Abstract
Neural tissue engineers are exploiting key mechanisms responsible for neural cell migration and axonal path finding during embryonic development to create living scaffolds for neuroregeneration following injury and disease. These mechanisms involve the combined use of haptotactic, chemotactic, and mechanical cues to direct cell movement and re-growth. Living scaffolds provide these cues through the use of cells engineered in a predefined architecture, generally in combination with biomaterial strategies. Although several hurdles exist in the implementation of living regenerative scaffolds, there are considerable therapeutic advantages to using living cells in conjunction with biomaterials. The leading contemporary living scaffolds for neurorepair are utilizing aligned glial cells and neuronal/axonal tracts to direct regenerating axons across damaged tissue to appropriate targets, and in some cases to directly replace the function of lost cells. Future advances in technology, including the use of exogenous stimulation and genetically engineered stem cells, will further the potential of living scaffolds and drive a new era of personalized medicine for neuroregeneration.
Keywords: Tissue engineering, Cell transplant, Biomaterials, Regeneration, Neurotrauma, Neurodegeneration, Axon pathfinding, Cell migration
1. Overview
The brain, spinal cord, and peripheral nervous system have limited capacity for regeneration, making the effects of neurotrauma or neurodegenerative disease particularly devastating and often permanent. Successful regeneration would involve a precisely orchestrated reestablishment of neural connections and reformation of cellular structure, often requiring directed long-distance axonal path finding and neuronal/glial migration. The objective of the field of neural tissue engineering is to utilize biomaterial-and cell-based strategies to augment endogenous regeneration and/or to provide direct replacement of neural cells and circuitry. A particularly promising tissue engineering approach involves the development of “living scaffolds”, which are regenerative scaffolds comprised of living neural cells in a preformed, often anisotropic, three-dimensional (3-D) architecture. Living scaffolds may facilitate targeted neural cell migration and axonal path finding by mimicking key developmental mechanisms. Indeed, directed axon growth and cell migration along pathways formed by other cells is a common tactic in nervous system development and is crucial to the proper formation of axonal connectivity and cellular localization. Growth and migration along living neural cells is driven by juxtracrine signaling involving the concurrent and often Q3 synergistic presentation of a panoply of cell-mediated haptotactic, chemotactic, and neurotrophic cues (Fig. 1). Living scaffolds exploiting these cues possess considerable advantages over more traditional acellular biomaterial approaches due to the ability to actively drive and direct regeneration rather than simply being permissive substrates. Moreover, living scaffolds have the ability for constitutive and sustained interactions rather than transient, often short-lived influence on the host. Importantly, living scaffolds may act based on feedback and cross talk with regenerating cells/axons and thus are able to modulate their signaling based on the state and progression of the regenerative process. On this front, there are a number of promising emerging strategies for the development of living regenerative scaffolds consisting of aligned glial cells and/or longitudinal axonal tracts that have driven robust and targeted axonal re-growth and neural cell migration. However, there are several significant challenges to the development and translation of living scaffolds, including advancing tissue engineering techniques for the creation of living cellular constructs in a defined 3-D architecture, establishing transplantation strategies to ensure preservation of construct vitality and architecture, and devising strategies for immunological tolerance at both acute and chronic time frames. As these challenges are overcome, living scaffolds have the potential to transform the field of neuroregenerative medicine by driving the re-establishment of complex neural structures and axonal connections, ultimately facilitating functional recovery following a range of currently untreatable traumatic and neurodegenerative disorders.
Fig. 1.

Structural and soluble cues directing axonal outgrowth along “living scaffolds”. (A) Axon growth is directed along existing axonal tracts (“pioneer” axons in development or tissue engineered axonal tracts in regeneration) due to a combination of a precise spatial presentation of cell-adhesion molecules (CAMs) and the intimate presentation of secreted chemotactic and neurotrophic factors. (B) Axon guidance may also progress along aligned astrocytic somata and processes, where the presentation and/or gradients of CAMS, extracellular matrix constituents, and secreted neurotrophic factors promote axon guidance. Similarly, “living scaffolds” may also be applied to facilitate and direct cell migration to reconstruct complex neural tissue structure (not shown).
2. Definition of living scaffolds
The field of regenerative medicine encompasses the use of biomaterials, cell replacement strategies, and tissue engineering to promote regeneration following injury or disease. Biomaterials can provide 3-D structure for host cell infiltration and organization, and may also serve as a means for drug administration (e.g., controlled release). Cell delivery strategies can replace lost cells in cases where endogenous cells are insufficient or unavailable (e.g., new neurons). Tissue engineering combines aspects of both biomaterial and cell replacement techniques to create 3-D constructs to facilitate regeneration of native tissue and/or to directly restore lost function based on permanent structural integration [1]. An emerging strategy in neural tissue engineering involves the development and application of “living scaffolds”, which are defined as constructs with a controlled, often heterogeneous and anisotropic 3-D cellular architecture and biomaterial composition. The objective of these living cellular-biomaterial scaffolds is to serve as chaperones to support, guide, and aid regenerating cells and/or processes (e.g. axons) − mimicking crucial aspects of developmental path finding. The cells impart the “living” component of the scaffold, and incorporated cell types may include primary, stem, differentiated, genetically engineered, autologous, allergenic, or heterogonous cells [1,2]. Biomaterials utilized within the scaffold often provide structure and produce an environment in which cells can adhere, migrate, differentiate, and signal to each other and to the host [3] The biomaterial composition often governs the mechanical properties of the construct and resulting tissue [3]. A crucial property of a living scaffold is that it must possess a defined architecture, encompassing both the structural composition as well as the organization of the cells/processes (Fig. 2). This architecture must be precisely engineered to match the structure and properties of the tissue it will integrate with, or to provide directionality for infiltration and targeted re-growth of host cells. Biomaterials may be synthesized to promote such a desired cellular organization or to give directional dependence to mechanical properties, such as rigidity and elasticity [3]. Likewise, gradients of co-delivered factors, such as growth factors and signaling molecules, may be used within living scaffolds to generate an anisotropic cytoarchitecture [4].
Fig. 2.
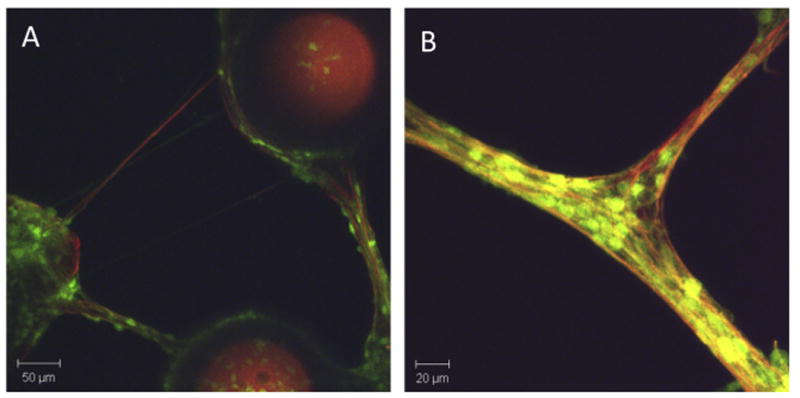
Glial and neuronal alignment in engineered 3-D microsystems In Vitro. Neuronal-astrocytic co-cultures formed in 3-D around engineered micro-towers (250 μm tall, 200 μm diameter). (A) Across the top 200 μm of these cultures, neural cells expressing green fluorescent protein (GFP) and astrocytic processes (glial-fibrillary acidic protein; red) coalesced into discrete tracts spanning the cellular populations directly adhered to the micro-towers. (B) In this system, neurons (GFP+) grew in alignment with long astrocytic processes (GFAP+; red) spanning the micro-towers. Adapted from [43].
3. The challenges to nervous system repair and regeneration
The nervous system, encompassing both the central nervous system (CNS) and peripheral nervous system (PNS), is comprised of two major cell types: neurons and glia. Neurons typically receive electrical signals via branched projections called “dendrites” and transmit these signals along fibers called “axons”. Glia (CNS: astrocytes, oligodendrocytes, microglia; PNS: Schwann cells) generally act as support cells to provide structure, protection, and nutrients to neurons and insulation to axonal projections. A variety of insults can lead to neuronal and glial cell loss, including traumatic injury, stroke, and neurodegenerative diseases. In addition, disconnection of axonal pathways is a common feature across multiple types of neurotrauma and neurodegenerative disorders. Unfortunately, functional regeneration of these connections rarely occurs due to long distances to appropriate targets and a lack of directed guidance. Injury to the CNS often initiates a robust inflammatory response, leading to a non-permissive environment for regeneration. Astrocytes convert to a “reactive” state and may form a dense barrier of hypertrophic processes and inhibitory molecules in order to protect the nervous system from further damage. This barrier, termed the “glial scar”, is long lasting and obstructs the growth of regenerating axons [5]. In contrast, following trauma to the PNS, tissue and axon regeneration is generally more successful owing to the pro-regenerative response of resident Schwann cells; however, functional restoration following major nerve lesions (e.g., several centimeters or greater in length) is generally poor due to insufficient axonal reinnervation of distal targets [6]. To date, neither cell replacement strategies nor acellular biomaterial-based approaches have been successful in orchestrating neural tissue formation and long-distance axonal path finding in the CNS or PNS.
