Abstract
Background
Pre-eclampsia (PE) is a risk factor for the development of peripartum cardiomyopathy (PPCM), but it is unknown whether PE impacts clinical or LV functional outcomes. This study sought to assess clinical and functional outcomes in women with PPCM complicated by PE.
Methods and Results
This retrospective cohort study included women diagnosed with PPCM delivering at Barnes-Jewish Hospital between 2004–2014. The primary outcome was one-year event-free survival rate for the combined endpoint of death and hospital readmission. The secondary outcome was recovery of LV ejection fraction (LVEF). Seventeen of 39 women (44%) with PPCM had PE. The groups had similar mean LVEF at diagnosis (29.6 with vs. 27.3 without PE, p=0.5). Women with PE had smaller mean left ventricular (LV) end diastolic diameters (5.2 vs. 6.0 cm, p=0.001), greater relative wall thickness (0.41 vs. 0.35 mmHg, p=0.009), and lower incidence of eccentric remodeling (12% vs. 48%, p=0.03). Clinical follow up was available for 32 women; five died of cardiovascular complications within one year of diagnosis (4/15 with vs. 1/17 without PE, p= 0.16). In time to event analysis, patients with PE had worse event-free survival during one–year follow up (p=0.047). Echocardiographic follow-up was available in 10 survivors with and 16 without PE. LVEF recovered in 80% survivors with vs. 25% without PE (p=0.014).
Conclusions
PPCM with concomitant PE is associated with increased morbidity and mortality and different patterns of LV remodeling and recovery of LV function when compared to patients with PPCM that is not complicated by PE.
Subject Terms: Pregnancy, Cardiomyopathy, Preeclampsia
Peripartum cardiomyopathy (PPCM) is a distinct type of heart failure that occurs within the last month of pregnancy or within 5 months following delivery.1, 2 It is defined as left ventricular ejection fraction ≤45% ± left ventricular (LV) cavity dilation occurring during the peripartum period in the absence of pre-existing heart disease or other identifiable causes of heart failure.1, 2 As many as 72% of women may have recovery of their LV ejection fraction.2, 3 Pre-eclampsia (PE) has been epidemiologically associated with PPCM, with a prevalence of PE in patients with PPCM more than four times the rate expected in the general population.4 This observation is consistent with recent evidence suggesting that the underlying mechanism of cardiac injury in PPCM may be vascular in nature, and that PPCM and PE may share a common underlying pathophysiologic mechanism.4, 5 Although the mechanisms of PE are not known, it has been suggested that PE is as vascular disease, likely related to the secretion of anti-angiogenic factors including soluble fms-like tyrosine kinase-1 (sFLT1) from the placenta in pregnancy.4, 6–10 While these anti-angiogenic factors are secreted by the placenta in all pregnancies, they are greatly up-regulated in women with PE.4 Epidemiologic studies have shown that PE is associated with PPCM in approximately 20% of cases.4 Given evidence that PE leads to LV diastolic dysfunction, and given that there is a strong epidemiological link between PE and PPCM, it has been suggested that PE and PPCM share a common pathophysiological mechanism(s) that leads to the clinical manifestation of heart failure.4, 11–17 However, the prior clinical and epidemiological studies that have associated PE with the development of PPCM have never separately compared and contrasted the longitudinal clinical and functional outcomes of PPCM associated with PE and PPCM that is not associated with PE. Accordingly, the objective of this study was to compare clinical and functional outcomes of PPCM patients with PE to those who did not have PE. To our surprise, we found the clinical and functional outcomes of PPCM with concomitant PE are distinctly different from those observed with PPCM that is not complicated by PE.
Methods
This is a retrospective cohort study performed at Barnes-Jewish Hospital (BJH) between 2004–2014. Patients with PPCM were identified via detailed chart review of the electronic medical record. Patients were included in the analysis if they delivered at BJH and were diagnosed with PPCM between one month prior to delivery and five months post-partum. Inclusion criteria included initial left ventricular ejection fraction (LVEF) less than or equal to 45% without any other identifiable causes of heart failure. Patients were excluded if their initial echocardiogram was performed elsewhere, or was otherwise unavailable for review. Echocardiograms were interpreted by a cardiologist board-certified in echocardiography. The reader was blinded to PE diagnosis. PE was diagnosed according to American College of Obstetricians and Gynecologists criteria (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on two occasions at least four hours apart after 20 weeks gestation and proteinuria [≥ 300 mg/24-hour urine collection or protein/creatinine ratio ≥ 0.3 or dipstick reading of 1+].18 Patients with preeclampsia without severe features, with severe features, hemolysis elevated liver enzymes low platelet syndrome (HELLP), and eclampsia were all included as having PE for the purposes of our study.
