6.67 ng/ml; mean stage II=7.38 ng/ml and mean stage III=7.40 ng/ml). APRIL serum levels were particularly elevated in stage IV patients (mean stage IV=9.32 ng/ml) (Figure 4a) and consistently correlated with the presence of metastatic disease and reduced survival (log-rank P-value 0.008) (Table 3, Figure 4b and Table 4a). Bivariate analysis in rectal cancer patients confirmed that the influence of APRIL as a prognostic factor was highly dependent on the stage of disease (HR 1.25; 95% CI 0.79–1.99; P-value 0.340) (Table 4b).
Table 3. Correlation between APRIL serum levels and clinicopathological parameters of rectal cancer patients.
| Factor |
APRIL serum levels |
P-value | |
|---|---|---|---|
| Low | High | ||
| Overall (n=209) | |||
| Gender | |||
| Male | 70 | 45 | 0.729 |
| Female | 55 | 39 | |
| Age | 0.901 | ||
| <67 | 74 | 49 | |
| ⩾67 | 51 | 35 | |
| Stage | 0.016 | ||
| I | 46 | 22 | |
| II | 22 | 13 | |
| III | 36 | 21 | |
| IV | 16 | 27 | |
| Unknown | 5 | 1 | |
| Grade | 0.792 | ||
| G1 | 4 | 2 | |
| G2 | 92 | 63 | |
| G3 | 21 | 16 | |
| Unknown | 8 | 3 | |
| Lymphatic metastasis | 0.031 | ||
| Negative | 68 | 35 | |
| Positive | 53 | 49 | |
| Unknown | 4 | 0 | |
| Distant metastasis | 0.002 | ||
| Negative | 104 | 56 | |
| Positive | 16 | 27 | |
| Unknown | |||
APRIL levels were divided into high and low using the median value as a cutoff. APRIL levels correlate significantly with stage, lymphatic and distant metastasis (χ2 test, χ2 test by trend was performed for analysis of stage).
Figure 4.
APRIL serum levels associate to advanced disease features and are prognostic in rectal cancer patients. High APRIL levels in serum of rectal cancer patients are associated with reduced overall survival. (a) APRIL is highly expressed in metastatic stage IV patients. Bee swarm representation of APRIL expression in the different stages of rectal cancer patients. (b) Kaplan–Meier analysis of overall survival in 209 rectal patients grouped according to their APRIL serum level as high and low (using median as cutoff). Patients with high APRIL levels had a significantly lower overall survival (Log-rank test).
Table 4. Cox proportional hazards regression model.
| Factors | HR | 95%CI | P-value |
|---|---|---|---|
| Gender | |||
| Male | 1 | 0.045 | |
| Female | 0.64 | 0.42–0.99 | |
| Age | |||
| 67< | 1 | 0.322 | |
| 67⩾ | 1.24 | 0.81–1.90 | |
| Grade | |||
| G1 | 1 | 0.745 | |
| G2 | 2.05 | 0.28–14.86 | |
| G3 | 2.45 | 0.33–18.48 | |
| Gx | 1.57 | 0.16–15.06 | |
| Stage | |||
| I | 1 | 9.93E−11 | |
| II | 2.43 | 1.21–4.88 | |
| III | 2.3 | 1.19–4.45 | |
| IV | 10.04 | 5.18–19.45 | |
| Unknown | 2.78 | 0.63–2.31 | |
| APRIL expression | |||
| Low | 1 | 0.009 | |
| High | 1.79 | 1.16–2.76 | |
| Lymphatic metastasis | |||
| Negative | 1 | 1.92E−05 | |
| Positive | 2.89 | 1.85–4.54 | |
| Unknown | 1.37 | 0.18–10.15 | |
| Distant metastasis | |||
| Negative | 1 | 2.66E−11 | |
| Positive | 5.75 | 3.52–9.41 | |
| Unknown | 1.61 | 0.39–6.65 | |
| APRIL expression | |||
| Low | 1 | 0.340 | |
| High | 1.25 | 0.79–1.99 | |
| Stage | |||
| I | 1 | 4.55E−09 | |
| II | 2.41 | 1.20–4.85 | |
| III | 2.26 | 1.17–4.37 | |
| IV | 9.24 | 4.67–18.28 | |
| Unknown | 2.93 | 0.66–12.99 | |
Univariate analysis for overall survival of rectal cancer patients showed that gender, stage, lymphatic metastasis, distant metastasis and APRIL levels are statistically significant prognostic factors in our study cohort. APRIL levels as prognostic factor are dependent on stage. Cox proportional hazards regression model. Bivariate analysis on APRIL expression and stage for overall survival on rectal cancer patients.