The emerging strategy of tissue engineered living scaffolds represents a promising approach for complex nervous system repair. Living scaffolds are generally created to fulfill one or both of the following objectives: (1) motivate and direct guidance of host processes, typically axons, and (2) facilitate migration and organization of host cells. The most common objective of living scaffolds is to guide the re-growth of axonal tracts damaged from disease or injury. Following disconnection of axonal tracts, regenerating axons frequently need to traverse extremely long distances across complicated 3-D environments to reach specific targets. In such cases, living scaffolds can be used to guide regenerating axons to precise targets and allow for functional reinnervation. In cases where neural cells are damaged due to trauma or neurodegenerative diseases, living scaffolds can be used to provide the necessary structural and chemical cues to encourage inward migration of existing or newly formed neurons or glial cells (or precursors). Anisotropy in the living scaffold can be used to organize neurons into networks and patterns. In some cases, the living constructs may be used to physically replace lost neural cells as well as their long distance connections.
4. Examples of living scaffolds during nervous system development
The mechanisms by which tissue engineered living scaffolds promote neuroregeneration are fundamentally based within developmental biology. Throughout embryogenesis and pre-natal development, there are many instances in which neuronal migration and axonal path finding are mediated by naturally occurring living scaffolds. Specifically, living scaffolds are used to facilitate the migration of neurons from the center of the brain to the forming neocortex [7]. Likewise, axons in both the peripheral and central nervous systems frequently extend along living scaffolds in order to reach and innervate their respective targets [8-11].
In a developing fetus, neural progenitor cells located in the ventricular zone produce the majority of neurons. However, these newborn neurons must migrate outward radially in order to take their place in the forming neocortex. As the size of the brain increases, neurons utilize scaffolding created by a population of cells called “radial glia” to aid them in traversing the greater distances. Radial glia extend processes connecting the ventricular zone to the pial surface of the brain. These glial processes create guided pathways along which the newborn neurons migrate to the neocortex [7]. In addition to migration, it has been observed that growing axons often use a glial scaffold to locate appropriate targets and guide their extension. In the CNS of Drosophila embryos, Jacobs et al. showed that glial cells exist in patterned configurations that outline axonal pathways before the growth of the axonal tracts themselves. Pioneering neurons make extensive contact with these glial scaffolds using their growth cones and filopodia prior to projecting their axons along the length of the glia [11]. Peripheral glial cells also direct path finding of axons in the transition zone between the PNS and CNS. Sepp et al. showed that during Drosophila development, peripheral glia formed funnel-shaped arrays that guided pioneering motor axons into the periphery. They observed that the axons made typical growth cone contact along the glia, and ablation of peripheral glia caused disruption of motor axon extension out of the transition zone [10]. Likewise, these same peripheral glia formed tubes that directed sensory axons into the CNS. When the glial tubes were ablated, the sensory axons displayed path finding deficits and failed to reach their appropriate target regions within the CNS. Thus, the peripheral glia functioned as a living scaffold and substrate to pre-pattern the transition zone for correct routing of axons between the CNS and PNS during development [10].
Pioneering axons themselves also serve as living scaffolds for the guidance of subsequent axons in both the PNS and CNS. Through their research with grasshopper embryos, a classic study by Raper et al. demonstrated that a small number of early pioneering axons fasciculate to form a reproducible scaffold of axonal pathways (Fig. 3). These initial pathways are differentially labeled and serve to direct the growth cones and extension of later axons. Specifically, Raper et al. showed that the growth cones of two sibling neurons located in the second thoracic ganglion extended along the same initial axonal tract until they diverged along a second tract of existing axons. This study not only demonstrated that later axons use pioneer axons as a map for their growth, but that they also recognize specific and differential labels on these scaffolds to navigate the existing pathways [9]. This phenomenon of pioneer axons functioning as a scaffold for later axons has also been shown to occur in the PNS. For example, Ho et al. observed that pioneering axons are responsible for forming the initial pathways that subsequently become nerves in the antenna and legs of grasshoppers [12]. Overall, these examples demonstrate the developmental basis for contemporary tissue engineered living scaffolds being used for neuroregeneration; specifically, tissue engineered living scaffolds aim to exploit crucial mechanisms by which the CNS and PNS are originally formed.
Fig. 3.
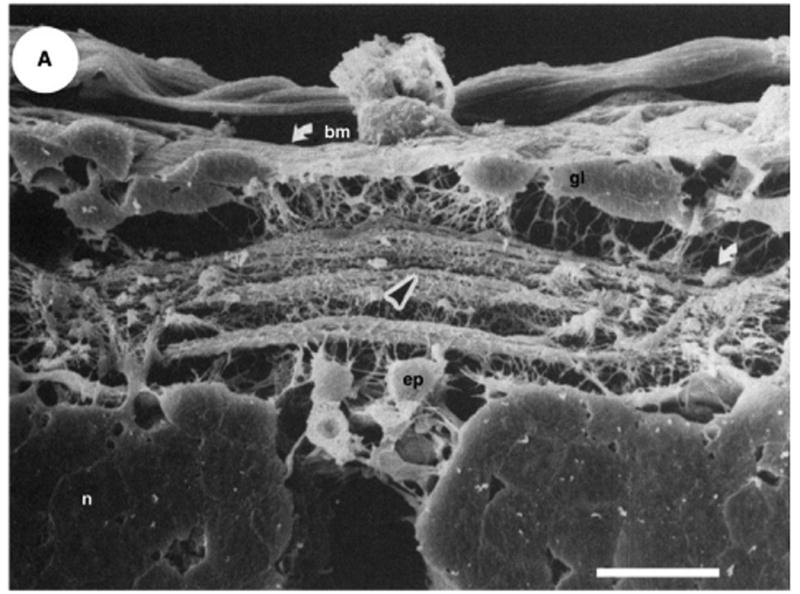
A living scaffold in nervous system development. Axon growth directly along “pioneer” axons that previously reached the appropriate target. Scanning electron micrograph of a cross-section of the posterior commissure in an embryo (41% of the way through gestation). Several discrete axon bundles are shown crossing in the posterior commissure (center of image). A black arrowhead indicates the previously formed bundle over which the growing axons travel. A white arrow indicates the axon bundle in the lateral neuropil upon which the various growth cones diverge and extend in the ganglionic connectives. Scale bar:20 μm. Reproduced with permission from [9].
5. Molecular mechanisms of neural cell migration and axon regeneration
In order to develop successful tissue engineered scaffolds for neuroregeneration, the mechanisms responsible for neural cell migration and axon guidance must be understood and recapitulated. Both cell motility and axon guidance have been shown to occur through one or more of the following mechanisms: contact signaling via structural cues presented along cells and/or on extracellular matrix (ECM), gradients created by diffusion of cell-secreted soluble factors, and substrate mechanical and geometric properties. Of note, living scaffolds possess the ability for “juxtracrine” signaling based on the simultaneous and often synergistic presentation of the aforementioned cues [4,13-17].
6. Neural cell migration
Neural migration is directed by contact signaling, gradients of soluble factors, and the mechanical properties of the substrate [18]. Changes in the cell cytoskeleton, specifically the rich network of microtubules in the soma, are the basis for nucleokinesis − movement of the nucleus [19]. Neurons migrate in response to contact cues with ECM components, cell adhesion molecules (CAMs), and direct contact with other cells. For instance, migration of olfactory epithelial neurons was preferentially stimulated and guided by the ECM protein laminin in vitro [20]. Studies have shown that neuron migration can also be modulated through cell-cell contact, specifically involving glial cells. Recently, it was found that the gap junction proteins connexin 46 and 23 are expressed by radial glia during glial-mediated migration of neurons [19] These proteins provide adhesive contacts between the radial glia and neurons which stabilize the processes of migrating neurons [19]. Direct cell contact mediated by CAMs, specifically L1-CAM and neural cell adhesion molecule (NCAM), also influence neuronal migration [21]. CAMs generally bind through homophilic and heterophilic interactions (i.e. binding to identical or similar, as well as different CAMs, respectively), and therefore provide structural cell-to-cell, axon-to-cell, or axon-to-axon linkages [22]. Aside from direct contact dependent neuronal migration, soluble neurotrophic factors along with neurotransmitters also play a role in influencing neuronal migration [18,23]. For example, astrocytes have been shown to secrete molecules that encourage neuronal migration [24]. Lastly, physical properties of the cell, such as polarity, can influence migration [25]. Thus, the incorporation of these various cues into living scaffolds can direct neural cell migration to the appropriate sites to promote regeneration.