Follow-up echocardiographic analysis was performed for all women with an echo performed between 6–24 months after diagnosis. If women had an echo between 1–6 months after diagnosis that documented recovery of LV function, they were also included in the analysis. The primary outcome variable was one-year event-free survival rate for the combined endpoint of death and hospital readmission. The secondary outcome was recovery of LV function, which was defined as LVEF ≥ 50% with an absolute improvement of ≥10%.2, 19, 20 This study was approved by the institutional review board, the Washington University Human Research Protection Office (institutional review board # 201107046). Informed consent was waived per IRB approval.
Statistical Analysis
All data are presented as mean ± SD. The composite outcome of interest was death/readmission within 1 year. Event counts were compared using Fisher’s exact test. Student’s two sample t-test for independent groups was used for analysis of continuous variables. Kaplan-Meier curves were created by preeclampsia status and compared using the log-rank test. Start time was date of diagnosis and patients were followed until 1st readmission, death, or were censored at last available follow-up or at 1 year. Analysis was conducted in SAS 9.4 (SAS Institute Inc., Cary, NC) and SPSS 23.0 (IBM Corp., Armonk, NY). Dr. Lindley had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Results
Patient Demographics
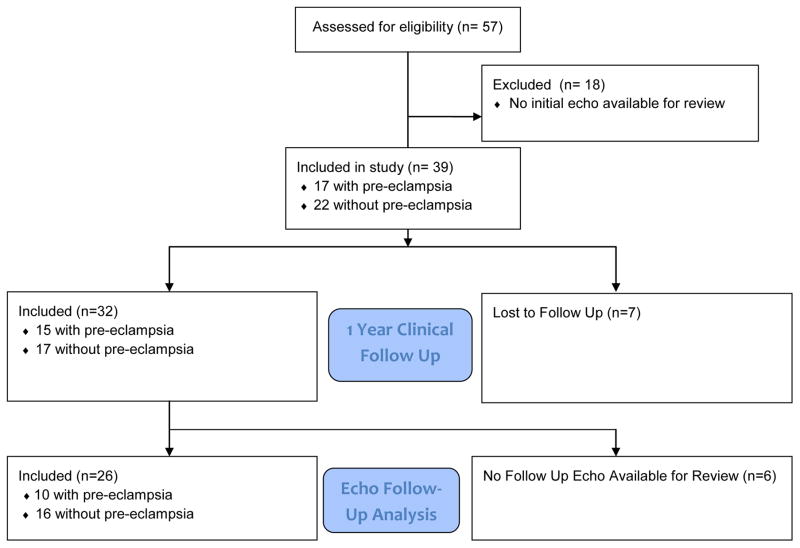
Fifty-seven women were identified with a diagnosis of PPCM, of whom thirty -nine had an initial echo available for review and were included in the study (Figure 1). Table 1 shows the baseline demographics for the patients in this study. Seventeen women (44%) with PPCM had concomitant pre-eclampsia (2 mild PE, 14 severe PE, 1 HELLP). As shown, the cohort was predominantly African American. There was no significant difference between groups with respect to underling chronic hypertension or diabetes. The patients with concomitant pre-eclampsia delivered at an earlier gestational age. Systolic and diastolic blood pressures were both significantly higher in the patients with pre-eclampsia (p < 0.001 and 0.004, respectively). As shown in Table 1, there were no significant differences in medical therapy after diagnosis, with the majority of both groups receiving angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers, beta-blockers, and furosemide.
Figure 1.
Flow diagram for development of study cohort.
TABLE 1.
