Abstract
Importance
Advances in the evaluation and treatment of breast cancer have made the clinical decision-making context much more complex. A second medical oncology opinion may facilitate decision-making for women with breast cancer, yet little is known about second opinion use.
Objective
We investigated the patterns and correlates of second opinion use, and their impact on chemotherapy decisions.
Design, Setting, and Participants
A total of 1901 women newly diagnosed with stages 0 to II breast cancer between July 2013 and September 2014 (response rate, 71%), accrued through two population-based SEER registries (Georgia and Los Angeles County, California) and surveyed about their experiences with medical oncologists, decision-making, and chemotherapy use.
Main Outcome Measures
Factors associated with second opinion use were evaluated using logistic regression. Also assessed was the association between second opinion and chemotherapy use, adjusting for chemotherapy indication and propensity for receiving a second opinion. Multiple imputation and weighting were used to account for missing data.
Results
A total of 1,901 patients with stage I to II breast cancer (mean [SD] age, 61.6 [11.0]; 1071 [56.3%] non-Hispanic white) saw any medical oncologist. Analysis of multiply imputed, weighted data (mean n=1866) showed that 168 (9.8%) (SE, 0.74%) received a second opinion and 54 (3.2%) (SE, 0.47%) received chemotherapy from the second oncologist. Satisfaction with chemotherapy decision-making was high and did not differ between those who did (mean [SD], 4.3 [0.08] on a 1- to 5-point scale) or did not (4.4 [0.03]) obtain a second opinion (p=.29). Predictors of second opinion use included college education vs less education (odds ratio [OR], 1.85; 95% CI, 1.24–2.75), frequent use of internet-based support groups (OR, 2.15; CI, 1.12–4.11), an intermediate result on the 21-gene recurrence score assay (OR, 1.85; CI, 1.11–3.09), and a variant of uncertain significance on hereditary cancer genetic testing (OR, 3.24; CI, 1.09–9.59). After controlling for patient and tumor characteristics, second opinion use was not associated with chemotherapy receipt (OR, 1.04; 95% CI, 0.71–1.52).
Conclusion and Relevance
Second opinion use was low (<10%) among patietns with early-stage breast cancer, and high decision satisfaction regardless of second opinion use suggests little unmet demand. Along with educational level and use of internet support groups, uncertain results on genomic testing predicted second opinion use. Patient demand for second opinions may grow as increasingly complex genomic tests are disseminated.
INTRODUCTION
Advances in the evaluation and treatment of breast cancer have made the clinical decision-making context much more complex.1,2 Options for all modalities of treatment, including surgery, drug therapy, radiation and reconstruction have markedly expanded, as have preventive options for women at high genetic risk for second cancers. This is particularly true for decisions about systemic therapies as patients now must consider choices about 3 different medication categories: endocrine, chemotherapy and biologic. Examples include whether to take tamoxifen or an aromatase inhibitor, with or without ovarian suppression, and for how long;3–6 whether to take chemotherapy, with or without anthracyclines,7–9 and before or after surgery;10,11 and whether to take a new biologic agent such as pertuzumab.12,13 Moreover, diagnostic algorithms that guide treatment recommendations have become increasingly technical as genomic analyses, including germline genetic testing,14 are integrated into routine care.15–18 This complicated decision context can quickly overwhelm a patient seeking to understand her new diagnosis and choose a comprehensive care plan. Furthermore, most patients have only recently met the specialist physicians who are now in charge of their cancer care. Thus at the same time when she must deliberate between treatment options, a patient must also appraise the quality of one or more therapeutic relationships. These simultaneous demands may especially burden patients with limited educational, social or financial resources.19,20
Second opinions can facilitate treatment decision-making, and should be encouraged when patients are uncertain about their options or lack confidence in the treatment decision process. Given the growing complexity of treatment decision-making, second opinions may be an increasingly important opportunity for patients to gain confidence in their physicians and the proposed management plan. It is possible that a second opinion may indicate poor communication or care coordination, if, for instance, there are socioeconomic gradients in use, evidence of discordance in communication or decision-making, or differential use of indicated treatments in patients who do versus do not obtain second opinions.