7. Axonal outgrowth
7.1. Haptotaxis: contact dependent signaling
A combination of attractive and repulsive structural cues Q4 presented to growth cones direct axons to their targets. These haptotactic proteins appear both directly on cell surfaces as well as throughout ECM complexes, and serve as guideposts during development and regeneration [8,22]. Prominent structural cell contact cues involved in axon guidance include CAMs, most notably L1-CAM and NCAM (as with neural cell migration), amongst others [8,22]. These molecules play a key role during development, are involved in axon fasciculation, and are expressed on axons and Schwann cells during limb bud innervation. Indeed, genetic deficits in CAMs have a severe impact on the proper formation of corticospinal tracts and corpus callosum, potentially resulting in mental retardation, hydrocephalus, and difficulties in limb movement [22]. Moreover, deficits in CAM expression have also been associated with later-onset disorders such as schizophrenia, Alzheimer's disease, and bipolar disease [26,27]. CAMs have been shown to affect glial activity and axonal outgrowth in both in vitro and in vivo models. Axons growing in vitro have shown a preference for specific CAMs patterned onto a substrate, with distal axons (greater than 55 μm from the cell body) selectively following L1-CAM patterning and proximal axons recognizing both L1-CAM and N-cadherin [28]. In addition, conduits coated with recombinant human L1-CAM promoted superior axon regeneration and myelination in rat optic nerve transection models [13]. Moreover, functionalized CAM biomimetics have been shown to increase Schwann cell activity and myelination of regenerated axons [29,30]. Interestingly, CAMs are highly upregulated under regenerative conditions [22]; therefore, CAMs naturally existing along living scaffolds may present unique homo- and heterophilic domains in optimal patterns to promote targeted axonal regeneration. Other structural guidance cues involve matrix proteins such as collagen, fibrin and laminin, which may occur on cell surfaces but are most commonly associated with ECM (see [31] for recent review). The presence and density of these ECM ligands have been shown to be critical factors for axonal outgrowth in a number of model systems, including models of peripheral nerve injury and spinal cord injury (SCI) [32-36]
7.2. Chemotaxis: soluble factor signaling
The effects of chemotactic cues on axon guidance have been studied extensively (see [8,37,38] for recent reviews). While many trophic factors enhance axon guidance during regeneration, nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), glial derived neurotrophic factor (GDNF), and insulin growth factor (IGF) have received particular attention. Multiple studies have shown that incorporating these factors into nerve conduits either individually or in combination significantly enhances nerve regeneration [4,14-16]. Addition of neurotrophic factors into nerve conduits was shown to increase regenerating axon density, as well as Schwann cell migration, alignment, and eventual myelination at the injury site [4,16]. The spatial presentation and gradient of both soluble factors and membrane-bound structural cues play a significant role in axon guidance. For instance, it was reported that axons extend in the direction of increasing substrate-bound laminin concentrations, and that NGF gradients of at least 133 ng/mL per mm and less than 995 ng/mL per mm are needed to promote neurite outgrowth and guide growth cone extension in PC12 cells [39,40]. Likewise, collagen scaffolds cross-linked with laminin and loaded with ciliary neurotrophic factor resulted in enhanced axonal guidance, regeneration, and functional recovery in a rodent model of peripheral nerve injury [41]. Living scaffolds are able to present a combination of intimate soluble and structural cues, which may together have significant and synergistic effects on axonal extension.
7.3. Mechanotaxis: physical/geometric influences
In addition to conventional chemotactic and haptotactic cues for axonal guidance, it is becoming well established that the mechanical and physical properties of a cell's environment has a significant impact on cell growth and behavior [17,42]. The effects of microenvironmental physical properties on neurite outgrowth may be referred to as “mechanotaxis”, and we previously reviewed interrelated and synergistic influences of chemotactic, haptotactic, and mechanotactic factors on neuronal survival and neurite outgrowth in 3-D engineered matrices [43]. Prior work has shown that parameters of neurite outgrowth such as growth rate and neurite branching depend on matrix mechanical properties (in 3-D and 2-D) [44,45]. For example, it was shown that agarose stiffness and pore size differentially influenced the rate and degree of neurite extension of dorsal root ganglia (DRG), with maximal neurite outgrowth occurring in low concentration (<1.00%) gels [33,44,46]. DRG neurite outgrowth has also been studied in collagen matrices of varying concentrations (and hence stiffness), finding that neurite extension was maximized in lower (0.6 mg/mL) rather than higher (2 mg/mL) concentration gels [36]. However, low concentration hydrogels have been shown to be unsuitable for the survival and neurite outgrowth of cortical neurons [47,48]. Additionally, improved cortical neuron viability was seen on substrates of modulus similar to that of the intact brain [42]. These studies underscore that different intrinsic mechanisms exist between different neuronal sub-types. Moreover, within 3-D matrices a complex interplay exists between matrix stiffness, porosity, and ligand presentation that affects neuronal survival and neurite outgrowth [47].
Additionally, the importance of geometric guidance cues, such as surface curvature, is increasingly being recognized [17,49-51]. For example, DRG axons in culture were shown to have enhanced longitudinal growth along microfibers with diameters of 35 μm or less − a diameter range similar to that of axon fascicles − due to the mechanics of minimizing process bending [17]. Additional surface features such as scratches, ridges, and grooves can aid in guiding neurite outgrowth. For instance, it was shown that growth cone branching was directly related to the number of potential paths at an intersection [52]. Similarly, the shape of the substrate affects neuron morphology and neuritogenesis. When neurons were cultured on varying micro-patterned shapes, their cytoskeletons deformed to imitate the shape of the substrate, revealing that neuritogenesis was increased at the vertex of angles, especially at 60° angles [53]. Therefore, the physical/mechanical properties and the geometric presentation of surface/binding domains on a substrate greatly impact neurite behavior, and can be used to manipulate neurite outgrowth. Of note, living scaffolds comprised of aligned glial cells/processes and axonal tracts may recapitulate the ideal mechanical and geometric factors for targeted axonal re-growth.
8. Advantages of tissue engineered living scaffolds
A tissue engineered living scaffold with the highest capacity for regeneration will possess all of the aforementioned factors, including chemotaxic, haptotaxic, and mechanical cues, which will work synergistically to promote targeted axonal guidance and/or cellular infiltration. Although creating 3-D living scaffolds represents a tremendously complex endeavor, significant progress has been made in the past decade. These living scaffolds show great promise for neuroregeneration and hold significant advantages over competing regenerative therapies. Living cells possess the ability to secrete thousands of neurotrophic factors and control CAM expression. They may actively respond to their environment and modulate pro-regenerative cues such that they remain optimal for axon guidance and sprouting, myelination, and the restoration of complex 3-D tissue structures. In contrast to living scaffolds, most current axon guidance conduits or acellular scaffolds are fabricated from synthetic or naturally occurring biomaterials. These constructs are sometimes loaded or coated with soluble factors to promote neural regeneration [1,4,54]. Such therapies are limited in recreating and maintaining the optimal concentrations of soluble factors and presentation of structural cues for regeneration; they weakly simulate the conditions that exist during embryonic development when neurogenesis and axogenesis first occur. At present, non-living scaffolds can only deliver a relatively small number of factors involved in regeneration, and, although controlled release of soluble factors is a common objective [1,37,54] acellular scaffolds are not currently capable of modulating secreted factors based on the progression and state of the regenerative process. As such, the mechanisms and efficacy of numerous acellular constructs have not successfully translated to in vivo environments despite being “optimized” in vitro, suggesting that endogenous processes and signals may override the regenerative effects of many contemporary acellular biomaterials.