Patient Demographics
| Variable | No Pre-Eclampsia (n=22) | Pre-Eclampsia (n=17) | p-value |
|---|---|---|---|
| Age at Delivery (years) | 29.3 (5.9) | 27.4 (7.4) | 0.18 |
| Gravidity (# pregnancies) | 3.1 (1.9) | 2.6 (2.2) | 0.58 |
| Race = African American (n,%) | 17 (77) | 13 (77) | 1 |
| Race = White (n,%) | 5 (23) | 4 (24) | 1 |
| Pre-pregnancy Weight (pounds) | 200 (59.9) | 177 (59.6) | 0.47 |
| Tobacco Use (n,%) | 4 (18) | 4 (24) | 0.71 |
| Chronic Hypertension (n,%) | 5 (23) | 5 (29) | 0.72 |
| Diabetes (n,%) | 2 (9) | 3 (18) | 0.64 |
| Gestational DM (n,%) | 4 (18) | 1 (6) | 0.36 |
| Cesarean Delivery (n,%) | 11 (50) | 11 (65) | 0.59 |
| Birth Weight (grams) | 3273 (775) | 1875 (984) | 0.26 |
| Gestational Age at Delivery (weeks) | 38.7 (2.6) | 32.1 (4.7) | <0.001 |
| Systolic Blood Pressure (mmHg) | 130 (14.7) | 151 (27.1) | 0.004 |
| Diastolic Blood Pressure (mmHg) | 82 (8.7) | 97 (20.1) | 0.003 |
| Creatinine at delivery (mg/dl) (10 no pre-e, 15 pre-e) | 0.57 (0.10) | 0.70 (0.20) | 0.08 |
| BNP ( 15 no pre-e, 9 pre-e) | 525 (345) | 888 (510) | 0.049 |
| Diagnosis Data | |||
| Diagnosed Prior to Delivery (n,%) | 5 (23) | 4 (24) | 1.0 |
| Post-partum Day | 40.7 (43.7) | 15.6 (46.2) | 0.09 |
| Medical Therapy Initiated After Diagnosis (n,%) | |||
| Furosemide | 12(55) | 12(71) | 0.3 |
| Other Diuretic | 1(5) | 2(12) | 0.6 |
| Spironolactone | 4(18) | 5(29) | 0.5 |
| Beta Blocker | 17(77) | 15(88) | 0.4 |
| Calcium Channel Blocker | 3(14) | 2(12) | 1 |
| Digoxin | 5(23) | 3(18) | 1 |
| ACEI/ARB | 18(82) | 16(94) | 0.4 |
| Anticoagulation | 11(50) | 5(29) | 0.3 |
| Aspirin | 6(27) | 3(18) | 0.7 |
| Warfarin | 7(32) | 2(12) | 0.3 |
Values reported as mean(SD) except where indicated
LV Structure and Function at Baseline
Table 2 shows the 2-D echocardiographic variables at the time of entry in the study. Measurements of LV function, including LV ejection fraction (LVEF), LV mean global longitudinal strain and LV outflow tract velocity time integral were all depressed at baseline, but were not significantly different between the two groups. Importantly, women with pre-eclampsia had significantly smaller LV end-diastolic diameter (5.2 ±0.51 vs. 6.0 ±0.70 cm, p=0.001) and increased LV relative wall thickness (0.41 ±0.09 vs. 0.35 ±0.06, p=0.009) when compared to patients without pre-eclampsia. Patients with PE were less likely to have an eccentric remodeling phenotype than patients without PE (12% vs. 48%,p=0.03). Both groups of patients had increased estimated LV filling pressures (p=0.06), and patients with pre-eclampsia had significantly higher mean estimated pulmonary artery pressures (p=0.04).
TABLE 2.
Initial Echo Findings
| Echocardiographic Parameter | No Pre-Eclampsia (n=22) | Pre-Eclampsia (n=17) | p-value |
|---|---|---|---|
| LV Ejection Fraction | 27.3 (10.5) | 29.6 (8.7) | 0.5 |
| LV Mean Global Longitudinal Strain | −9.4 (3.4) | −7.7 (4.8) | 0.5 |
| LVOT VTI | 13.8 (4.7) | 15.5 (3.8) | 0.3 |
| LVEDD (cm) | 6.0 (0.70) | 5.2 (0.51) | 0.001 |
| Septal Wall Thickness (cm) | 0.97 (0.12) | 1.05 (0.15) | 0.08 |
| Posterior Wall Thickness (cm) | 1.02 (0.12) | 1.07 (0.16) | 0.27 |
| Relative Wall Thickness | 0.35 (0.06) | 0.41 (0.09) | 0.009 |
| LV Mass Index | 112.2 (28.6) | 101.3 (18.1) | 0.18 |
| Relative Wall Thickness ≥0.42 (n,%) | 4 (18) | 9 (53) | 0.04 |
| Eccentric Remodeling Phenotype (n,%) | 10 (48) | 2 (12) | 0.03 |
| Average E/e′ | 16.9 (5.5) | 21.9 (9.7) | 0.06 |
| E/A ratio | 3.2 (2.6) | 2.8 (1.4) | 0.6 |
| Estimated PASP (mmHg) | 36.8 (8.7) | 45.1 (9.0) | 0.04 |
| LA Volume Index | 36.2 (14.5) | 34.8 (10.4) | 0.8 |
| Normal RV Function (n,%) | 15 (71) | 12 (71) | 0.3 |
Values reported as mean(SD) except where indicated
Effect of Pre-eclampsia on Clinical and Functional Outcomes
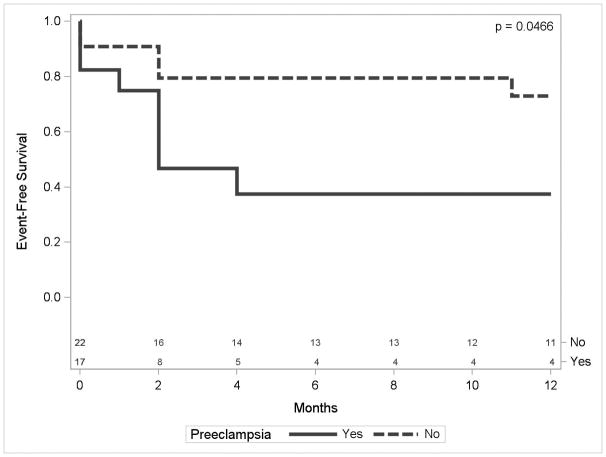
We were able to obtain vital status on 32 of 39 women in this study, including 15 women with pre-eclampsia and 17 women without preeclampsia. Five women died of cardiovascular complications within one year of diagnosis, of whom four had pre-eclampsia and one did not have pre-eclampsia (p= 0.16). Figure 2 shows the one-year event-free survival for the combined endpoint of death or hospital readmission in women with and without pre-eclampsia, and includes patients immediately lost to follow-up (n=7; 2 PE and 5 no PE) among those at risk. As shown, there was a significantly lower (p = 0.047) event-free survival in the patients with pre-eclampsia than patients without pre-eclampsia during 1-year follow-up.