However, little is known about how patients are referred to a medical oncologist after diagnosis and surprisingly, virtually nothing is known about the patterns and correlates of second opinions in community practice nor the implications for quality of care. Also unknown are the characteristics of the patient-oncologist encounter, whether related to the patient, physician, or clinical situation, that prompt patients to seek a second opinion. Understanding these aspects of treatment decision-making is necessary to inform interventions that can improve breast cancer care delivery and outcomes. We examined the patterns and correlates of second medical oncology opinions, and patients’ perspectives on chemotherapy decision-making and communication with oncologists, in a large, diverse contemporary population-based sample of patients newly diagnosed with breast cancer.
METHODS
Study Sample
We selected from the iCanCAre study women aged 20 to 79 years diagnosed with stages 0 to II breast cancer who were reported to the Surveillance Epidemiology and End Results (SEER) registries of Georgia and Los Angeles County, California. Eligible patients were identified approximately 2 months after surgery via pathology reports from “definitive” surgical procedures (those intended to remove the entire tumor with clear margins). To ensure a relatively homogeneous sample of patients with early-stage disease, patients with stages III to IV metastatic disease, tumors larger than 5 cm, or 3 or more involved lymph nodes were excluded. Black, Asian, and Hispanic women were oversampled in Los Angeles as previously described.21 Patients were selected between July 2013 and September 2014. This studyw as approved by the University of Michigan Instituteional Review Board and received a waiver of written informed consent. All data were deidentified before research use.
Questionnaire Design and Content
Questionnaire content was developed using a conceptual framework, research questions, and hypotheses. We developed measures drawing from the literature and our prior research.15,22 We used standard techniques to assess content validity, including systematic review by design experts, cognitive pre-testing with patients, and pilot studies in relevant populations.
Data Collection
Surveys were mailed approximately 2 months after surgery. To encourage response, we provided a $20 cash incentive and used a modified Dillman method,23 including reminders to nonrespondents. All materials were sent in English. We added Spanish-translated materials for all women with surnames suggesting Hispanic ethnicity.21 Each SEER registry provided limited SEER data (stripped of all identifiers) for participants to the University of Michigan: these data were then merged to survey data under institutional review board approval from partnering universities and the Public Health Departments of of Georgia and California.
Measures
Patients provided information about chemotherapy decisions, including how strongly the oncologist recommended chemotherapy on a 1- to 5-point scale (1, very strongly; 2, weakly; 3, left it up to me; 4, weakly against it; 5, very strongly against it), whether they saw a second oncologist (the question was worded as “Did you see a second medical oncologist for an opinion about chemotherapy?” [yes or no]) and, if so, whether that second oncologist administered chemotherapy (yes or no). Patients reported their satisfaction with their amount of involvement and information about chemotherapy decisions (on a 1- to 5-point scale, with 1 indicating not enough; 3, just right; and 5, too much) and the chemotherapy decision itself (on a 1- to 5-point scale, with 1 indicating not at all satisfied; 2, a little; 3, somewhat; 4, quite; and 5, totally). Patients rated their decision-making preferences on a 1- to 5-point scale (1 indicating not at all true; 2, a little; 3, somewhat; 4, quite; and 5, very) as follows: “preferred to be told what to do”, “wanted my doctor to tell me”, or “wanted to make my own decisions”. Patients rated oncologists on a 1- to 5-point scale (1, not at all true; 2, a little; 3, somewhat; 4, quite; and 5, very) according to the Health Care Climate Questionnaire,24 which measures perceived physician support of patient autonomy with questions as follows: “provided me with choices”, “understood how I saw things”, “expressed confidence in my decision making”, “listened to how I would like to be treated”, “encouraged me to ask questions”, and “tried to understand how I saw things”.
Patients provided information on the following: race/ethnicity, insurance, education, travel time to the nearest hospital, comorbidities, marital status, employment and household income. Patients reported on whether they received germline genetic testing for BRCA1 and BRCA2 (BRCA1/2) and/or other genes (yes or no) and results (positive, negative, or variant of uncertain significance [VUS]). Patients reported whether they received 21-gene recurrence score testing (yes or no) and results (low, intermediate or high). Patients reported on use of internet-based support groups (1- to 5-point scale: 1, almost never; 2, rarely; 3, sometimes; 4, often; and 5, almost always). SEER registries provided age (years), cancer stage (I, II), grade (1–3), and biomarkers including expression of estrogen receptor (ER), progesterone receptor (PR), and the erb-b2 receptor tyrosine kinase 2 gene/human epidermal growth factor receptor 2 gene (ERBB2/HER2).