9. Disadvantages of tissue engineered living scaffolds
While living cell-based scaffolds provide several key advantages for promoting neuroregeneration, there are also significant drawbacks to this approach. Living cells may elicit an immune response from host tissue leading to inflammation or rejection of the graft, depending on the source and phenotype of the cells [55]. This response can be circumvented through the use of autologous cells from the patient. Additionally, whereas glial cells elicit a vigorous immune response and show poor attrition upon transplantation, constructs consisting of pure neurons appear to be well tolerated by the immune system and survive at least several months [56-58]. Certain undifferentiated stem cells also may be immune privileged and only elicit a delayed or significantly attenuated immune response [59]. Although undesirable, immunosuppression can be utilized to mitigate an immune response. While this approach leaves the host susceptible to other infections, a unique feature of living neuroregenerative scaffolds is that they do not need to be permanent; once a living scaffold has facilitated axonal regeneration and tissue reformation, immunosuppression can be ceased. Interestingly, in some cases adjunct immunosuppression has actually been shown to accelerate axonal regeneration and functional restoration [60]. An additional challenge with living scaffolds is that increasing the regenerative capacity of the cellular environment may lead to excessive or aberrant regeneration, such as over-proliferation of host cells as well as formation of deleterious axonal connections. Although the challenge of aberrant regeneration is not limited to living scaffolds, regenerative constraints may be necessary in certain applications. However, the nervous system does maintain tremendous capacity for plasticity, creating the possibility of effective use of novel connectivity. Finally, while living scaffolds offer the advantage of being “personalized”, i.e. fabricated using a patients own cells to treat their specific neurological affliction, it may be desirable in other cases to have generalized scaffolds available “off the shelf.” This prospect creates several challenges associated with the scale-up and storage of live constructs, as well as the quality control measures following production and immediately prior to implantation [61]. These challenges are shared with all emerging tissue engineering strategies for living grafts and organ replacement, and thus there are a number of companies focused on various strategies to address these issues [61,62].
10. Examples of living scaffolds in neuroregeneration
Several early in vitro studies paved the way for the later production and implementation of living scaffolds for neuroregeneration in vivo. These studies confirmed that the mechanisms of action observed during organism development could be reproduced in culture. For example, Fawcett et al. looked at the ability of cellulose ester tubes lined with either Schwann cells or astrocytes to guide axonal growth, revealing that axons from both embryonic DRG and embryonic retina neurons grew quickly and profusely through the Schwann cell tubes [63] Similarly, astrocyte-containing tubes were also capable of guiding axon growth [63]. In another seminal study, Chang et al. examined the growth of neurites along an axonal substrate in vitro. They found preferential neurite extension along the axonal substrate that was mediated by specific axon surface glycoproteins on the axonal substrate [64].
In the last two decades, extensive progress has been made in the field of neural tissue engineering. The mechanisms of neural growth and axonal path finding discovered in the 1980s are now being fully utilized in tissue engineered living scaffolds. For example, in groundbreaking studies, East et al. at The Open University developed 3-D collagen gels containing aligned astrocytes. Astrocytes were first seeded in a tethered collagen gel and allowed to grow. Transforming growth factor was then added to the cultures, which caused the astrocytes to activate and align within the tethered gel [65]. Plastic compression was used to swiftly remove fluid from the gels in order to form stable, collagenous sheets containing the aligned cells. The sheets were then rolled to create cylindrical constructs. In order to test their ability to promote neuronal growth, dissociated DRG neurons were seeded within these constructs along with the astrocytes. East el al. found that neurites preferentially grew along the aligned astrocytes (Fig. 4), and that their growth was enhanced in comparison to control tubes [65]. Similarly, another group created 3-D nanofiber scaffolds containing aligned astrocytes. Here, Weightman et al. utilized electrospinning to produce acetate frames containing poly-lactic acid nanofibers. Astrocytes and collagen were added to these nanofiber meshes, after which the frames were stacked in layers. This process caused the astrocytes to align and proliferate upon the nanofibers [66]. Both of these approaches to create scaffolds of aligned astrocytes were engineered for repair of CNS injury, and represent promising approaches that may subsequently support neuronal re-growth and regeneration.
Fig. 4.
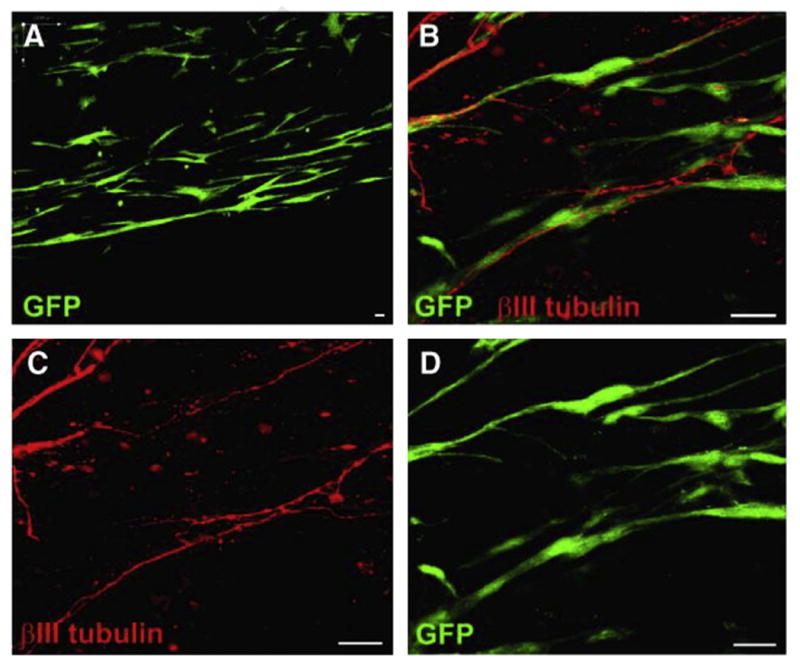
Tissue engineered constructs with aligned astrocytes. (A) Astrocytes expressing green fluorescent protein (GFP) grown in plastic compressed collagen constructs survived and maintained alignment within the matrix. (B–D) Confocal micrographs of aligned astrocytic constructs in co-culture with DRG neurons. Neurites stained for β-tubulin III (red) could be seen extending along GFP+ astrocyte processes. This demonstrated that neurite outgrowth was guided by aligned astrocytes in these implantable constructs. Scale bars: 25 μm. Reproduced with permission from [65].
The Phillips laboratory at The Open University also applied techniques to fabricate collagen constructs containing aligned rat Schwann cells for peripheral nerve regeneration, which they designated “engineered neural tissue” (EngNT). Aligned Schwann cells specifically recreate the Bands of Bungner − naturally occurring columns of Schwann cells that form following peripheral nerve injury to guide and accelerate axonal regeneration. In order to test the efficacy of their EngNT constructs in vivo, Georgiou et al. first placed rolled constructs into commercially available nerve guidance tubes, and then transplanted these tubes into a 15 mm gap rat sciatic nerve model. At 8 weeks post-repair, they found that there was considerably more neural tissue in the nerve guidance tubes containing their constructs as compared with empty tubes [67]. This group plans to further optimize their constructs and test their effectiveness across critical gap defects. The use of aligned Schwann cells for peripheral nerve regeneration is also being pursued by Bozkurt et al. through the use of nerve guides containing pore channels. This research group engineered a collagen-based micro-structured scaffold comprised of longitudinally oriented and interconnected pores, designated “Perimaix”. In vitro, they found that their construct was capable of inducing Schwann cell alignment and supporting longitudinal axonal outgrowth. To investigate the ability of the Perimaix nerve guides to promote nerve regeneration, they first seeded the scaffolds with rat Schwann cells and then transplanted them into a 20 mm gap rat sciatic nerve model. At 6 weeks, they found that the density of axons in the Schwann cell-seeded Perimaix group was close to the density of axons in their autograft group, and that the two groups demonstrated comparable myelination [68]. In the future, Bozkurt et al. intend to optimize their nerve guide for better stability and longevity in preparation for chronic nerve regeneration studies. In addition to oriented nanofibers, tethered gels, and structured channels, several other techniques are being used to align cell populations. These include electrically and magnetically aligned matrices, micropatterned surfaces, gradients of neurotrophic factors, and fibers containing longitudinal grooves [4,69-71]. Collectively, these constructs aim to exploit glial-mediated axonal regeneration to facilitate regeneration following major neurotrauma or neurodegeneration.
An alternative approach to engineering scaffolds containing aligned glial cells is to create constructs containing long, aligned axonal tracts. These constructs are designed to utilize “axon-mediated axonal outgrowth” to support neural repair. This mechanism involves the growth of regenerating axons along pre-formed (i.e. tissue engineered) axonal pathways, mimicking axonal growth along “pioneer” axons during nervous system development. Our group at the University of Pennsylvania has employed constructs containing longitudinally aligned axonal tracts as the basis for living scaffolds to repair axonal connections in the CNS and PNS. In one application, we engineered a micron-scale tubular construct consisting of an inner ECM core and an outer hydrogel shell. In order to generate the 3-D axonal tracts, we plated populations of primary neurons on either end of the constructs and optimized conditions to permit axonal projections across the tubular constructs [72]. These “micro-tissue engineered neural networks” (micro-TENNs) exhibited robust neuronal survival and axonal extension in vitro, recapitulating the systems-level neuroanatomy of the brain: discrete neuronal populations spanned by long axonal tracts (Fig. 5). In future applications, these miniature constructs − roughly three times the diameter of a human hair and extending several centimeters in length − may be used for targeted reconstruction of neural circuitry lost due to injury or disease in the CNS (Fig. 5).
Fig. 5.

