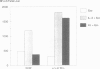Abstract
The c-kit proto-oncogene encodes a transmembrane tyrosine kinase receptor for an unidentified ligand and is allelic with the murine white-spotting locus (W). W mutations affect melanogenesis, gametogenesis and hematopoiesis during development and in adult life. Cellular targets of W mutations in hematopoiesis include distinct cell populations in the erythroid and mast cell lineages as well as stem cells. In the absence of interleukin-3 (IL-3) mast cells derived from normal mice but not from W mutant mice can be maintained by co-culture with 3T3 fibroblasts. Based on the defective proliferative response of W mast cells in the 3T3 fibroblast co-culture system it had been proposed that fibroblasts produce the c-kit ligand. We have used a mast cell proliferation assay to purify a 30 kd protein, designated KL, from conditioned medium of Balb/3T3 fibroblasts to apparent homogeneity. KL stimulates the proliferation of normal bone marrow derived mast cells but not mast cells from W mice, although both normal and mutant mast cells respond similarly to IL-3. Connective tissue-type mast cells derived from the peritoneal cavity of normal mice were found to express a high level of c-kit protein on their surface and to proliferate in response to KL. The effect of KL on erythroid progenitor cells was investigated as well. In combination with erythropoietin, KL was found to stimulate early erythroid progenitors (BFU-E) from fetal liver and spleen cells but not from bone marrow cells of adult mice and from fetal liver cells of W/W mice.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Barker J. E., McFarland E. C. Hemopoietic precursor cell defects in nonanemic but stem cell-deficient W44/W44 mice. J Cell Physiol. 1988 Jun;135(3):533–538. doi: 10.1002/jcp.1041350324. [DOI] [PubMed] [Google Scholar]
- Besmer P., Murphy J. E., George P. C., Qiu F. H., Bergold P. J., Lederman L., Snyder H. W., Jr, Brodeur D., Zuckerman E. E., Hardy W. D. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986 Apr 3;320(6061):415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Brown M. A., Pierce J. H., Watson C. J., Falco J., Ihle J. N., Paul W. E. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987 Aug 28;50(5):809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- Chabot B., Stephenson D. A., Chapman V. M., Besmer P., Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988 Sep 1;335(6185):88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Das S. K., Stanley E. R. Structure-function studies of a colony stimulating factor (CSF-1). J Biol Chem. 1982 Nov 25;257(22):13679–13684. [PubMed] [Google Scholar]
- Dayton E. T., Pharr P., Ogawa M., Serafin W. E., Austen K. F., Levi-Schaffer F., Stevens R. L. 3T3 fibroblasts induce cloned interleukin 3-dependent mouse mast cells to resemble connective tissue mast cells in granular constituency. Proc Natl Acad Sci U S A. 1988 Jan;85(2):569–572. doi: 10.1073/pnas.85.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck L. Berberine sulphate binding to mast cell polyanions: a cytofluorometric method for the quantitation of heparin. Histochemistry. 1974;42(4):301–313. doi: 10.1007/BF00492678. [DOI] [PubMed] [Google Scholar]
- Fujita J., Nakayama H., Onoue H., Kanakura Y., Nakano T., Asai H., Takeda S., Honjo T., Kitamura Y. Fibroblast-dependent growth of mouse mast cells in vitro: duplication of mast cell depletion in mutant mice of W/Wv genotype. J Cell Physiol. 1988 Jan;134(1):78–84. doi: 10.1002/jcp.1041340109. [DOI] [PubMed] [Google Scholar]
- Fujita J., Onoue H., Ebi Y., Nakayama H., Kanakura Y. In vitro duplication and in vivo cure of mast-cell deficiency of Sl/Sld mutant mice by cloned 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2888–2891. doi: 10.1073/pnas.86.8.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., McFarland E. C., Russell E. S. Analysis of pleiotropism at the dominant white-spotting (W) locus of the house mouse: a description of ten new W alleles. Genetics. 1981 Feb;97(2):337–361. doi: 10.1093/genetics/97.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Williams R. L. The pleiotropic actions of leukemia inhibitory factor. Cancer Cells. 1989 Nov;1(3):77–80. [PubMed] [Google Scholar]
- Gregory C. J., Eaves A. C. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood. 1978 Mar;51(3):527–537. [PubMed] [Google Scholar]
- Hamaguchi Y., Kanakura Y., Fujita J., Takeda S., Nakano T., Tarui S., Honjo T., Kitamura Y. Interleukin 4 as an essential factor for in vitro clonal growth of murine connective tissue-type mast cells. J Exp Med. 1987 Jan 1;165(1):268–273. doi: 10.1084/jem.165.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980 Jan;55(1):77–81. [PubMed] [Google Scholar]
- Jarboe D. L., Marshall J. S., Randolph T. R., Kukolja A., Huff T. F. The mast cell-committed progenitor. I. Description of a cell capable of IL-3-independent proliferation and differentiation without contact with fibroblasts. J Immunol. 1989 Apr 1;142(7):2405–2417. [PubMed] [Google Scholar]
- Kitamura Y., Fujita J. Regulation of mast cell differentiation. Bioessays. 1989 Jun;10(6):193–196. doi: 10.1002/bies.950100604. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Go S. Decreased production of mast cells in S1/S1d anemic mice. Blood. 1979 Mar;53(3):492–497. [PubMed] [Google Scholar]
- Levi-Schaffer F., Austen K. F., Caulfield J. P., Hein A., Bloes W. F., Stevens R. L. Fibroblasts maintain the phenotype and viability of the rat heparin-containing mast cell in vitro. J Immunol. 1985 Nov;135(5):3454–3462. [PubMed] [Google Scholar]