We constructed a measure of chemotherapy indication according to guidelines of the National Comprehensive Cancer Network (eTables 1 and 2 in the Supplement).25 Patients were categorized as having a high chemotherapy indication if they had a tumor larger than 1 centimeter (cm) and/or involved lymph nodes, and also had ER- and PR-negative and/or ERBB2-positive disease. They were categorized as having a low chemotherapy indication if they had all of the following: age of 50 years or older, post-menopausal status, and stage I, grade 1, ER- and/or PR-positive, ERBB2-negative disease. All others were categorized as having an intermediate chemotherapy indication.
Statistical Methods
Weights
Survey design and non-response weights were created to compensate for the differential probability of selecting patients by race, stage and SEER site and to adjust for potential bias attributable to survey nonresponse. The weights were normalized to equal the observed sample size. Unless otherwise noted, all analyses were weighted so that statistical inferences are representative of our target population.26
Multiple Imputation
To account for item nonresponse and missing data, we multiply imputed data using a sequential regression multiple imputation framework.26 We generated 5 independently-imputed datasets and then computed inferential statistics that combined estimates across the data sets.27
Statistical Analyses
We described the unadjusted association of second opinion receipt with patient and tumor characteristics and patient appraisal of care yielded by observed unweighted data. A total of 436 patients (22.9%) had 1 or more missing values. We then multiply imputed data, to which we applied inclusion and exclusion criteria to select an analytic sample (average N=1,866). We constructed a multivariable weighted logistic regression model to examine the association between the probability of second opinion receipt and SEER site, age at survey, race/ethnicity, comorbidities, education level, employment, insurance, household income, marital status, travel time to nearest hospital, germline genetic testing receipt, 21-gene recurrence score testing receipt, chemotherapy indication, internet-based support group use and treatment decision-making preferences. We estimated the effect of second opinion receipt on the likelihood of chemotherapy receipt using an inverse probability of treatment weighting28,29 approach, adopted to address confounding. For each patient, we estimated the propensity of receiving a second opinion. Weighting each subject by the inverse propensity of her second opinion receipt, we created a synthetic sample in which second opinion receipt is independent of patient characteristics. After examining the properties of the weights,30 we estimated the mean effect of second opinion receipt on the probability of chemotherapy receipt. In a separate model using the F test for multiply imputed data, we tested for the presence of a joint effect of second opinion receipt and its interaction with chemotherapy indication. All analyses were conducted using SAS Version 9.4 [Cary, NC, USA]. P<.05 was considered statistically significant (2-sided joint Wald test). Reported results were generated using multiply imputed, weighted data.