Micro-tissue engineered neural networks (Micro-TENNs) consisting of aligned axons and/or glia for CNS repair. We have developed “micro-TENNs”, which are miniature, living, preformed constructs grown in vitro that consist of discrete neuronal population(s) spanned by long axonal tracts. These living micro-TENNs reconstitute the neuroanatomy of brain pathways, and therefore may be used to physically reconstruct lost or dysfunctional neural circuits. (A-D) Confocal reconstructions of micro-TENNs labeled via immunocytochemistry to denote neuronal somata/axons (β-tubulin III; green), cell nuclei (Hoechst; blue), and glial somata/processes (glial-fibrillary acidic protein; red) at 7 days in vitro. (A) Axons projected longitudinally into the construct core, (B) while both neuronal and glial (when present) somata remained in a dense cluster on the end. (C) When glial processes were present, they also presented aligned 3-D growth, but did not project as far as the axons. (D) Overlay (scale bar = 100 um). (E) These miniature living scaffolds are designed for minimally invasive injection into the brain to simultaneously replace neurons and reconstruct long-distance axonal connections lost due to trauma, stroke, or neurodegenerative disease. Note that while (A-D) illustrates a unidirectional micro-TENN, (E) depicts the implantation of a bi-directional micro-TENN. Adapted with permission from [72].
Our research group also utilizes much larger constructs containing long, integrated, axonal tracts for peripheral nerve repair. To generate our “tissue engineered nerve grafts” (TENGs), we used the recently discovered process of axonal “stretch-growth.” This process mimics the developmental mechanism by which axons are extended in length due to tension as an organism grows from embryogenesis to adulthood [73,74]. This process involves plating two neuronal populations on either side of an interface, allowing axonal networks to form between them, and then slowly separating the populations in micron-size increments using custom mechano-bioreactors. These integrated axons respond to the forces by increasing in length as well as diameter, and this process also encourages fasciculation [73,74]. To date, stretch-grown axonal constructs have been generated at lengths of 5−10 cm in 14−21 days, with even longer lengths likely attainable [73,74]. To test the efficacy of the TENGs in vivo, we encapsulated the stretch-grown axons in collagenous matrices for stability and transferred them into nerve guidance tubes for transplantation into a 10 mm gap rat sciatic nerve model. At 6 weeks post transplantation, we found that the TENGs survived, maintained their cytoarchitecture, and had integrated with the host nerve tissue. In particular, host axons were in intimate proximity with TENG axons, suggesting the transplanted axon tracts mediated host axonal growth across the lesion (Fig. 6). At 16 weeks post transplantation, the segments of neural tissue bridging the gap appeared grossly normal, with a significant density of myelinated host axons [56]. Similar constructs containing “stretch-grown” axonal tracts were also used for spinal cord repair. Here living scaffolds consisting of neurons and stretch-grown axonal tracts were grown to 10 mm in length, encapsulated in collagenous matrices, and transplanted to repair equally sized lateral hemisection spinal cord lesions in rats. Remarkably, at one-month post surgery, the constructs had survived and integrated with the host by extending axons into the spinal cord [57]. This promising approach may permit host axon regeneration across major spinal cord lesions.
Fig. 6.

Living axonal scaffolds for peripheral nerve regeneration. Survival and integration of implanted living tissue engineered nerve grafts (TENGs) at 6 weeks following transplantation to bridge excised segments of sciatic nerve in rats. Constructs consisted of longitudinally aligned axonal tracts (GFP+) generated based on axonal “stretch-growth.” (A) Longitudinal section of continuous proximal (top) and distal (bottom) nerve across the repair site (scale bars = 0.5 mm). Note multiple transplanted ganglia on the proximal and distal ends with aligned axonal tracts spanning those neuronal populations (GFP+). (B) Regenerating host axons (neurofilament; red) entered into the proximal end of the constructs and were not co-localized with GFP. (C) However, host axons (red) across the center of the grafts were co-localized with GFP+ transplanted axons, suggesting host regeneration occurred along the transplanted axonal tracts. (D) A subset of GFP+ TENGs were transplanted across the excised sciatic nerve in transgenic rats expressing alkaline phosphatase (AP; red). Here, neurites from transplanted neurons (GFP+, short arrow) were observed in intimate contact with axons from the host (AP+, arrow heads) and were often intertwined (long arrows). These observations suggested that host axonal regeneration occurred directly along the living scaffold of aligned axonal tracts presented by TENGs. Adapted with permission from [56].
11. Future applications
Living scaffolds for neuroregeneration are evolving quickly and merging with other areas of biology and biotechnology. A prime example of this is the inclusion of autologous stem cells within living scaffolds. This approach will converge with personalized medicine in order to deliver scaffolds with the precise architecture and cell phenotype(s) necessary for a given patient and their disorder. Many different stem cells have already been isolated and used for purposes of neural regeneration; these include precursor cells for neurons, oligodendrocytes, astrocytes, and Schwann cells differentiated from human embryonic stem cells (hESCs), induced pluripotent stem cells (iPSCs), and adipose-derived stem cells (ASCs) among others [75-79]. In addition to being an eternal source of autologous cells, stem cells have been shown to encourage neural regeneration through their unique release of trophic factors. For example, neural stem cells demonstrated functional improvement in rat and mice models of Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis [80-82]. Transplanted neural progenitor cells derived from iPSCs improved neurological outcomes in a rat stoke model, while oligodendrocyte progenitor cells derived from iPSCs demonstrated robust myelination of myelin deficient mice and prolonged their survival [77,78]. NG2-expressing oligodendrocyte precursor cells, or polydendrocytes proliferate and differentiate into oligodendrocytes in response to demyelinating lesions and promote functional recovery and remyelination, which was shown in a mouse model of encephalomyelitis [83-85]. ASCs promoted nerve regeneration and axon myelination following peripheral nerve lesions in rats [86]. Although autologous stem cells have drastically increased the feasibility of clinical translation, it is important to note that the mechanisms by which they stimulate regeneration are not well understood. Furthermore, they have the potential to differentiate into undesirable phenotypes and/or result in tumorigenesis [76].
In addition to, or in combination with, the use of stem cells, the inclusion of genetically engineered cells within living scaffolds is gaining recognition as a means to enhance the regenerative response. The genetic material of these cells can be altered to provide the cells with increased durability upon transplant or to better promote axonal regeneration and function. The approaches being pursued to provide these capabilities vary greatly and range from augmenting the intrinsic functions of the cells to engineering cells that can constitutively secrete trophic factors [2]. For example, Akerud et al. injected mouse neural stem cells engineered to over express glial cell line-derived neurotropic factor (GDNF) into a mouse model of Parkinson's disease. At four months post surgery, the engineered cells had maintained therapeutic levels of GDNF in vivo and prevented degeneration of dopaminergic neurons in the substantia nigra [87]. In a different approach, Gravannis et al. genetically modified Schwann cells to express sialyltransferase X (STX), an enzyme responsible for mediating the properties of the cell adhesion molecule NCAM. The altered Schwann cells were seeded into nerve guidance tubes for transplant into a rat model of peripheral nerve injury, and resulted in increased fiber diameter and myelin thickness of regenerating axons [88]. However, it is possible to over-engineer cells to the point that they actually inhibit regeneration. For example, Santosa et al. found that allografts supplemented with Schwann cells overexpressing GDNF were overly attractive and prevented regenerating axons from leaving the allografts, thus blunting regeneration [89].
Genetically engineered cells are also being used to control neural circuitry via optogenetic stimulation. Optogenetics refers to the technique of controlling neural activity through light sensitive ion channels called channelrhodopsins. For example, Weick et al. used light to exclusively stimulate transplanted neurons expressing channelrhodopsins. They showed that the transplanted cells formed functional circuits with host neurons, and that they could drive the activity of the host neurons by optically stimulating the transplanted neurons [90]. It has similarly been shown that neurons expressing channelrhodopsins could modulate a range of neural activity, including activating high frequency oscillations when injected into the hippocampus as well as control of muscle function when engrafted into the sciatic nerve [91,92]. As the applications of optogenetics evolve, the inclusion of cells expressing channelrhodopsins will afford living scaffolds the ability to drive local circuitry for purposes of neuromodulation and/or neuroregeneration.
Bioreactor technology is similarly beginning to converge with the development of novel living scaffolds. Bioreactors are devices engineered to mimic a specific physiological environment for the purpose of influencing the growth of cells or tissue [93]. Bioreactors may guide or control the growth of cells through the use of mechanical, electrical, biological, or chemical stimuli. In particular, it is expected that electrical conditioning will be used to impact the function of neural cells in living scaffolds for neuroregeneration. It was shown that exogenous electrical activity generated by piezoelectric substrates resulted in increased neurite length and branching complexity of rat spinal cord neurons [94]. Likewise, it was shown that the application of an electrical field induced preferential directionality to neurite outgrowth [95] Additionally, electrical stimulation has been seen to play a significant role in fate determination of stem cells [96]. Once the consequences of various stimulation paradigms are better understood, it is likely these signals will be incorporated into bioreactors to condition living scaffolds for enhanced performance in vivo.