Figure 2.
Kaplan-Meier survival curve for combined endpoint of death or heart failure hospitalization over the course of one year following diagnosis of peripartum cardiomyopathy.
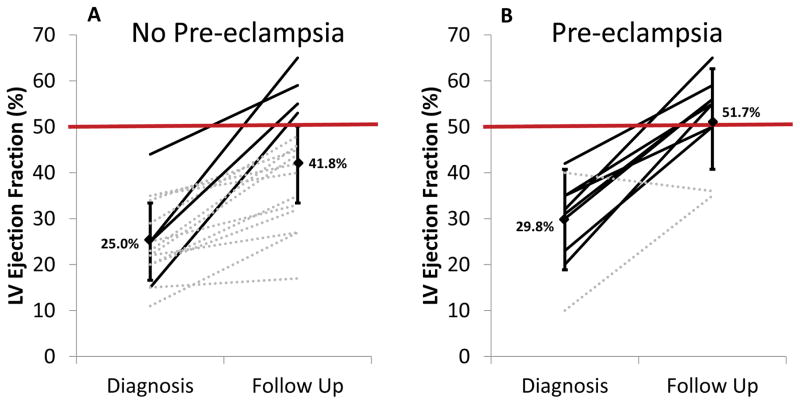
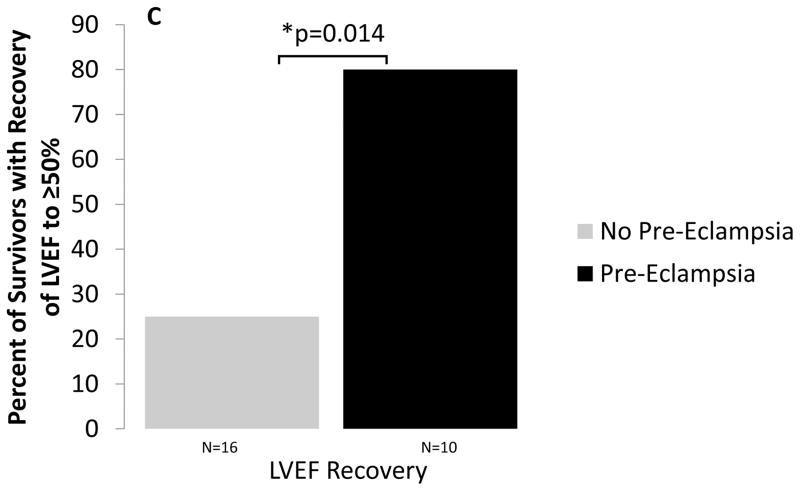
Echocardiographic follow-up was available in a total of 26 of the 39 patients (67%), of whom 10 had pre-eclampsia and 16 did not have pre-eclampsia. As shown in Figures 3a and b, all but one patient with PE had some degree of improvement in LVEF. Overall, patients with PE had higher mean LV ejection fraction on follow-up echo (p=0.046). Patients with PE were also significantly more likely to meet criteria for recovery of LV function (defined as LVEF ≥50% with absolute increase of ≥10%) at follow up (Figure 3c, p=0.014). Persistent diastolic dysfunction was common in both groups of patients (60% vs. 81%; p=0.5, Table 3). There was no significant difference between the two groups in mean systolic or diastolic blood pressures or in being diagnosed with chronic hypertension at one-year follow up (p=0.8, 0.5, 0.7; Table 3).
Figure 3.