RESULTS
Patient Characteristics
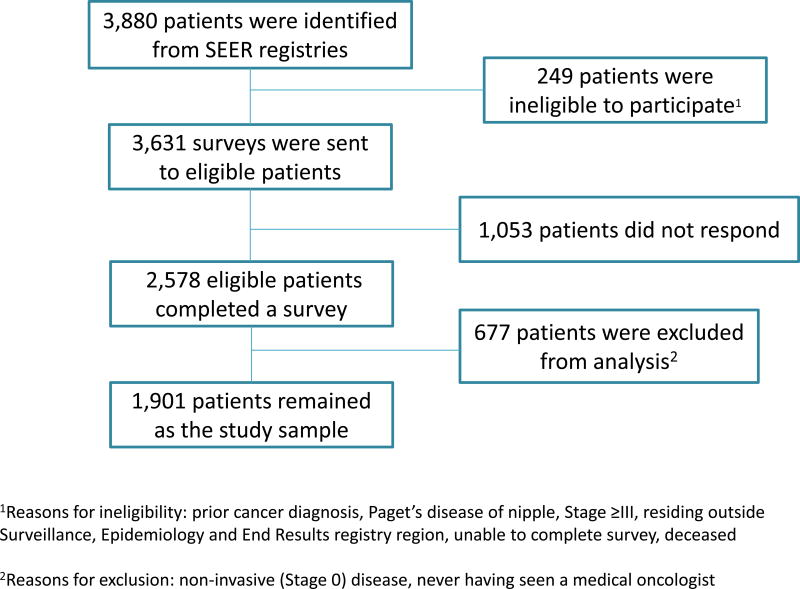
We selected 3,880 patients diagnosed with early-stage breast cancer (mean [SD] age, 61.6 [11.0] years), of whom 3,631 (93.6%) were eligible for the study. The survey response rate was 71% (N=2,578). We excluded 677 patients from this analysis (497 with non-invasive disease and 164 who never saw a medical oncologist, Figure 1). Table 3 in the Supplement indicates that the 1,901 remaining patients were racially and socioeconomically diverse: 1,071 (56.3%) were non-Hispanic white, 306 (16.1%) were non-Hispanic black, 328 (17.3%) were Hispanic, and 141 (7.4%) were Asian. For 1,160 (61.0%), high school was the highest educational level (Table 3 in the Supplement). A total of 1,194 (62.8%) had stage I disease, 518 (27.2%) had grade 1 disease, and 471 (24.8%) had grade 3 disease. A total of 1,597 (84.0%) had ER- and PR-positive tumors; 211 (11.1%) had ERBB2-positive tumors (Supplemental Table 1). A total of 610 (32.1%) reported germline genetic testing and 716 (37.7%) reported 21-gene recurrence score (RS) testing.
Figure 1.
Patient flow into the study. This figure depicts the flow of patients into the study from those initially identified to the final analytic sample.
Factors Associated with Receipt of Second Opinions
Multiple imputation (mean n=1866 patients) yielded an estimated mean (SD) prevalence of second opinion receipt of 168 (9.8% [0.74%]). Figure 2 shows that factors significantly associated with second opinion receipt were a college education vs less (odds ratio [OR], 1.85; 95% CI, 1.24–2.75), a preference for making one’s own treatment decisions quite a bit of the time or always vs never or sometimes (OR, 1.15; 95% CI, 1.01–1.31), frequent use of internet-based support groups vs none (OR, 2.15; CI, 1.12–4.11), an intermediate result on the 21-gene RS assay vs not tested (OR, 1.85; 95% CI, 1.11–3.09), and a VUS (OR, 3.24; 95% CI, 1.09–9.59) or negative result (OR, 1.58; 95% CI, 1.04–2.42) on germline genetic testing vs not tested. Odds of second opinion receipt were significantly lower in Georgia vs Los Angeles County (OR, 0.58; 95% CI, 0.39–0.87), but there were no interactions between site and other model covariates. No other factor, including comorbidities, employment, income, or race/ethnicity was associated with second opinion receipt.
Figure 2.
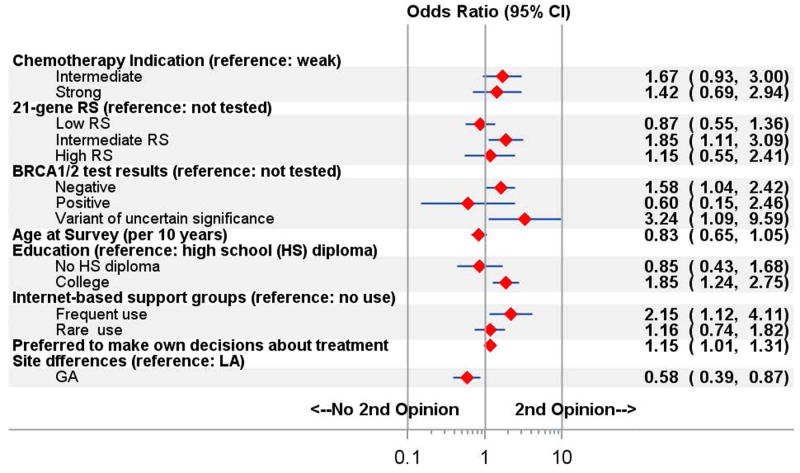
Multivariable model of characteristics associated with receipt of a second opinion. Abbreviation: RS, recurrence score.