Novel fabrication methods, including 3-D printing and “cell electrospinning,” are also being utilized to generate living scaffolds. 3-D printing has the capacity to integrate proteins, growth factors, biomaterial, and live cells into a singular scaffold with increased control over deposition location and amount. Therefore, 3-D printing can fabricate anisotropic scaffolds that more closely mimic in vivo tissue [97]. However, few neuronal cell types have been tested in 3-D printers, and there is widespread concern regarding the potentially harmful vibration frequencies and power levels used in various printers. Lorber et al. recently found that the viabilities of piezoelectric inkjet-printed retinal ganglia and glial cells were significantly reduced in comparison to controls [98]. Within the last decade, researchers have also used “cell electros-pinning” to generate fibers containing cells for use in living scaffolds. While these fibers can be fabricated with anisotropic properties, it remains unclear how the process influences cell gene expression [99]. To date, living neurons and glia have yet to be incorporated into electrospun fibers for regeneration therapies. Thus, although 3-D printing and cell electrospinning hold great promise for neural tissue engineering applications, further characterization of their effects on cell viability and gene expression is necessary.
In the future, living scaffolds may also be used as interfaces between the nervous system and machines. One of the greatest obstacles facing neural interface technology is the immune response elicited by implanted electrodes. An immune response generally results in fibrous encapsulation of the electrodes and, ultimately, the attenuation of signal transmittance. Living scaffolds may be able to prevent an immune response by acting as biological intermediates between the body and the electrodes. In this case, the body would only perceive the biological portion of the interface, while the living scaffold would interact directly with the electrodes [100,101].
Finally, once the mechanisms that lead to successful neuroregeneration are fully understood they could be recapitulated onto acellular biomaterials to fabricate a universal, cost-efficient scaffold. For example, the CAMs responsible for nerve guidance could be presented in the appropriate pattern on a biomaterial with the correct mechanical properties, such as stiffness, fiber diameter, etc. Neurotrophic factors could be loaded into the scaffold at varying distances to create a gradient for controlled release that would promote axon guidance and regeneration. Although a biomimetic, acellular scaffold presents many benefits, it is unlikely that it would match the advantages, ability, and robustness of the “living scaffold” defined herein.
12. Summary
Directed axon growth and cell migration along pathways formed by other cells is a common tactic in nervous system development. Indeed, this concept has long been appreciated in developmental neurobiology as crucial to the proper formation of the nervous system, including necessary axonal connectivity and localization of cellular constituents. However, only recently has this idea been embraced as a strategy to facilitate nervous system regeneration by the relatively new field of neural tissue engineering. Tissue engineers are growing cells and creating living constructs with specific geometrical, mechanical, and biological cues to allow for targeted and orchestrated neural tissue regeneration across three-dimensional space. Although there are significant challenges to implementation, this approach is extremely promising for neuroregenerative medicine and may ultimately facilitate functional recovery for a number of currently intractable neurotrauma and neurodegenerative diseases.
Acknowledgments
This work was made possible due to financial support provided by the U.S. Army Medical Research and Materiel Command Q5 through the Armed Forces Institute of Regenerative Medicine Q6 (#W81XWH-08-2-0034) and the Congressionally Directed Medical Research Program (#W81XWH-10-1-0941); Axonia Medical, Inc., through a sponsored research agreement; Department of Veterans Affairs (RR&D Merit Review #B1097-I); National Institutes of Health/NINDS (T32-NS043126); and the National Science Foundation (Graduate Research Fellowship DGE-1321851).
References
- 1.Eberli D, Atala A. Tissue engineering using adult stem cells. Methods Enzymol. 2006;420:287–302. doi: 10.1016/S0076-6879(06)20013-2. [DOI] [PubMed] [Google Scholar]
- 2.Korecka JA, Verhaagen J, Hol EM. Cell-replacement and gene-therapystrategies for Parkinson's and Alzheimer's disease. Regenerative Med. 2007;2:425–46. doi: 10.2217/17460751.2.4.425. [DOI] [PubMed] [Google Scholar]
- 3.Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2008;17(Suppl.4):467–79. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madduri S, di Summa P, Papaloizos M, Kalbermatten D, Gander B. Effect of controlled co-delivery of synergistic neurotrophic factors on early nerveregeneration in rats. Biomaterials. 2010;31:8402–9. doi: 10.1016/j.biomaterials.2010.07.052. [DOI] [PubMed] [Google Scholar]
- 5.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Cullen DK. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39:81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 7.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–48. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raper J, Mason C. Cellular strategies of axonal path finding. Cold Spring Harb Perspect Biol. 2010;2:a001933. doi: 10.1101/cshperspect.a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raper JA, Bastiani M, Goodman CS. Path finding by neuronal growth cones in grass hopper embryos. II. Selective fasciculation onto specific axonal pathways. J Neurosci Off J Soc Neurosci. 1983;3:31–41. doi: 10.1523/JNEUROSCI.03-01-00031.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sepp KJ, Schulte J, Auld VJ. Peripheral glia direct axon guidance across the CNS/PNS transition zone. Develop Biol. 2001;238:47–63. doi: 10.1006/dbio.2001.0411. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs JR, Goodman CS. Embryonic development of axon pathways in the Drosophila CNS. I. A glial scaffold appears before the first growth cones. J Neurosci: Off J Soc Neurosci. 1989;9:2402–11. doi: 10.1523/JNEUROSCI.09-07-02402.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho RK, Goodman CS. Peripheral pathways are pioneered by an array of central and peripheral neurones in grasshopper embryos. Nature. 1982;297:404–6. doi: 10.1038/297404a0. [DOI] [PubMed] [Google Scholar]
- 13.Xu G, Nie DY, Wang WZ, Zhang PH, Shen J, Ang BT, et al. Optic nerve regeneration in polyglycolic acid-chitosan conduits coated with recombinant L1-Fc. Neuroreport. 2004;15:2167–72. doi: 10.1097/00001756-200410050-00004. [DOI] [PubMed] [Google Scholar]
- 14.Yan Q, Yin Y, Li B. Use new PLGL-RGD-NGF nerve conduits for promoting peripheral nerve regeneration. Biomed Eng Online. 2012;11:36. doi: 10.1186/1475-925X-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine EG, Decosterd I, Papaloizos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 16.Apel PJ, Ma J, Callahan M, Northam CN, Alton TB, Sonntag WE, et al. Effect of locally delivered IGF-1 on nerve regeneration during aging: an experimental study in rats. Muscle Nerve. 2010;41:335–41. doi: 10.1002/mus.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smeal RM, Rabbitt R, Biran R, Tresco PA. Substrate curvature influences the direction of nerve outgrowth. Ann Biomed Eng. 2005;33:376–82. doi: 10.1007/s10439-005-1740-z. [DOI] [PubMed] [Google Scholar]
- 18.Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–83. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- 19.Valiente M, Marin O. Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol. 2010;20:68–78. doi: 10.1016/j.conb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Calof AL, Lander AD. Relationship between neuronal migration and cell-substratum adhesion: laminin and merosin promote olfactory neuronal migration but are anti-adhesive. J Cell Biol. 1991;115:779–94. doi: 10.1083/jcb.115.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmid RS, Maness PF. L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Curr Opin Neurobiol. 2008;18:245–50. doi: 10.1016/j.conb.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiencken-Barger AE, Mavity-Hudson J, Bartsch U, Schachner M, Casagrande VA. The role of L1 in axon path finding and fasciculation. Cereb Cortex. 2004;14:121–31. doi: 10.1093/cercor/bhg110. [DOI] [PubMed] [Google Scholar]