As serum samples were derived at time of surgery, our cohort of rectal cancer patients was heterogeneous with respect to neoadjuvant treatment. To investigate the impact of neoadjuvant treatment on APRIL serum levels and advanced disease features in our cohort of rectal cancer patients, we corrected for treatment status. We observed that elevated APRIL serum levels are prognostic of poor outcome in rectal cancer patients that have not received neoadjuvant treatment (log-rank analysis P-value 0.013; Supplementary Figure 4b). In the group of patients that had undergone neoadjuvant treatment the prognostic effect of elevated APRIL serum levels was not observed (Supplementary Figure 4a; descriptive characteristics of patients that received or not neoadjuvant treatment in Supplementary Table 2). This may indicate that timing of sample collection is critical for the use of APRIL as a serum marker for poor outcome.
Discussion
Development of patient-tailored therapies is critically dependent on easy-to-implement biomarkers that reliably predict outcome and response to therapy. Serum markers are particularly attractive because they allow relatively noninvasive longitudinal sampling in a uniform fashion. We previously found that transgenic elevation of systemic APRIL levels supports intestinal tumorigenesis in preclinical tumor models.5 The assessment of the impact of APRIL on tumor growth in the clinical context is complex as many antibodies are limited in their ability to detect APRIL levels against a background of serum epitopes. To overcome this limitation and study the impact of APRIL levels on patient prognosis, we derived a panel of anti-APRIL mAbs and screened for their capacity to recognize APRIL against a background of human serum. We selected one candidate, hAPRIL.133, based on its ability to detect APRIL in samples from chronic lymphocytic leukemia patients. We used this newly derived hAPRIL.133 to test APRIL levels in a cohort of colorectal cancer patients. We found that elevated circulating APRIL levels were associated with features of advanced disease such as stage and lymphatic and distant metastasis, which supports our earlier preclinical work.
There are clear differences in epidemiology, molecular biology, and prognosis between right and left-sided tumors.16 The cohort of colon tumors in this study represents a heterogeneous population of left and right-sided lesions. It is currently unclear whether there are differences in APRIL serum values between patients with left and those with right-sided CRC. For these reasons we decided to investigate colon and rectal cancer patients separately. We observed that the association with advanced disease features relied mostly on the rectal cancer patient population, whereas in colon cancer patients this association was not seen. We cannot exclude that APRIL might have an effect in defined groups of patients with colon cancer as well, but further stratification of patients may be required to investigate this.
APRIL levels associate with poor outcome and metastatic disease in rectal cancer patients. Previously, using a different ELISA setup, it was also shown that APRIL serum levels increase in colorectal disease and are associated with stage, although a difference between rectal and colon carcinoma was not tested.11 This suggests that systemic levels of APRIL might be a useful tool to select, in a noninvasive fashion, poor prognosis patients that benefit most from (neo)adjuvant treatment. About 25–65% of patients with locally advanced (cT3 and/or N1/N2) rectal cancer develop distant metastases. These patients may benefit most from alterations in treatment schedule to allow local control through surgery following radiotherapy and optimal combination chemotherapy. In this context, serum APRIL levels may be useful to indicate patients with a higher likelihood of a poor outcome or monitor treatment response in a relatively noninvasive fashion.
