Abstract
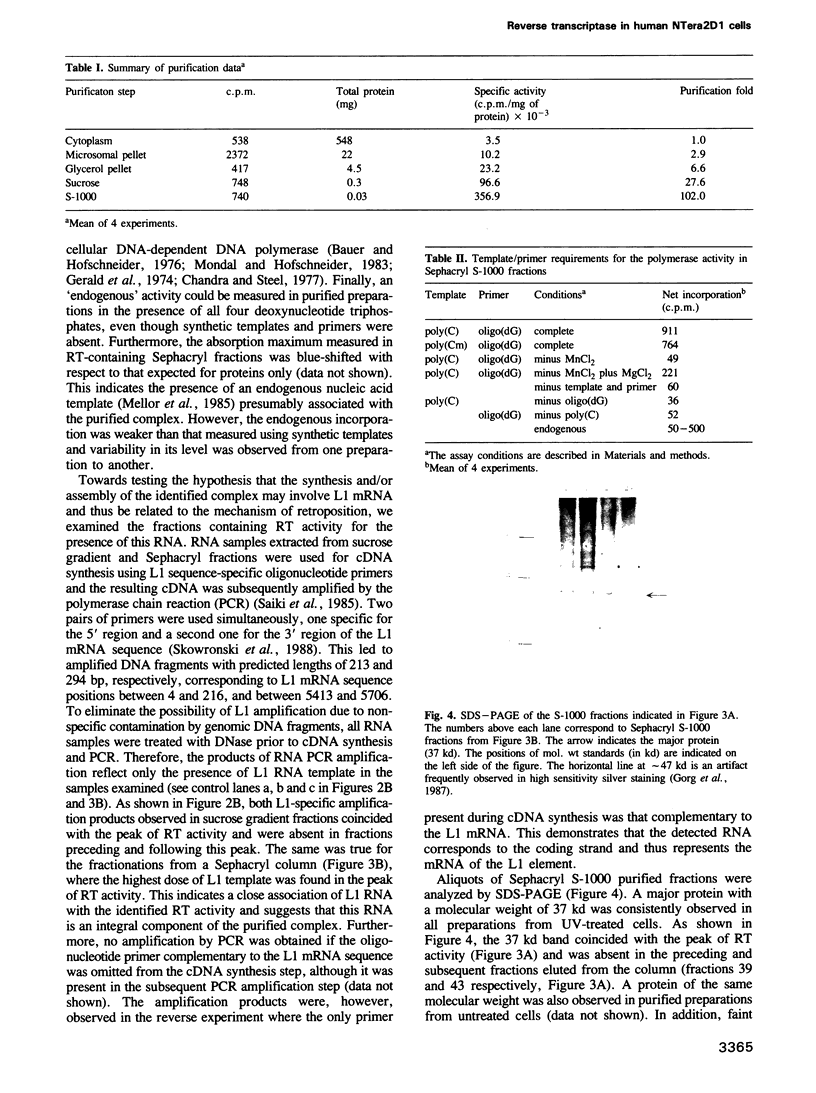
We have identified an RNA-dependent DNA polymerase activity in the microsomal fraction of human pluri-potential embryonal carcinoma cells NTera2D1, which are known to express the full length coding strand of the genomic Line-1 (L1) elements. This activity was classified as a reverse transcriptase (RT) based on its utilization of an RT specific synthetic poly(Cm) template in the presence of Mn2+ ions. Treatment of the cell by ultraviolet irradiation (200 erg/mm2) which resulted in a 2- to 3-fold enhancement of the RT activity, was required for the reproducible detection of the activity throughout the entire purification procedure. More than a 100-fold enrichment in RT activity was obtained by centrifugation in a glycerol step gradient and a linear sucrose density gradient followed by Sephacryl S-1000 gel filtration. These experiments demonstrated that the RT activity was associated with a macromolecular complex having the characteristics of a viral-like particle with a major protein component of 37 kd. The presence of L1 mRNA in RT-containing fractions suggests that the activity identified could originate from L1 elements and/or be involved in the mechanism of retroposition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W., Damjanov I., Simon D., Banting G. S., Carlin C., Dracopoli N. C., Føgh J. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab Invest. 1984 Feb;50(2):147–162. [PubMed] [Google Scholar]
- Andrews P. W. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984 Jun;103(2):285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Bauer G., Hofschneider P. H. An RNA-dependent DNA polymerase, different from the known viral reverse transcriptases, in the chicken system. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3025–3029. doi: 10.1073/pnas.73.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra P., Steel L. K. Purification, biochemical characterization and serological analysis of cellular deoxyribonucleic acid polymerases and a reverse transcriptase from spleen of a patient with myelofibrotic syndrome. Biochem J. 1977 Dec 1;167(3):513–524. doi: 10.1042/bj1670513f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton A. J., Hellman A., Kalter S. S., Helmke R. J. Ultrastructural comparison of placental virus with several type-c- oncogenic viruses. J Natl Cancer Inst. 1974 Apr;52(4):1379–1381. doi: 10.1093/jnci/52.4.1379. [DOI] [PubMed] [Google Scholar]
- Dirksen E. R., Levy J. A. Virus-like particles in placentas from normal individuals and patients with systemic lupus erythematosus. J Natl Cancer Inst. 1977 Oct;59(4):1187–1192. doi: 10.1093/jnci/59.4.1187. [DOI] [PubMed] [Google Scholar]
- Fanning T., Singer M. The LINE-1 DNA sequences in four mammalian orders predict proteins that conserve homologies to retrovirus proteins. Nucleic Acids Res. 1987 Mar 11;15(5):2251–2260. doi: 10.1093/nar/15.5.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Boeke J. D., Fink G. R. Ty element transposition: reverse transcriptase and virus-like particles. Cell. 1985 Sep;42(2):507–517. doi: 10.1016/0092-8674(85)90108-4. [DOI] [PubMed] [Google Scholar]