- 23.Keenan TM, Grinager JR, Procak AA, Svendsen CN. In vitro localization of human neural stem cell neurogenesis by engineered FGF-2 gradients. Integrative Biol: Quant Biosci Nano Macro. 2012;4:1522–31. doi: 10.1039/c2ib20074k. [DOI] [PubMed] [Google Scholar]
- 24.Mason HA, Ito S, Corfas G. Extracellular signals that regulate the tangential migration of olfactory bulb neuronal precursors: inducers, inhibitors, and epellents. J Neurosci: Off J Soc Neurosci. 2001;21:7654–63. doi: 10.1523/JNEUROSCI.21-19-07654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin super family: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 27.Enriquez-Barreto L, Palazzetti C, Brennaman LH, Maness PF, Fairen A. Neural cell adhesion molecule, NCAM, regulates thalamocortical axon path finding and the organization of the cortical somatosensory representation in mouse. Front Mol Neurosci. 2012;5:76. doi: 10.3389/fnmol.2012.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi P, Shen K, Kam LC. Local presentation of L1 and N-cadherin in multicomponent, microscale patterns differentially direct neuron function in vitro. Develop Neurobiol. 2007;67:1765–76. doi: 10.1002/dneu.20553. [DOI] [PubMed] [Google Scholar]
- 29.Haile Y, Haastert K, Cesnulevicius K, Stummeyer K, Timmer M, Berski S, et al. Culturing of glial and neuronal cells on polysialic acid. Biomaterials. 2007;28:1163–73. doi: 10.1016/j.biomaterials.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 30.Mehanna A, Mishra B, Kurschat N, Schulze C, Bian S, Loers G, et al. Polysialic acid glycomimetics promote myelination and functional recovery after peripheral nerve injury in mice. Brain: J Neurol. 2009;132:1449–62. doi: 10.1093/brain/awp128. [DOI] [PubMed] [Google Scholar]
- 31.Myers JP, Santiago-Medina M, Gomez TM. Regulation of axonal outgrowth and path finding by integrin-ECM interactions. Develop Neurobiol. 2011;71:901–23. doi: 10.1002/dneu.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellamkonda R, Ranieri JP, Aebischer P. Laminin oligopeptide derivatized agarose gels allow three-dimensional neurite extension in vitro. J Neurosci Res. 1995;41:501–9. doi: 10.1002/jnr.490410409. [DOI] [PubMed] [Google Scholar]
- 33.Bellamkonda R, Ranieri JP, Bouche N, Aebischer P. Hydrogel-based three-dimensional matrix for neural cells. J Biomed Mater Res. 1995;29:663–71. doi: 10.1002/jbm.820290514. [DOI] [PubMed] [Google Scholar]
- 34.Krewson C, Chung S, Dai W, Saltzman W. Cell-aggregation and neurite growth in gels of extracellular-matrix molecules. Biotechnol Bioeng. 1994;43:555–62. doi: 10.1002/bit.260430704. [DOI] [PubMed] [Google Scholar]
- 35.Schense JC, Hubbell JA. Three-dimensional migration of neurites is mediated by adhesion site density and affinity. J Biol Chem. 2000;275:6813–8. doi: 10.1074/jbc.275.10.6813. [DOI] [PubMed] [Google Scholar]
- 36.Willits RK, Skornia SL. Effect of collagen gel stiffness on neurite extension. J Biomater Sci Polym Ed. 2004;15:1521–31. doi: 10.1163/1568562042459698. [DOI] [PubMed] [Google Scholar]
- 37.Thorne RG, Frey WH., 2nd Delivery of neurotrophic factors to the centralnervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2001;40:907–46. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- 38.Mortimer D, Fothergill T, Pujic Z, Richards LJ, Goodhill GJ. Growth cone chemotaxis. Trends Neurosci. 2008;31:90–8. doi: 10.1016/j.tins.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Cao X, Shoichet MS. Defining the concentration gradient of nerve growth factor for guided neurite outgrowth. Neuroscience. 2001;103:831–40. doi: 10.1016/s0306-4522(01)00029-x. [DOI] [PubMed] [Google Scholar]
- 40.Dertinger SK, Jiang X, Li Z, Murthy VN, Whitesides GM. Gradients of substrate-bound laminin orient axonal specification of neurons. Proc Natl Acad Sci USA. 2002;99:12542–7. doi: 10.1073/pnas.192457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao J, Sun C, Zhao H, Xiao Z, Chen B, Gao J, et al. The use of laminin modified linear ordered collagen scaffolds loaded with laminin-binding ciliary neurotrophic factor for sciatic nerve regeneration in rats. Biomaterials. 2011;32:3939–48. doi: 10.1016/j.biomaterials.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–8. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cullen DK, Wolf JA, Vernekar VN, Vukasinovic J, LaPlaca MC. Neural tissue engineering and biohybridized microsystems for neurobiological investigation in vitro (Part 1) Crit Rev Biomed Eng. 2011;39:201–40. doi: 10.1615/critrevbiomedeng.v39.i3.30. [DOI] [PubMed] [Google Scholar]
- 44.Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–84. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 45.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–5. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dillon GP, Yu X, Sridharan A, Ranieri JP, Bellamkonda RV. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. J Biomater Sci Polym Ed. 1998;9:1049–69. doi: 10.1163/156856298x00325. [DOI] [PubMed] [Google Scholar]
- 47.Cullen DK, Lessing MC, LaPlaca MC. Collagen-dependent neurite outgrowth and response to dynamic deformation in three-dimensional neuronal cultures. Ann Biomed Eng. 2007;35:835–46. doi: 10.1007/s10439-007-9292-z. [DOI] [PubMed] [Google Scholar]
- 48.O'Connor SM, Stenger DA, Shaffer KM, Ma W. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci Lett. 2001;304:189–93. doi: 10.1016/s0304-3940(01)01769-4. [DOI] [PubMed] [Google Scholar]
- 49.Cullen DK, Patel AR, Doorish JF, Smith DH, Pfister BJ. Developing a tissue-engineered neural-electrical relay using encapsulated neuronal constructs on conducting polymer fibers. J Neural Eng. 2008;5:374–84. doi: 10.1088/1741-2560/5/4/002. [DOI] [PubMed] [Google Scholar]
- 50.Smeal RM, Tresco PA. The influence of substrate curvature on neurite outgrowth is cell type dependent. Exp Neurol. 2008;213:281–92. doi: 10.1016/j.expneurol.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 51.Wen X, Tresco PA. Effect of filament diameter and extracellular matrix molecule precoating on neurite outgrowth and Schwann cell behavior on multifilament entubulation bridging device in vitro. J Biomed Mater Res, Part A. 2006;76:626–37. doi: 10.1002/jbm.a.30520. [DOI] [PubMed] [Google Scholar]
- 52.Withers GS, James CD, Kingman CE, Craighead HG, Banker GA. Effects of substrate geometry on growth cone behavior and axon branching. J Neurobiol. 2006;66:1183–94. doi: 10.1002/neu.20298. [DOI] [PubMed] [Google Scholar]
- 53.Jang MJ, Nam Y. Geometric effect of cell adhesive polygonal micropatterns on neuritogenesis and axon guidance. J Neural Eng. 2012;9:046019. doi: 10.1088/1741-2560/9/4/046019. [DOI] [PubMed] [Google Scholar]
- 54.Rosenstein JM, Krum JM, Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6:107–14. doi: 10.4161/org.6.2.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx: J Am Soc Exp Neurotherapeutics. 2004;1:472–81. doi: 10.1602/neurorx.1.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang JH, Cullen DK, Browne KD, Groff R, Zhang J, Pfister BJ, et al. Long-term survival and integration of transplanted engineered nervous tissue constructs promotes peripheral nerve regeneration. Tissue Eng Part A. 2009;15:1677–85. doi: 10.1089/ten.tea.2008.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iwata A, Browne KD, Pfister BJ, Gruner JA, Smith DH. Long-term survival and outgrowth of mechanically engineered nervous tissue constructs implanted into spinal cord lesions. Tissue Eng. 2006;12:101–10. doi: 10.1089/ten.2006.12.101. [DOI] [PubMed] [Google Scholar]
- 58.Liu W, Ren Y, Bossert A, Wang X, Dayawansa S, Tong J, et al. Allotransplanted neurons used to repair peripheral nerve injury do not elicit overt immunogenicity. PLoS ONE. 2012;7:e31675. doi: 10.1371/journal.pone.0031675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12:407–12. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Jensen JN, Brenner MJ, Tung TH, Hunter DA, Mackinnon SE. Effect of FK506 on peripheral nerve regeneration through long grafts in inbred swine. Ann Plast Surg. 2005;54:420–7. doi: 10.1097/01.sap.0000151461.60911.c0. [DOI] [PubMed] [Google Scholar]
- 61.Jayo MJ, Watson DD, Wagner BJ, Bertram TA. Tissue engineering and regenerative medicine: role of toxicologic pathologists for an emerging medical technology. Toxicol Pathol. 2008;36:92–6. doi: 10.1177/0192623307311405. [DOI] [PubMed] [Google Scholar]
- 62.Parenteau N. Skin: the first tissue-engineered products. Sci Am. 1999;280:83–4. doi: 10.1038/scientificamerican0499-83. [DOI] [PubMed] [Google Scholar]
- 63.Fawcett JW, Housden E, Smith-Thomas L, Meyer RL. The growth of axons in three-dimensional astrocyte cultures. Develop Biol. 1989;135:449–58. doi: 10.1016/0012-1606(89)90193-0. [DOI] [PubMed] [Google Scholar]