The association between circulating APRIL levels and patient prognosis was not observed in rectal cancer patients who had previously undergone neoadjuvant treatment. It is conceivable that APRIL is a direct readout of tumor load (and metastatic potential). Previous work on a set of tumor biopsies from CRC patients using complementary DNA expression arrays has suggested that APRIL expression may be induced in tumor cells following neoadjuvant treatment.10 Similar results were shown in cell lines.17 Our results clearly show that total circulating APRIL levels decrease after neoadjuvant treatment. This may indicate that although neoadjuvant treatment induces APRIL expression in viable tumor cells, the total burden of disease reflected accurately the amount of circulating APRIL. Prior neoadjuvant treatment therefore obscured prognostic differences between patients by reducing circulating APRIL. This result indicates that timing of serum collection is critical to investigate the role of circulating APRIL as a marker for poor outcome in patients scheduled for neoadjuvant treatment. Future prospective studies should address whether circulating APRIL serum levels before initiation of neoadjuvant treatment predict prognosis in rectal cancer patients, particularly in high-risk patients with locally advanced disease. These findings may appear counterintuitive as it was reported before that neoadjuvant therapy rather induced the expression of APRIL in the tumor microenvironment and that specifically the stromal-expressed APRIL was predictive for response to therapy.10 However, we believe that levels of circulating APRIL reflect a combined reading between tumor burden and APRIL production and a reduction in the circulating levels of APRIL may simply reflect the reduction in tumor size and may not be related to the local response to therapy. Further analysis is needed to align these apparently contradictory observations. APRIL levels in serum may also indicate a higher level of immune infiltration in the tumors. Previous immunohistochemical studies showed that the accumulation of APRIL in tumor tissues was mainly the cause of tumor-infiltrating neutrophils.9 However, whether the levels of APRIL in serum are a direct reflection of neutrophil involvement and a pro-tumorigenic inflammation remains to be investigated. In this context, the lack of prognostic value of APRIL levels upon neoadjuvant treatment might reflect an efficient reduction of the immune infiltrate.
Conclusions
We designed a reliable assay to quantify levels of APRIL in the serum of patients. This revealed that APRIL serum levels at time of surgery were associated with features of advanced disease and prognosis in rectal cancer patients. This result strengthens the previously reported preclinical observation of increased APRIL levels correlating with progressive disease. It remains to be tested whether APRIL acts as a driver in this context or as a secondary marker of increased inflammatory infiltration. APRIL levels are particularly increased in patients with metastatic disease. Future studies should focus on the value of APRIL serum levels for selecting rectal cancer patients with a high risk of local failure and occult metastasis at the beginning of neoadjuvant therapy.
Materials and methods
Production and purification of anti-APRIL antibodies
APRIL mAbs (hAPRIL.130, hAPRIL.132, hAPRIL.133, hAPRIL.135 and hAPRIL.138) were essentially generated according to the procedures described by Guadagnoli et al.18 Antibodies were isolated by selecting anti-hAPRIL antibody-producing B cells that bind APRIL when bound to BCMA-Fc. For this purpose, lymph node cells and erythrocyte-depleted splenocytes were subjected to a selection on APRIL–BCMA complexed tosyl-activated magnetic DynaBeads (Thermo Fisher Scientific Inc, Breda, The Netherlands) in a beads: cells ratio of 1:1.5. Nonspecific binding splenocytes were washed away by 15 × washes with 5 ml of Dulbecco's Modified Eagle's F12/P/S/10%BCS medium. Next, selected B cells were cultured as described previously.19 After 9 days APRIL-reactive supernatants were screened for binding to FLAG-APRIL, when captured by BCMA-Fc. B-cell clones were selected and immortalized by mini-electrofusion to generate hybridomas. Selected hybridomas were cultured in DMEM F12 modified media (Sigma-Aldrich, Zwijndrecht, The Netherlands) containing 10% BCS, 0.5 mm sodiumpyruvate, 50 μm β-mercaptoethanol, 80 U/ml penicillin/streptomycin, HT supplement and 2% (v/v) T24 conditioned media (CM) and IL-6 containing supernatant of a human bladder carcinoma cell line T24. For purification, hybridomas were cultured in H-SFM culture medium (Gibco, Bleiswijk, The Netherlands) containing penicllin/streptomycin in roller bottles at 37 °C. Following 7 days in culture, the supernatants were collected, filtered and purified with mAb Select SuRe ProtA resin according to the manufacturer’s instructions (GE Healthcare, Piscataway, NJ, USA). Buffer was exchanged for PBS using PD-10 gelfiltration columns (GE Healthcare). Antibodies were quantified using spectrophotometry. Using a mouse mAb isotyping test kit (Roche, # 11493027001, Almere, The Netherlands), the (sub)-isotype of all hAPRIL antibodies was determined to be IgG1, Kappa. Purity was analyzed by size-exclusion chromatography, SDS–PAGE and coomassie staining.