Receipt of Chemotherapy and Use of Second Opinions
Based on an analysis using multiply imputed, weighted data (average n=1866), 823 patients (44.0% [SE 1.2%]) reported chemotherapy receipt, with somewhat higher rates among patients who did (94 [52.1%]) vs did not (729[43.2%]) receive a second opinion on univariate analysis (OR, 1.45; 95% CI, 1.07–1.97). However, several patient characteristics were correlated with both second opinion and chemotherapy use, raising concern about confounding. To test whether second opinion use had a significant impact on chemotherapy use, we used a inverse probability of treatment weighting model to control for differences in the distribution of characteristics between patients who did versus did not receive a second opinion, and thus to reduce bias from confounding. We found no significant interaction between second opinion receipt and chemotherapy indication on the probability of receiving chemotherapy (p-value=0.45): high indication (OR, 0.42; 95% CI, 0.11–1.56), low indication (OR, 0.41; 95% CI, 0.06–2.77), and intermediate indication (OR, 1.28; 95% CI, 0.83–1.97). After controlling for patient and tumor characteristics, second opinion use was not associated with chemotherapy receipt (OR, 1.04; 95% CI, 0.71–1.52). Among patients who received a second opinion, 54 (3.2%) (SE, 0.47%) received chemotherapy from the second oncologist.
Patient Appraisal of Decision-Making and Second Opinions
Satisfaction with chemotherapy decisions was high and did not differ between those who did (mean [SD], 4.3[.08] on a 1- to 5-point scale) or did not (4.4[.03]) obtain a second opinion (P=.29) (Supplemental Table 4). Patients rated oncologists highly (mean score, 4.1 of 5) on the Health Care Climate Questionnaire, signifying perceived clinician support of patient autonomy (Supplemental Table 4).
DISCUSSION
Second opinions may substantially affect a breast cancer treatment plan. Recent studies have focused on second opinions provided by one clinician to another: for example, reports that discordant reads in pathology and radiology change treatment in 10% to 25% of cases.30–33 Tumor boards, which enable clinicians to synthesize a combined multidisciplinary opinion, are associated with improved care quality.34–36 We previously reported on the role of second opinions in surgical decision-making.37,38 Yet despite this evidence of impact, very little is known about the prevalence and consequences of second opinions that oncologists provide directly to patients.
In this large, diverse contemporary cohort, second opinion use was remarkably low: less than 10%, with fewer than 5% of all patients receiving chemotherapy from a second oncologist. There were regional differences, with second opinions less common in Georgia than LA. Reassuringly, we did not observe racial/ethnic or socioeconomic gradients; patient-reported dissatisfaction with communication or decision-making; or differential chemotherapy use by patients who did versus did not receive second opinions. We conclude that there is little evidence of unmet demand for second opinions, and that their potential impact on chemotherapy decisions in community practice appears to be small.
Overall, our findings are encouraging with regard to the quality of breast cancer care. However, we identified key predictors of second opinion use that suggest opportunities for improvement. We observed a distinct profile of patients who were more likely to obtain second opinions. These patients were more often college-educated, more frequently used internet-based support groups, and preferred to make their own treatment decisions. Such patients may desire greater engagement in their care, and pursue second opinions for more information and support. This may constitute an appropriate use of second opinions; yet interventions that enable the first oncologist to recognize and address these patients’ needs may also be desirable.
Along with patient demographics and preferences, we identified a clinical predictor of second opinion use: uncertain results of genomic tests. Patients who reported having a variant of uncertain significance (VUS) on germline genetic testing were three-times more likely to obtain a second medical oncology opinion. These unclassified results may confuse patients; moreover, one study found that very few (<15%) physicians who order BRCA1/2 testing understand how to manage a VUS.39 Oncologists confronted by VUS may struggle to explain them to patients’ satisfaction, prompting patients to seek another oncologist who can. While VUS rates are low (2%–5%) when BRCA1/2 are the only genes sequenced, they rise ten-fold (35%) with the multiple-gene panels that are rapidly emerging into breast cancer care.40–44 Furthermore, VUS rates are significantly higher in racial/ethnic minorities than non-Hispanic whites.45,46 Thus the demand for second opinions may rise with dissemination of more comprehensive genetic testing, and to a greater extent among vulnerable populations. This raises concern about future access disparities and emphasizes the need to follow trends in second opinion use over time. Studies are urgently needed to improve the interpretation of VUS and physicians’ ability to manage them.