Initial and one-year follow up left ventricular ejection fraction for women without (A) and with (B) pre-eclampsia. (C) Percentage of survivors in each group meeting criteria for recovery of LV ejection fraction. (key: solid black solid line = recovered EF (EF ≥50% with absolute increase of ≥10%); dotted gray dotted line = non-recovered LVEF)
TABLE 3.
Clinical and Echocardiographic Follow Up
| One Year Clinical Follow Up (n=32) | No Pre-eclampsia (17) | Pre-eclampsia (15) | p value |
|---|---|---|---|
| Composite Death/Readmission 1 year (n, %) | 5 (29) | 8 (53) | 0.28 |
| Death 1 year (n,%) | 1 (6) | 4 (27) | 0.16 |
| Readmission 1 year (n,%) | 5 (29) | 6 (40) | 0.71 |
| Chronic Hypertension Diagnosis (n,%) | 5/16 (31) | 2/10 (20) | 0.7 |
| Mean Systolic Blood Pressure | 123 (19.1) | 126 (29.7) | 0.8 |
| Mean Diastolic Blood Pressure | 79 (13.9) | 83 (20.3) | 0.5 |
| Diagnosed Prior to Delivery (n=7) | 4 (57) | 3 (43) | 1.0 |
| LV Recovery | 0 (0) | 0 (0) | n/a |
| Diagnosed Post-Partum (n=25) | 13 (52) | 12 (48) | 1.0 |
| LV Recovery | 4 (30.8) | 9 (75.0) | 0.047 |
| Survivors with Echo Follow Up (n=26) | No-pre-eclampsia (16) | Pre-eclampsia (10) | p value |
| LV Recovery (n,%) (LVEF ≥50% with absolute increase ≥10%) | 4 (25) | 8 (80) | 0.014 |
| LV Ejection Fraction | 41.8 (12.9) | 51.7 (9.5) | 0.046 |
| % Improvement from baseline LVEF | 16.8 (11.5) | 21.9 (11.1) | 0.27 |
| Average Global Strain | −16.3% (4.1) | −13.8% (4.6) | 0.4 |
| LV End Diastolic Diameter (cm) | 5.2 (0.63) | 5.1 (0.72) | 0.13 |
| LVOT VTI | 18.0 (4.6) | 19.3 (4.3) | 0.5 |
| Diastolic Dysfunction (n,%) | 13 (81) | 6 (60) | 0.5 |
| Average E/e′ | 12.7 (7.4) | 10.47 (5.2) | 0.3 |
| E/A Ratio | 1.3 (0.7) | 1.7 (0.8) | 0.1 |
| Estimated PASP (mmHg) | 25.3 (5.8) | 32.4 (12.3) | 0.6 |
| LA Volume Index | 26.8 (9.8) | 29.7 (14.9) | 0.5 |
| Normal RV Function (n,%) | 15 (94) | 8 (80) | 0.5 |
Values reported as mean(SD) except where indicated
Although our analysis included only outcomes to 1 year follow-up, it is important to note that there were three late heart failure deaths – one in the group without PE at 64 months, and the two PE patients who failed to recover their EF on follow up (69 and 70 months). The patient without PE had partial recovery of her LVEF to 47%, but then was noncompliant with medical therapy and had subsequent decline in her LV function and ultimately died of heart failure while on palliative home inotropes. One patient with PE developed mixed functional/degenerative severe mitral regurgitation and died of post-operative complications following valve repair. The other patient with PE had partial recovery of LVEF and then marked decompensation following a subsequent pregnancy complicated by PE. She ultimately died of infectious complications of her left ventricular assist device.
Effect of Timing of Diagnosis on Clinical Outcomes
Five (23%) of the patients without pre-eclampsia and 4 (24%) of patients with pre-eclampsia were diagnosed prior to delivery (p=1.0). Patients without pre-eclampsia were diagnosed a mean 40.7 (±43.7) days post-partum, compared to patients with pre-eclampsia who were diagnosed a mean 15.6 (±46.2) days post-partum, p=0.09. Of the 32 patients with one year clinical follow up, none of the seven patients who were diagnosed prior to delivery (3 with PE, 4 without PE) had recovery of LVEF, whereas 13 of 25 (52%) of patients diagnosed post-partum had recovery of LVEF, p=0.03. PE patients diagnosed post-partum were more likely to have recovery of LVEF than patients without PE diagnosed post-partum (75% vs 31%, p=0.047).