- 64.Chang S, Rathjen FG, Raper JA. Extension of neurites on axons is impaired by antibodies against specific neural cell surface glycoproteins. J Cell Biol. 1987;104:355–62. doi: 10.1083/jcb.104.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.East E, de Oliveira DB, Golding JP, Phillips JB. Alignment of astrocytes increases neuronal growth in three-dimensional collagen gels and is maintained following plastic compression to form a spinal cord repair conduit. Tissue Eng Part A. 2010;16:3173–84. doi: 10.1089/ten.tea.2010.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weightman A, Jenkins S, Pickard M, Chari D, Yang Y. Alignment of multiple glial cell populations in 3D nanofiber scaffolds: toward the development of multicellular implantable scaffolds for repair of neural injury. Nanomed Q7 Nanotechnol Biol Med. 2013 doi: 10.1016/j.nano.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Georgiou M, Bunting SC, Davies HA, Loughlin AJ, Golding JP, Phillips JB. Engineered neural tissue for peripheral nerve repair. Biomaterials. 2013;34:7335–43. doi: 10.1016/j.biomaterials.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 68.Bozkurt A, Lassner F, O'Dey D, Deumens R, Bocker A, Schwendt T, et al. The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves. Biomaterials. 2012;33:1363–75. doi: 10.1016/j.biomaterials.2011.10.069. [DOI] [PubMed] [Google Scholar]
- 69.Nichterwitz S, Hoffmann N, Hajosch R, Oberhoffner S, Schlosshauer B. Bioengineered glial strands for nerve regeneration. Neurosci Lett. 2010;484:118–22. doi: 10.1016/j.neulet.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 70.Verdu E, Labrador RO, Rodriguez FJ, Ceballos D, Fores J, Navarro X. Alignment of collagen and laminin-containing gels improve nerve regeneration within silicone tubes. Restor Neurol Neurosci. 2002;20:169–79. [PubMed] [Google Scholar]
- 71.Hsu SH, Su CH, Chiu IM. A novel approach to align adult neural stem cells on micro patterned conduits for peripheral nerve regeneration: a feasibility study. Artif Organs. 2009;33:26–35. doi: 10.1111/j.1525-1594.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 72.Cullen DK, Tang-Schomer MD, Struzyna LA, Patel AR, Johnson VE, Wolf JA, et al. Microtissue engineered constructs with living axons for targeted nervous system reconstruction. Tissue Eng Part A. 2012;18:2280–9. doi: 10.1089/ten.tea.2011.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfister BJ, Iwata A, Meaney DF, Smith DH. Extreme stretch growth of integrated axons. J Neurosci: Off J Soc Neurosci. 2004;24:7978–83. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfister BJ, Iwata A, Taylor AG, Wolf JA, Meaney DF, Smith DH. Development of transplantable nervous tissue constructs comprised of stretch-grown axons. J eurosci Methods. 2006;153:95–103. doi: 10.1016/j.jneumeth.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 75.Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87:2183–200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 76.Faroni A, Terenghi G, Reid AJ. Adipose-derived stem cells and nerve regeneration: promises and pitfalls. Int Rev Neurobiol. 2013;108:121–36. doi: 10.1016/B978-0-12-410499-0.00005-8. [DOI] [PubMed] [Google Scholar]
- 77.Tornero D, Wattananit S, Gronning Madsen M, Koch P, Wood J, Tatarishvili J, et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain: J Neurol. 2013;136:3561–77. doi: 10.1093/brain/awt278. [DOI] [PubMed] [Google Scholar]
- 78.Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–64. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335–40. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, et al. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease. J Neurosci: Off J Soc Neurosci. 2006;26:12497–511. doi: 10.1523/JNEUROSCI.3719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee ST, Park JE, Lee K, Kang L, Chu K, Kim SU, et al. Noninvasive method of immortalized neural stem-like cell transplantation in an experimental model of Huntington's disease. J Neurosci Method. 2006;152:250–4. doi: 10.1016/j.jneumeth.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 82.Xu L, Yan J, Chen D, Welsh AM, Hazel T, Johe K, et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82:865–75. doi: 10.1097/01.tp.0000235532.00920.7a. [DOI] [PubMed] [Google Scholar]
- 83.Bai L, Hecker J, Kerstetter A, Miller RH. Myelin repair and functional recovery mediated by neural cell transplantation in a mouse model of multiple sclerosis. Neurosci Bull. 2013;29:239–50. doi: 10.1007/s12264-013-1312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Honsa P, Pivonkova H, Dzamba D, Filipova M, Anderova M. Polydendrocytes display large lineage plasticity following focal cerebral ischemia. PLoS ONE. 2012;7:e36816. doi: 10.1371/journal.pone.0036816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zuo H, Nishiyama A. Polydendrocytes in development and myelin repair. Neurosci Bull. 2013;29:165–76. doi: 10.1007/s12264-013-1320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu GB, Cheng YX, Feng YK, Pang CJ, Li Q, Wang Y, et al. Adipose-derived stem cells promote peripheral nerve repair. Arch Med Sci: AMS. 2011;7:592–6. doi: 10.5114/aoms.2011.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease. J Neurosci: Off J Soc Neurosci. 2001;21:8108–18. doi: 10.1523/JNEUROSCI.21-20-08108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gravvanis AI, Lavdas A, Papalois AE, Franceschini I, Tsoutsos DA, Dubois-Dalcq M, et al. Effect of genetically modified Schwann cells with increased motility in end-to-side nerve grafting. Microsurgery. 2005;25:423–32. doi: 10.1002/micr.20141. [DOI] [PubMed] [Google Scholar]
- 89.Santosa KB, Jesuraj NJ, Viader A, MacEwan M, Newton P, Hunter DA, et al. Nerve allografts supplemented with Schwann cells overexpressing glial-cell-line-derived neurotrophic factor. Muscle Nerve. 2013;47:213–23. doi: 10.1002/mus.23490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weick JP, Johnson MA, Skroch SP, Williams JC, Deisseroth K, Zhang SC. Functional control of transplantable human ESC-derived neurons via optogenetic targeting. Stem Cells. 2010;28:2008–16. doi: 10.1002/stem.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bryson JB, Machado CB, Crossley M, Stevenson D, Bros-Facer V, Burrone J, et al. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science. 2014;344:94–7. doi: 10.1126/science.1248523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pina-Crespo JC, Talantova M, Cho EG, Soussou W, Dolatabadi N, Ryan SD, et al. High-frequency hippocampal oscillations activated by optogenetic stimulation of transplanted human ESC-derived neurons. J Neurosci: Off J Soc Neurosci. 2012;32:15837–42. doi: 10.1523/JNEUROSCI.3735-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barron V, Lyons E, Stenson-Cox C, McHugh PE, Pandit A. Bioreactors for cardiovascular cell and tissue growth: a review. Ann Biomed Eng. 2003;31:1017–30. doi: 10.1114/1.1603260. [DOI] [PubMed] [Google Scholar]
- 94.Royo-Gascon N, Wininger M, Scheinbeim JI, Firestein BL, Craelius W. iezoelectric substrates promote neurite growth in rat spinal cord neurons. Ann Biomed Eng. 2013;41:112–22. doi: 10.1007/s10439-012-0628-y. [DOI] [PubMed] [Google Scholar]
- 95.Rajnicek AM. The physiology of bioelectricity in development, tissue regeneration and cancer. CRC Press; 2011. [Google Scholar]
- 96.Yamada M, Tanemura K, Okada S, Iwanami A, Nakamura M, Mizuno H, et al. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem Cells. 2007;25:562–70. doi: 10.1634/stemcells.2006-0011. [DOI] [PubMed] [Google Scholar]
- 97.Lee JS, Hong JM, Jung JW, Shim JH, Oh JH, Cho DW. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6:024103. doi: 10.1088/1758-5082/6/2/024103. [DOI] [PubMed] [Google Scholar]
- 98.Lorber B, Hsiao WK, Hutchings IM, Martin KR. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication. 2013;6:015001. doi: 10.1088/1758-5082/6/1/015001. [DOI] [PubMed] [Google Scholar]
- 99.Arumuganathar S, Jayasinghe SN. Living scaffolds (specialized and unspecialized) for regenerative and therapeutic medicine. Biomacromolecules. 2008;9:759–66. doi: 10.1021/bm701322k. [DOI] [PubMed] [Google Scholar]
- 100.Cullen DK, Smith DH. Bionic connections: living bridges to connect bionic limbs to the nervous system. Sci Am. 2013;308:52–7. doi: 10.1038/scientificamerican0113-52. [DOI] [PubMed] [Google Scholar]
- 101.Cullen DK, Wolf JA, Smith DH, Pfister BJ. Neural tissue engineering for neuroregeneration and biohybridized interface microsystems in vivo (Part 2) Rit Rev Biomed Eng. 2011;39:241–59. doi: 10.1615/critrevbiomedeng.v39.i3.40. [DOI] [PubMed] [Google Scholar]