Patient cohort and serum collection
The records of 432 patients diagnosed with colorectal cancer who underwent primary resection between 1990 and 2009 were reviewed. Sampling of biomaterials was approved by the local ethics committee (AZ 99/110). Serum samples were collected at the time of the operation, but before any invasive manipulation. The observation time in this collected cohort was the interval between diagnosis and last contact (death or last follow-up). The survival time interval was calculated between operation and last contact.
Overview ELISA
To test whether the newly produced antibody hAPRIL.133 recognizes APRIL in the presence of the serum, we generated two different standard curves. We used APRIL, which was produced by transfection of a plasmid construct leading to expression of wt APRIL in HEK293T cells. This APRIL was diluted in either PBS+fetal calf serum 10%+human serum 20% (Sigma, cat# H4522), or in PBS+BSA 1%. These two standard curves were tested using several commercially available antibodies or ELISA kits and the newly designed hAPRIL.133-based ELISA. The sensitivity of the hAPRIL.133 ELISA was calculated as the analyte concentration resulting in an absorbance significantly higher than that of dilution media (PBS–fetal calf serum–human seum). hAPRIL.133-based ELISA. The anti-APRIL hAPRIL.133 was used for the detection of endogenous levels of APRIL in the serum of our patient cohort. ELISA plates were coated with 100 μl of 0.5 μg/ml BCMA-Fc (EBC0512081; R&D, Abingdon, UK), in coating buffer (0.2 m sodium phosphate (11.8 g Na2PO4, 16.1 g NaH2PO4, H2O ad 1 l, pH=6.5) and incubated overnight at 4 °C. Later, plates were blocked to prevent unspecific binding using 150 μl PBS–BSA 1% for 1 h at 37 °C. Next, 100 μl of samples and standard curves concentrations were incubated for 2 h at room temperature. After the incubation time, 100 μl of the anti-APRIL hAPRIL.133 were added to the plates at a concentration of 1 μg/ml diluted in PBS–BSA 1%, this was done for 1 h at 37 °C. The following step included the addition of a 100 μl of goat anti-mouse IgG (H&L; Southern Biotech, cat# 1031-05, Uithoorn, The Netherlands) diluted 1:1000 in PBS–BSA 1% for 1 h at 37 °C. In between every step, plates were washed three times with PBS–Tween 0.2% (80 g NaCl, 11.6 g Na2PO4, 2 g KH2PO4, 2 g KCl H2O ad 1 l, pH=6.5+0.2% Tween). The enzymatic development reaction was done using TMB substrate (3,3′,5,5-tetramethylbenzidine). The reaction was stopped by adding an equal amount of 1 m hydrochloric acid to the reaction volume. This yielded a yellow color that was measured in an ELISA reader at 450 nm. The two different APRIL standard curves were tested in the following ELISA procedures. Biolegend (Uithoorn, The Netherlands) MAX ELISA Kit with PRE-coated Plates (cat# 439307). We followed the protocol provided by the manufacturer. ELISA performed by coating with BCMA-Fc and detection with APRILY-5 bio. ELISA plates were coated with 100 μl of 0.5 μg/ml BCMA-Fc (EBC0512081; R&D), in coating buffer (previously described) and incubated overnight at 4 °C. Plates were blocked using 150 μl PBS–BSA 1% for 1 h at 37 °C. Hundred microliter