Discussion
The results of this study show for the first time that the clinical and functional outcomes of patients with PPCM with concomitant PE are distinctly different from those observed with PPCM that is not complicated by PE. Two distinct lines of evidence support this statement. First, despite a similar degree of LV dysfunction at the time of initial diagnosis, PPCM diagnosed in the setting of PE was associated with significantly worse one-year morbidity and mortality in this predominantly African American cohort (Figure 2). Importantly, the differences in clinical event rates between these two groups emerged as early as 4–5 months after the time of diagnosis. While the mortality rate in this study is relatively high, it has previously been identified that African American patients with PPCM have worse outcomes than patients of other races3. The second major finding of this study is that the pattern of LV remodeling in PPCM with PE is distinctly different from the pattern of remodeling in PPCM that is not associated with PE. Patients with PPCM without PE underwent greater LV dilation and had a decrease in relative LV wall thickness consistent with the classic “eccentric” LV remodeling. In contrast the decrease in LV ejection fraction in patients with PPCM with PE was not associated with LV dilation nor a decrease in relative wall thickness, which is more consistent with a concentric pattern of LV remodeling. Viewed together the results of this study raise the intriguing question of whether the PE-induced LV dysfunction and PPCM represent two different disease processes that are share a common clinical presentation, namely heart failure.
Pre-eclampsia and Peripartum Cardiomyopathy
PE is a common hypertensive disorder of pregnancy that is associated with short-term as well as long-term postpartum morbidity and mortality secondary to cardiovascular dysfunction.11, 12, 21–24 Although LV ejection fraction is generally unchanged or minimally decreased in patients with PE, subtle echocardiographic changes LV function have been observed repeatedly in preeclampsia.14 Indeed, previous studies have shown that women with PE have a greater degree of diastolic dysfunction and greater reductions in LV global strain when compared to age-matched pregnant women with PE,11, 14 despite preservation of global LV ejection fraction. Moreover, PE-induced LV dysfunction persists for at least 1–2 years after delivery, even after normalization of blood pressure.23
Epidemiologic studies have shown that PE is present in approximately 20% of PPCM cases.4 Given that PE leads to LV dysfunction, and given that there is a strong epidemiological link between PE and PPCM, it has been suggested that PE and PPCM share a common pathophysiological mechanism leading to cardiomyopathy.25 However, it is important to recognize that none of the prior clinical and epidemiological studies that conflated PE and PPCM examined longitudinal clinical and functional outcomes in patients with PPCM and PE. As noted above, the results of this study show that the clinical outcomes and patterns of LV remodeling are distinctly different in PPCM patients with PE versus patients with PPCM without PE. Moreover, the percentage of PPCM patients with recovery of LV function is ~ 3-fold greater in patients with PE than without PE, which has also not been reported for PPCM. Viewed together, these results suggest that PE-induced LV dysfunction that meets the diagnostic criteria for PPCM may be a different pathophysiological disease process than PPCM that is not associated with PE. An alternative interpretation of our data is that PPCM with PE represents a more severe PPCM phenotype with worse outcome. However, it is difficult to reconcile this interpretation with the differing patterns of LV remodeling and the greater degrees of recovery of LV function in PPCM associated with PE.
There is one additional unique aspect of this study that warrants further discussion. As noted, our data suggest that women with PPCM with PE were more likely to recover LV ejection fraction to a normal range than women with PPCM without PE. While the reason(s) for this finding are not known, there are several potential explanations. First, this finding may be the result of survival bias, since we only obtained 2-D echoes on patients who were alive at the time of follow-up. It is likely that the PPCM patients with PE who died did not have recovery of LV function. A second explanation is that LV wall stress (i.e. relative wall thickness) was not increased in patients with PPCM with PE. Given that LV ejection fraction is “load-sensitive,” the increased LV ejection fraction in the patients with PE-induced LV dysfunction may have been because they were able to normalize their wall stress, whereas the PPCM patients with increased wall stress were not. A third explanation could be related to timing of disease onset. While patients with PE were as likely to be diagnosed prior to delivery as those without PE, there was a trend toward diagnosis at an earlier post-partum date for those who were diagnosed after delivery. The lack of recovery of LVEF in any patient diagnosed prior to delivery is consistent with prior studies, suggesting that earlier presentation may represent a more aggressive form of the disease.26 However, of patients diagnosed post-partum in our cohort, those with PE remained more likely to have LVEF recovery despite the trend towards an earlier diagnosis date.
A final, albeit speculative, potential explanation for the differences in LV functional recovery is that the systemic angiogenic imbalance that occurs in PPCM is accentuated by pre-eclampsia.5 In humans, the placenta secretes VEGF inhibitors such as soluble FLT1 (sFLT1).5 While sFLT1 levels are elevated above controls in women with PPCM, they are elevated to a much greater degree in patients with PE.5, 27 sFLT1 levels decline rapidly after delivery.28 A recent analysis of the IPAC (Investigators of Pregnancy-Associated Cardiomyopathy) study identified that higher sFLT1 levels correlated with more severe symptoms and major adverse events in women with PPCM.28 Accordingly, higher levels of sFLT1 would be expected in the setting of PE, and could account for the increased early mortality and heart failure hospitalizations in the PE group in our study, as well as the greater recovery of LV function following the rapid decline in sFLT1 levels following pregnancy. Thus, resolution of the anti-angiogenic insult in PE-induced LV dysfunction may result in higher likelihood of recovering LV function than in patients with PPCM in the absence of PE. Additional studies will be necessary to address this interesting possibility.