Patients with intermediate results on the 21-gene RS assay were two-times more likely than untested patients to receive a second opinion. While the clinical utility of low and high RS is well established,14,47–49 the appropriate management of intermediate RS remains unknown, pending results of clinical trials.14 A recent study of oncologists reported low “genomic confidence”: namely, the ability to use genomic testing results effectively for patient care.50 Some patients may perceive their oncologists’ low confidence about treatment recommendations in the setting of uncertain germline or tumor genomic results, and seek greater confidence through a second opinion. This finding underscores the need for educational interventions that help oncologists’ knowledge and competence to keep pace with the rapid expansion of precision medicine technology.
Aspects of this study warrant comment. Its strengths include a large, racially/ethnically diverse, contemporary sample of breast cancer patients enrolled from two population-based cancer registries; specific measures of patients’ clinical decision-making; and a high response rate. Furthermore, weighting and multiple imputation techniques were used to account for potential bias due to missing data and to ensure that results were representatitve of the overall population. Its limitations include restriction to two geographic areas (Georgia and LA), so results may not apply fully to all United States breast cancer patients. Furthermore, the sample was selected for earlier stage (I-II) and had generally favorable tumor biology. The patterns, correlates and outcomes of second opinion use may be different in patients whose stage or tumor biology renders them at higher risk for metastatic recurrence. We have not yet validated patients’ reports of genetic testing, nor oncologists’ perspectives on second opinions and other aspects of treatment. There has been insufficient follow-up time to ascertain long-term outcomes of cancer recurrence and survival. Nonetheless, this study offers a novel and clinically relevant view of breast cancer treatment decision-making.
Conclusion
In an era of concern about the cost and value of cancer care, guidelines advise that we choose wisely before ordering diagnostic tests.51 Yet there are no guidelines as to whether a second opinion (with costs similar to those of diagnostic tests) is potentially valuable or merely redundant. Given the subjective and personal nature of the therapeutic encounter, second opinions may sometimes be necessary to address a poor fit between patient and physician. Encouragingly, we found high endorsement of perceived autonomy supportiveness of medical oncologists, with very few patients (<10%) seeking a second opinion and little evidence of an unmet need. Our results indicate that a patient’s preference for greater engagement is one predictor of second opinion use, and uncertain results of diagnostic testing are another. As treatment options proliferate and molecular diagnostic tests expand, physicians may face growing pressure to enable patients’ preferences about treatment decision-making and to navigate the increasingly murky landscape of genomic testing. These tasks demand effective physician-patient communication, and developing interventions to enhance the quality of such communication is a high priority.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute (NCI) of the National Institutes of Health under award number P01CA163233 to the University of Michigan.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the NCI’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California (USC), and contract HHSN261201000034C awarded to the Public Health Institute. The collection of cancer incidence data in Georgia was supported by contract HHSN261201300015I, Task Order HHSN26100006 from the NCI and cooperative agreement 5NU58DP003875-04-00 from the CDC. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the NCI, and the CDC or their Contractors and Subcontractors is not intended nor should be inferred.
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Allison W. Kurian, M.D., M.Sc. and Irina Bondarenko, M.S., had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
We acknowledge the outstanding work of our project staff (Mackenzie Crawford and Kiyana Perrino from the Georgia Cancer Registry; Jennifer Zelaya, Pamela Lee, Maria Gaeta, Virginia Parker, and Renee Bickerstaff-Magee from the University of Southern California; Rebecca Morrison, Rachel Tocco, Alexandra Jeanpierre, Stefanie Goodell, Rose Juhasz, Paul Abrahamse, Kent Griffith from the University of Michigan). These people were compensated for their contributions.
We acknowledge with gratitude our survey respondents.
Footnotes
The authors have no disclaimers or conflicts of interests related to this work to report.