- Gerard G. F., Rottman F., Green M. Poly(2'-O-methylcytidylate)-oligodeoxyguanylate as a template for the ribonucleic acid directed deoxyribonucleic acid polymerase in ribonucleic acid tumor virus particles and a specific probe for the ribonucleic acid directed enzyme in transformed murine cells. Biochemistry. 1974 Apr 9;13(8):1632–1641. doi: 10.1021/bi00705a012. [DOI] [PubMed] [Google Scholar]
- Goblet C., Prost E., Whalen R. G. One-step amplification of transcripts in total RNA using the polymerase chain reaction. Nucleic Acids Res. 1989 Mar 11;17(5):2144–2144. doi: 10.1093/nar/17.5.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Hattori M., Kuhara S., Takenaka O., Sakaki Y. L1 family of repetitive DNA sequences in primates may be derived from a sequence encoding a reverse transcriptase-related protein. Nature. 1986 Jun 5;321(6070):625–628. doi: 10.1038/321625a0. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loh T. P., Sievert L. L., Scott R. W. Proviral sequences that restrict retroviral expression in mouse embryonal carcinoma cells. Mol Cell Biol. 1987 Oct;7(10):3775–3784. doi: 10.1128/mcb.7.10.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwer J., Wondrak E. M., Kurth R. Genome analysis and reverse transcriptase activity of human teratocarcinoma-derived retroviruses. J Gen Virol. 1987 Nov;68(Pt 11):2807–2815. doi: 10.1099/0022-1317-68-11-2807. [DOI] [PubMed] [Google Scholar]
- Löwer R., Löwer J., Frank H., Harzmann R., Kurth R. Human teratocarcinomas cultured in vitro produce unique retrovirus-like viruses. J Gen Virol. 1984 May;65(Pt 5):887–898. doi: 10.1099/0022-1317-65-5-887. [DOI] [PubMed] [Google Scholar]
- Mellor J., Malim M. H., Gull K., Tuite M. F., McCready S., Dibbayawan T., Kingsman S. M., Kingsman A. J. Reverse transcriptase activity and Ty RNA are associated with virus-like particles in yeast. Nature. 1985 Dec 12;318(6046):583–586. doi: 10.1038/318583a0. [DOI] [PubMed] [Google Scholar]
- Mondal H., Hofschneider P. H. Demonstration of free reverse transcriptase in the nuclei of embryonic tissues of the Japanese quail. Biochem Biophys Res Commun. 1983 Oct 14;116(1):303–311. doi: 10.1016/0006-291x(83)90415-1. [DOI] [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Skowronski J., Fanning T. G., Singer M. F. Unit-length line-1 transcripts in human teratocarcinoma cells. Mol Cell Biol. 1988 Apr;8(4):1385–1397. doi: 10.1128/mcb.8.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski J., Singer M. F. Expression of a cytoplasmic LINE-1 transcript is regulated in a human teratocarcinoma cell line. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6050–6054. doi: 10.1073/pnas.82.18.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Taketo M., Tanaka M. A cellular enhancer of retrovirus gene expression in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3748–3752. doi: 10.1073/pnas.84.11.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]