of samples or APRIL standard curves were incubated for 2 h at room temperature. Next, the commercially available APRILY-5 biotinylated (ALX-804-801-C100, Enzo Life Sciences BVBA, Antwerpen, Belgium) was added to the wells (100 μl of 1 μg/ml diluted in PBS–BSA 1%) and incubated for 1 h at 37 °C. Subsequently, 100 μl of Streptavidin-horseradish peroxidase (cat# 890803, R&D) diluted 1:1000 in PBS–BSA 1% was added for 1 h at 37 °C. In between every step, the plates were washed the times with PBS–Tween 0.2%. The enzymatic substrate reaction was carried out using TMB. ELISA using coated Sascha-2 anti-APRIL Ab and detection with biotinylated APRILY-5. ELISA plates were coated with 100 μl of anti-APRIL antibody Sascha-2 (804-804-C100, Enzo Life Sciences BVBA, Antwerpen, Belgium) in coating buffer (previously described) and incubated overnight at 4 °C. Later, plates were blocked using 150 μl PBS–BSA 1% for 1 h at 37 °C. Hundred microliter of samples or APRIL standard curves were incubated for 2 h at room temperature. Next, the commercially available biotinylated APRILY-5 was added to the plates (100 μl of 1 μg/ml diluted in PBS–BSA 1%) and incubated 1 h at 37 °C. After, 100 μl of Streptavidin-horseradish peroxidase diluted 1:1000 in PBS–BSA 1% was added for 1 h at 37 °C. The enzymatic substrate reaction was carried out using TMB.
Statistics
As a cutoff for high versus low APRIL serum levels we used the mean for colorectal and colon cancer patients (8.24 ng/ml and 8.89 ng/ml, respectively), whereas for rectal cancer patients we used the median expression level (7.51 ng/ml). We used a χ2 test to evaluate the frequencies of APRIL high and low serum level patients in the different subgroups for clinicopathologic parameters. Kaplan–Meier curves were used to assess the impact on overall survival rate. The follow-up times in the total cohort are minimum 4 days, median 1257 days and maximum of 6278 days. The significance of APRIL expression in the survival analysis was assessed by the log-rank test. We used a Cox proportional hazards model to test the individual and simultaneous influence on cumulative survival. All covariates found to be significant in the univariate analysis were tested in a multivariate model. The analysis of Wilcoxon–Mann–Whitney was the nonparametric tests used to analyze whether the distribution of APRIL expression differs in patient populations. All tests were two sided. A P-value of <0.05 was considered to indicate statistical significance marked at times as (**); a P-value of <0.01 was marked as (***). All analyses were performed with the use IBM SPSS 20 (Amsterdam, The Netherlands).
Acknowledgments
We thank the statistics help desk from the AMC for expertize advice on the statistical methods employed in this study. Collection of clinical data and serum samples was supported by the popgen 2.0 network, funded by a grant from the German Ministry for Education and Research (01EY1103).
Footnotes
Supplementary Information accompanies this paper on the Oncogenesis website (http://www.nature.com/oncsis)
The newly designed antibodies were developed by BioNovion B.N., Oss, The Netherlands. Patent number NL2011406.