This manuscript has not been published previously. Preliminary results were presented in partial form at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 29–June 2, 2015
References
- 1.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 2.Munoz D, Near AM, van Ravesteyn NT, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst. 2014;106(11) doi: 10.1093/jnci/dju289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J. Med. 2015;372(5):436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goss PE, Ingle JN, Pritchard KI, et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J. Med. 2016 doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J. Med. 2014;371(2):107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum JL, Flynn PJ, Yothers G, et al. Interim joint analysis of the ABC (anthracyclines in early breast cancer) phase III trials (USOR 06-090, NSABP B-46I/USOR 07132, NSABP B-49 [NRG Oncology]) comparing docetaxel + cyclophosphamide (TC) v anthracycline/taxane-based chemotherapy regimens (TaxAC) in women with high-risk, HER2-negative breast cancer; American Society of Clinical Oncology Annual Meeting; June 4, 2016; Chicago, IL. [Google Scholar]
- 8.Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30(18):2232–2239. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones S, Holmes FA, O'Shaughnessy J, et al. Docetaxel With Cyclophosphamide Is Associated With an Overall Survival Benefit Compared With Doxorubicin and Cyclophosphamide: 7-Year Follow-Up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27(8):1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 10.DeMichele A, Yee D, Berry DA, et al. The Neoadjuvant Model Is Still the Future for Drug Development in Breast Cancer. Clin Cancer. Res. 2015;21(13):2911–2915. doi: 10.1158/1078-0432.CCR-14-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19(5):1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 12.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24(9):2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 13.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. The Lancet. Oncology. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 14.Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J. Med. 2015;373(21):2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jagsi R, Griffith KA, Kurian AW, et al. Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. J Clin Oncol. 2015;33(14):1584–1591. doi: 10.1200/JCO.2014.58.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jagsi R, Kurian AW, Griffith KA, et al. Genetic testing decisions of breast cancer patients: Results from the iCanCare study; American Society of Clinical Oncology Annual Meeting; May 29–June 2, 2015; Chicago, IL. [Google Scholar]
- 17.Dinan MA, Mi X, Reed SD, et al. Initial trends in the use of the 21-gene recurrence score assay for patients with breast cancer in the Medicare population. JAMA Oncology. 2015;1(2):158–166. doi: 10.1001/jamaoncol.2015.43. [DOI] [PubMed] [Google Scholar]
- 18.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30(18):2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livaudais JC, Franco R, Fei K, Bickell NA. Breast cancer treatment decision-making: are we asking too much of patients? J Gen Intern. Med. 2013;28(5):630–636. doi: 10.1007/s11606-012-2274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez KA, Kurian AW, Hawley ST, Jagsi R. How can we best respect patient autonomy in breast cancer treatment decisions? Breast Cancer Manag. 2015;4(1):53–64. doi: 10.2217/bmt.14.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton AS, Hofer TP, Hawley ST, et al. Latinas and breast cancer outcomes: population-based sampling, ethnic identity, and acculturation assessment. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(7):2022–2029. doi: 10.1158/1055-9965.EPI-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawley ST, Jagsi R, Morrow M, et al. Social and Clinical Determinants of Contralateral Prophylactic Mastectomy. JAMA surgery. 2014;149(6):582–589. doi: 10.1001/jamasurg.2013.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dillman DASJ, Christian LM. Internet, Mail, and Mixed-Mode Surveys: The Tailored Design Method. Third. Hoboken, New Jersey: John Wiley & Sons; 2009. [Google Scholar]
- 24.Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. Journal of personality and social psychology. 1996;70(1):115–126. doi: 10.1037//0022-3514.70.1.115. [DOI] [PubMed] [Google Scholar]
- 25.Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(3):324–354. doi: 10.6004/jnccn.2016.0037. [DOI] [PubMed] [Google Scholar]
- 26.Kish L. Survey Sampling. New York: John Wiley & Sons; 1965. [Google Scholar]
- 27.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NY: John Wiley & Sons; 1987. [Google Scholar]