Our study has several limitations. First, our findings are limited by the small sample size and the retrospective nature of the study design which was conducted in a single tertiary care center. As such, we did not have adequate power to detect moderate differences between groups. Further, we were not able to obtain vital status for all patients who comprised the initial patient cohort. Accordingly, these results of this study must be regarded as provisional until they can be confirmed by additional studies.
Conclusions
This study is the first to investigate the impact of PE on outcomes in women with PPCM. We observed that there was a high incidence of pre-eclampsia in this predominantly African American population of women diagnosed with PPCM. Despite comparable LV ejection fractions in the two groups, PPCM with PE is associated with excess early morbidity and mortality. Moreover, the patterns of LV remodeling and recovery of LV function were distinctly different in PPCM patients with PE than in PPCM patients without PE. Apart from the novelty of these findings, this study has a number of important clinical implications. First, while future pregnancies have been considered absolutely contraindicated only in women with PPCM with residual LV dysfunction, it is unknown if the risk of future pregnancy is equivalent for women with prior PPCM with vs. without associated PE, and whether residual diastolic dysfunction affects this risk.20, 29, 30,1 Our results suggest that despite complete normalization of LV ejection fraction in PPCM associated with PE, these patients remain at high risk of recurrent hospitalization and/or death. If our results are replicated in a larger patient cohort, they may lead to a rethinking of recommendations for future pregnancies in PPCM with PE. Second, although the incidence of recovery of LV ejection ≥ 50% was as high as 72% in the recent IPAC study, 45% of the patients in this study had hypertensive disorders of pregnancy. As noted above, the high incidence of recovery of LV ejection fraction in PPCM associated with PE is potentially misleading because the resolution of LV ejection fraction appears to be dissociated from increased cardiovascular events. Although the optimal duration of medical therapy for PPCM patients with recovered LV ejection fraction is unclear, a minimum of one year has been considered reasonable.1 If the results of our study are replicated in a larger patient cohort, they may also lead to changes in recommendations for the optimal duration of medical therapy in PPCM associated with PE. In summary, while the results of this study must be regarded as provisional because of the inherent limitations, this study serves the heuristic purpose of emphasizing the need to re-evaluate the complex relationship between PPCM and pre-eclampsia, in an effort to better understand how these two disease processes are interrelated, as well as how they may be different.
Clinical Perspective.
Despite the co-occurrence of pre-eclampsia (PE) in ~20% of peripartum cardiomyopathy (PPCM) cases, there is surprisingly little known about how PE impacts outcomes in women with PPCM. Recent evidence suggests that PPCM may be a vascular disease, and that PPCM and PE may share a common underlying vascular pathophysiologic mechanism. Accordingly, we sought to determine whether these two disease processes are related, or whether they are different diseases with a common phenotype (i.e. heart failure). We observed that despite comparable decreases in LV ejection fraction in the two groups, PPCM with PE is associated with excess early morbidity and mortality. Moreover, the patterns of LV remodeling and recovery of LV function were distinctly different in PPCM patients with PE. PPCM patients with PE had less eccentric remodeling, and appear to be more likely to recover LV ejection fraction at follow up. Both groups had a high incidence of persistent diastolic dysfunction at follow up. Given the relatively small sample size of this study, these results will need to be replicated in a larger patient cohort before counseling patients regarding prognosis and recommendations for future pregnancies in women with PPCM associated with PE. Nonetheless, our results emphasize the need to further evaluate the complex relationship between PPCM and pre-eclampsia.
Footnotes
Disclosures: None.