- Levi-Schaffer F., Austen K. F., Gravallese P. M., Stevens R. L. Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6485–6488. doi: 10.1073/pnas.83.17.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. L., Johnson G. R. Stimulation of multipotential, erythroid and other murine haematopoietic progenitor cells by adherent cell lines in the absence of detectable multi-CSF (IL-3). Nature. 1985 Aug 15;316(6029):633–636. doi: 10.1038/316633a0. [DOI] [PubMed] [Google Scholar]
- Little C. C., Cloudman A. M. The Occurrence of a Dominant Spotting Mutation in the House Mouse. Proc Natl Acad Sci U S A. 1937 Oct;23(10):535–537. doi: 10.1073/pnas.23.10.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCULLOCH E. A., SIMINOVITCH L., TILL J. E. SPLEEN-COLONY FORMATION IN ANEMIC MICE OF GENOTYPE WW. Science. 1964 May 15;144(3620):844–846. doi: 10.1126/science.144.3620.844. [DOI] [PubMed] [Google Scholar]
- Majumder S., Brown K., Qiu F. H., Besmer P. c-kit protein, a transmembrane kinase: identification in tissues and characterization. Mol Cell Biol. 1988 Nov;8(11):4896–4903. doi: 10.1128/mcb.8.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer T. C., Green M. C. An experimental analysis of the pigment defect caused by mutations at the W and S1 loci in mice. Dev Biol. 1968 Jul;18(1):62–75. doi: 10.1016/0012-1606(68)90023-7. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Kobayashi T., Ishiguro A., Tsuji K., Naganuma K., Ando O., Yagi Y., Tadokoro K., Akabane T. Extensive proliferation of mature connective-tissue type mast cells in vitro. Nature. 1986 Nov 6;324(6092):65–67. doi: 10.1038/324065a0. [DOI] [PubMed] [Google Scholar]
- Nicola N. A. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989;58:45–77. doi: 10.1146/annurev.bi.58.070189.000401. [DOI] [PubMed] [Google Scholar]
- Nocka K. H., Pelus L. M. Cell cycle specific effects of deferoxamine on human and murine hematopoietic progenitor cells. Cancer Res. 1988 Jul 1;48(13):3571–3575. [PubMed] [Google Scholar]
- Nocka K., Majumder S., Chabot B., Ray P., Cervone M., Bernstein A., Besmer P. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice--evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 1989 Jun;3(6):816–826. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- Nocka K., Tan J. C., Chiu E., Chu T. Y., Ray P., Traktman P., Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990 Jun;9(6):1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Qiu F. H., Ray P., Brown K., Barker P. E., Jhanwar S., Ruddle F. H., Besmer P. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family--oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988 Apr;7(4):1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Schrader J. W. In in vitro production and cloning of the P cell, a bone marrow-derived null cell that expresses H-2 and Ia-antigens, has mast cell-like granules, and is regulated by a factor released by activated T cells. J Immunol. 1981 Feb;126(2):452–458. [PubMed] [Google Scholar]
- Smith C. A., Rennick D. M. Characterization of a murine lymphokine distinct from interleukin 2 and interleukin 3 (IL-3) possessing a T-cell growth factor activity and a mast-cell growth factor activity that synergizes with IL-3. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1857–1861. doi: 10.1073/pnas.83.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. L., Austen K. F. Recent advances in the cellular and molecular biology of mast cells. Immunol Today. 1989 Nov;10(11):381–386. doi: 10.1016/0167-5699(89)90272-7. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Lee T. D., Seldin D. C., Austen K. F., Befus A. D., Bienenstock J. Intestinal mucosal mast cells from rats infected with Nippostrongylus brasiliensis contain protease-resistant chondroitin sulfate di-B proteoglycans. J Immunol. 1986 Jul 1;137(1):291–295. [PubMed] [Google Scholar]
- Takagi M., Nakahata T., Koike K., Kobayashi T., Tsuji K., Kojima S., Hirano T., Miyajima A., Arai K., Akabane T. Stimulation of connective tissue-type mast cell proliferation by crosslinking of cell-bound IgE. J Exp Med. 1989 Jul 1;170(1):233–244. doi: 10.1084/jem.170.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. C., Nocka K., Ray P., Traktman P., Besmer P. The dominant W42 spotting phenotype results from a missense mutation in the c-kit receptor kinase. Science. 1990 Jan 12;247(4939):209–212. doi: 10.1126/science.1688471. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Nakahata T., Takagi M., Kobayashi T., Ishiguro A., Kikuchi T., Naganuma K., Koike K., Miyajima A., Arai K. Effects of interleukin-3 and interleukin-4 on the development of "connective tissue-type" mast cells: interleukin-3 supports their survival and interleukin-4 triggers and supports their proliferation synergistically with interleukin-3. Blood. 1990 Jan 15;75(2):421–427. [PubMed] [Google Scholar]
- Tsuji K., Nakahata T., Takagi M., Kobayashi T., Ishiguro A., Kikuchi T., Naganuma K., Koike K., Miyajima A., Arai K. Synergistic action of phorbol ester and IL-3 in the induction of "connective tissue-type" mast cell proliferation. J Immunol. 1990 Jan 15;144(2):678–684. [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung Y. P., Eger R., Tertian G., Moore M. A. Long-term in vitro culture of murine mast cells. II. Purification of a mast cell growth factor and its dissociation from TCGF. J Immunol. 1981 Aug;127(2):794–799. [PubMed] [Google Scholar]
- Yung Y. P., Moore M. A. Long-term in vitro culture of murine mast cells. III. Discrimination of mast cells growth factor and granulocyte-CSF. J Immunol. 1982 Sep;129(3):1256–1261. [PubMed] [Google Scholar]
- Yurt R. W., Leid R. W., Jr, Austen K. F. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977 Jan 25;252(2):518–521. [PubMed] [Google Scholar]