- 28.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Statistics in medicine. 2004;23(19):2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 29.Joffe MM, Have TRT, Feldman HI, Kimmel SE. Model Selection, Confounder Control, and Marginal Structural Models: Review and New Applications. The American Statistician. 2004;58(4):272–279. [Google Scholar]
- 30.Elmore JG, Longton GM, Carney PA, et al. Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA. 2015;313(11):1122–1132. doi: 10.1001/jama.2015.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geller BM, Nelson HD, Carney PA, et al. Second opinion in breast pathology: policy, practice and perception. J Clin Pathol. 2014;67(11):955–960. doi: 10.1136/jclinpath-2014-202290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khazai L, Middleton LP, Goktepe N, Liu BT, Sahin AA. Breast pathology second review identifies clinically significant discrepancies in over 10% of patients. J Surg Oncol. 2015;111(2):192–197. doi: 10.1002/jso.23788. [DOI] [PubMed] [Google Scholar]
- 33.Spivey TL, Carlson KA, Janssen I, Witt TR, Jokich P, Madrigrano A. Breast Imaging Second Opinions Impact Surgical Management. Ann Surg Oncol. 2015;22(7):2359–2364. doi: 10.1245/s10434-014-4205-5. [DOI] [PubMed] [Google Scholar]
- 34.Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344:e2718. doi: 10.1136/bmj.e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrugia DJ, Fischer TD, Delitto D, Spiguel LR, Shaw CM. Improved Breast Cancer Care Quality Metrics After Implementation of a Standardized Tumor Board Documentation Template. J Oncol Pract. 2015;11(5):421–423. doi: 10.1200/JOP.2015.003988. [DOI] [PubMed] [Google Scholar]
- 36.Newman EA, Guest AB, Helvie MA, et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer. 2006;107(10):2346–2351. doi: 10.1002/cncr.22266. [DOI] [PubMed] [Google Scholar]
- 37.Katz SJ, Hofer TP, Hawley S, et al. Patterns and Correlates of Patient Referral to Surgeons for Treatment of Breast Cancer. Journal of Clinical Oncology. 2007;25(3):271–276. doi: 10.1200/JCO.2006.06.1846. [DOI] [PubMed] [Google Scholar]
- 38.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. Jama. 2009;302(14):1551–1556. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pal T, Cragun D, Lewis C, et al. A statewide survey of practitioners to assess knowledge and clinical practices regarding hereditary breast and ovarian cancer. Genet Test Mol Biomarkers. 2013;17(5):367–375. doi: 10.1089/gtmb.2012.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurian AW, Ford JM. Multigene Panel Testing in Oncology Practice: How Should We Respond? JAMA Oncol. 2015;1(3):277–278. doi: 10.1001/jamaoncol.2015.28. [DOI] [PubMed] [Google Scholar]
- 41.Desmond A, Kurian AW, Gabree M, et al. Clinical Actionability of Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Risk Assessment. JAMA Oncol. 2015;1(7):943–951. doi: 10.1001/jamaoncol.2015.2690. [DOI] [PubMed] [Google Scholar]
- 42.Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009. doi: 10.1200/JCO.2013.53.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurian AW, Antoniou AC, Domchek SM. Refining Breast Cancer Risk Stratification: Additional Genes, Additional Information. Am Soc Clin Oncol Educ Book. 2016;35:44–56. doi: 10.1200/EDBK_158817. [DOI] [PubMed] [Google Scholar]
- 44.Swisher EM. Usefulness of Multigene Testing: Catching the Train That's Left the Station. JAMA Oncol. 2015;1(7):951–952. doi: 10.1001/jamaoncol.2015.2699. [DOI] [PubMed] [Google Scholar]
- 45.Hall MJ, Reid JE, Burbidge LA, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115(10):2222–2233. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maxwell KN, Wubbenhorst B, D'Andrea K, et al. Prevalence of mutations in a panel of breast cancer susceptibility genes in BRCA1/2-negative patients with early-onset breast cancer. Genet. Med. 2015;17(8):630–638. doi: 10.1038/gim.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein LJ, Gray R, Badve S, et al. Prognostic Utility of the 21-Gene Assay in Hormone Receptor-Positive Operable Breast Cancer Compared With Classical Clinicopathologic Features. J Clin Oncol. 2008 doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer. Res. 2006;8(3):R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 50.Gray SW, Hicks-Courant K, Cronin A, Rollins BJ, Weeks JC. Physicians' attitudes about multiplex tumor genomic testing. J Clin Oncol. 2014;32(13):1317–1323. doi: 10.1200/JCO.2013.52.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnipper LE, Lyman GH, Blayney DW, et al. American Society of Clinical Oncology 2013 top five list in oncology. J Clin Oncol. 2013;31(34):4362–4370. doi: 10.1200/JCO.2013.53.3943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.