References
- 1.Blauwet LA, Cooper LT. Diagnosis and management of peripartum cardiomyopathy. Heart. 2011;97:1970–1981. doi: 10.1136/heartjnl-2011-300349. [DOI] [PubMed] [Google Scholar]
- 2.McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, Modi K, Alexis JD, Ramani GV, Semigran MJ, Haythe J, Markham DW, Marek J, Gorcsan J, III, Wu W-C, Lin Y, Halder I, Pisarcik J, Cooper LT, Fett JD. Clinical Outcomes for Peripartum Cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy) J Am Coll Cardiol. 2015;66:905–914. doi: 10.1016/j.jacc.2015.06.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58:659–670. doi: 10.1016/j.jacc.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 4.Bello N, Rendon IS, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:1715–1723. doi: 10.1016/j.jacc.2013.08.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, del Monte F, Hilfiker-Kleiner D, Karumanchi SA, Arany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–338. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 7.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 8.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, Mazor M. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 9.Schrey-Petersen S, Stepan H. Anti-angiogenesis and Preeclampsia in 2016. Curr Hypertens Rep. 2017;19:6. doi: 10.1007/s11906-017-0706-5. [DOI] [PubMed] [Google Scholar]
- 10.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18:2281–2284. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- 11.Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57:85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321. [DOI] [PubMed] [Google Scholar]
- 12.Melchiorre K, Thilaganathan B. Maternal cardiac function in preeclampsia. Curr Opin Obstet Gynecol. 2011;23:440–447. doi: 10.1097/GCO.0b013e32834cb7a4. [DOI] [PubMed] [Google Scholar]
- 13.Melchiorre K, Sutherland GR, Watt-Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Preg. 2012;31:454–471. doi: 10.3109/10641955.2012.697951. [DOI] [PubMed] [Google Scholar]
- 14.Shahul S, Rhee J, Hacker MR, Gulati G, Mitchell JD, Hess P, Mahmood F, Arany Z, Rana S, Talmor D. Subclinical left ventricular dysfunction in preeclamptic women with preserved left ventricular ejection fraction: a 2D speckle-tracking imaging study. Circ Cardiovasc Imaging. 2012;5:734–739. doi: 10.1161/CIRCIMAGING.112.973818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyldum EV, Backe B, Stoylen A, Slordahl SA. Maternal left ventricular and endothelial functions in preeclampsia. Acta Obstet Gynecol Scand. 2012;91:566–573. doi: 10.1111/j.1600-0412.2011.01282.x. [DOI] [PubMed] [Google Scholar]
- 16.Bamfo JE, Kametas NA, Chambers JB, Nicolaides KH. Maternal cardiac function in normotensive and pre-eclamptic intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32:682–686. doi: 10.1002/uog.5311. [DOI] [PubMed] [Google Scholar]
- 17.Sep SJ, Schreurs MP, Bekkers SC, Kruse AJ, Smits LJ, Peeters LL. Early-pregnancy changes in cardiac diastolic function in women with recurrent pre-eclampsia and in previously pre-eclamptic women without recurrent disease. BJOG. 2011;118:1112–1119. doi: 10.1111/j.1471-0528.2011.02951.x. [DOI] [PubMed] [Google Scholar]
- 18.American College of Obstetricians and Gynecologists. Hypertension in Pregnancy. 2013 http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy.
- 19.Givertz MM, Mann DL. Epidemiology and natural history of recovery of left ventricular function in recent onset dilated cardiomyopathies. Curr Heart Fail Rep. 2013;10:321–330. doi: 10.1007/s11897-013-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, Wani OR, Hameed A, Gviazda I, Shotan A. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344:1567–1571. doi: 10.1056/NEJM200105243442101. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-Eclampsia and Future Cardiovascular Risk Among Women: A Review. J Am Coll Cardiol. 2014;63:1815–1822. doi: 10.1016/j.jacc.2014.02.529. [DOI] [PubMed] [Google Scholar]
- 22.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58:709–715. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 24.Ray JG, Schull MJ, Kingdom JC, Vermeulen MJ. Heart failure and dysrhythmias after maternal placental syndromes: HAD MPS Study. Heart. 2012;98:1136–1141. doi: 10.1136/heartjnl-2011-301548. [DOI] [PubMed] [Google Scholar]
- 25.Bello NA, Arany Z. Molecular mechanisms of peripartum cardiomyopathy: A vascular/hormonal hypothesis. Trends Cardiovasc Med. 2015;25:499–504. doi: 10.1016/j.tcm.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, Staniloae C. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol. 2012;154:27–31. doi: 10.1016/j.ijcard.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 27.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damp J, Givertz MM, Semigran M, Alharethi R, Ewald G, Felker GM, Bozkurt B, Boehmer J, Haythe J, Skopicki H, Hanley-Yanez K, Pisarcik J, Halder I, Gorcsan J, 3rd, Rana S, Arany Z, Fett JD, McNamara DM. Relaxin-2 and Soluble Flt1 Levels in Peripartum Cardiomyopathy: Results of the Multicenter IPAC Study. JACC Heart Fail. 2016;4:380–388. doi: 10.1016/j.jchf.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart. 2006;92:1520–1525. doi: 10.1136/hrt.2006.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goland S, Weinstein JM, Zalik A, Kuperstein R, Zilberman L, Shimoni S, Arad M, Ben Gal T, George J. Angiogenic Imbalance and Residual Myocardial Injury in Recovered Peripartum Cardiomyopathy Patients. Circ Heart Fail. 2016;9:e003349. doi: 10.1161/CIRCHEARTFAILURE.116.003349. [DOI] [PubMed] [Google Scholar]