Supplementary Material
References
- Xia XZ, Treanor J, Senaldi G, Khare SD, Boone T, Kelley M et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med 2000; 192: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov 2006; 5: 235–246. [DOI] [PubMed] [Google Scholar]
- Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG et al. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med 2005; 201: 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberley FC, van Bostelen L, Cameron K, Hardenberg G, Marquart JA, Hahne M et al. The proteoglycan (heparan sulfate proteoglycan) binding domain of APRIL serves as a platform for ligand multimerization and cross-linking. FASEB J 2009; 23: 1584–1595. [DOI] [PubMed] [Google Scholar]
- Lascano V, Zabalegui LF, Cameron K, Guadagnoli M, Jansen M, Burggraaf M et al. The TNF family member APRIL promotes colorectal tumorigenesis. Cell Death Differ 2012; 19: 1826–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennert P, Schneider P, Cachero TG, Thompson J, Trabach L, Hertig S et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J Exp Med 2000; 192: 1677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne M, Kataoka T, Schröter M, Hofmann K, Irmler M, Bodmer JL et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med 1998; 188: 1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreaux J, Veyrune JL, De Vos J, Klein B. APRIL is overexpressed in cancer: link with tumor progression. BMC Cancer 2009; 9: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhawech-Fauceglia P, Kaya G, Sauter G, McKee T, Donze O, Schwaller J et al. The source of APRIL up-regulation in human solid tumor lesions. J Leukoc Biol 2006; 80: 697–704. [DOI] [PubMed] [Google Scholar]
- Petty RD, Samuel LM, Murray GI, MacDonald G, O'Kelly T, Loudon M et al. APRIL is a novel clinical chemo-resistance biomarker in colorectal adenocarcinoma identified by gene expression. BMC Cancer 2009; 9: 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Wang J, Wang F, Wang G, Wu Q, Ju S et al. Serum sAPRIL: a potential tumor-associated biomarker to colorectal cancer. Clin Biochem 2013; 46: 1590–1594. [DOI] [PubMed] [Google Scholar]
- Planelles L, Castillo-Gutiérrez S, Medema JP, Morales-Luque A, Merle-Béral H, Hahne M. APRIL but not BLyS serum levels are increased in chronic lymphocytic leukemia: prognostic relevance of APRIL for survival. Haematologica 2007; 92: 1284–1285. [DOI] [PubMed] [Google Scholar]
- Went P, Tzankov A, Schwaller J, Passweg J, Roosnek E, Huard B. Role of the tumor necrosis factor ligand APRIL in Hodgkin's lymphoma: a retrospective study including 107 cases. Exp Hematol 2008; 36: 533–534. [DOI] [PubMed] [Google Scholar]
- Novak AJ, Grote DM, Stenson M, Ziesmer SC, Witzig TE, Habermann TM et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood 2004; 104: 2247–2253. [DOI] [PubMed] [Google Scholar]
- Schwaller J, Schneider P, Mhawech-Fauceglia P, McKee T, Myit S, Matthes T et al. Neutrophil-derived APRIL concentrated in tumor lesions by proteoglycans correlates with human B-cell lymphoma aggressiveness. Blood 2007; 109: 331–338. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012; 61: 847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YW, Wei XQ, Li YW, Zheng FP, Yang SJ. [Effects of matrine, 5-fluorouracil and cisplatin on the expression of APRIL in hepatocellular carcinoma HepG2 cells]. Zhonghua Gan Zang Bing Za Zhi 2008; 16: 532–533. [PubMed] [Google Scholar]
- Guadagnoli M, Kimberley FC, Phan U, Cameron K, Vink PM, Rodermond H et al. Development and characterization of APRIL antagonistic monoclonal antibodies for treatment of B-cell lymphomas. Blood 2011; 117: 6856–6865. [DOI] [PubMed] [Google Scholar]
- Steenbakkers PG, Hubers HA, Rijnders AW. Efficient generation of monoclonal antibodies from preselected antigen-specific B cells. Efficient immortalization of preselected B cells. Mol Biol Rep 1994; 19: 125–134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.