Abstract
Histones are intra-nuclear cationic proteins that are present in all eukaryotic cells and are highly conserved across species. Within the nucleus, they provide structural stability to chromatin and regulate gene expression. Histone may be released into the extracellular space in three forms: freely, as a DNA-bound nucleosome or as part of neutrophil extracellular traps, and all three can be detected in serum after significant cellular death such as sepsis, trauma, ischaemia/reperfusion injury and autoimmune disease. Once in the extracellular space, histones act as damage-associated molecular pattern proteins, activating the immune system and causing further cytotoxicity. They interact with Toll-like receptors (TLRs), complement and the phospholipids of cell membranes inducing endothelial and epithelial cytotoxicity, TLR2/TLR4/TLR9 activation and pro-inflammatory cytokine/chemokine release via MyD88, NFκB and NLRP3 inflammasome-dependent pathways. Drugs that block the release of histone, neutralise circulating histone or block histone signal transduction provide significant protection from mortality in animal models of acute organ injury but warrant further research to inform future clinical applications.
Facts
Histone is a highly cationic intra-nuclear protein that supports the normal structural development of chromatin and regulation of gene expression.
Histone and DNA-bound histone may be released into the extracellular space during cell death processes including necrosis, apoptosis and NETosis.
In acute sterile organ injury, cell death occurs due to various toxic stimuli including ischaemic, traumatic and autoimmune pathology.
In the extracellular space, histones act as cytotoxic damage-associated molecular pattern (DAMP) proteins by activating Toll-like receptors (TLRs), promoting pro-inflammatory cytokine pathways and altering phospholipid membrane permeability.
In animal models of acute organ injury (AOI), anti-histone monoclonal antibodies and endogenous molecules (C-reactive protein and activated protein C) provide significant protection from mortality.
Open Questions
How can we accurately delineate the effects of free histone versus DNA-bound histone?
How do different histone subtypes affect different tissue types? Does cytotoxicity vary by histone subtype in different tissues?
How does the protective action of anti-histone antibodies in animal models of AOI compare to their action in human patients with AOI? Can we potentiate the protective effects of endogenous proteins such as CRP and aPC?
Acute organ injury (AOI) occurs after a toxic insult such as sepsis, trauma, ischaemia/reperfusion (I/R) injury and autoimmune disease.1, 2, 3 AOI is characterised by a highly pro-inflammatory environment potentiated by cytokine release, leukocyte migration, microvascular thromboses and cellular death.2, 4 ‘Traumatic injury’ activates systemic immune responses, characterised by a pro-inflammatory phase where cytokines, chemokines and damage-associated molecular pattern (DAMP) proteins predominate and an anti-inflammatory immunosuppressive phase.5 DAMP proteins, including high-mobility group box 1 (HMGB-1), purines such as adenosine triphosphate (ATP), DNA/RNA and, more recently, histones, have been found to act as immune activating ‘endogenous danger signals’ in these disease states1 (Figure 1). I/R develops after a period of interrupted blood flow and lack of tissue perfusion.1 A reduction in oxygen delivery and metabolic substrate clearance creates a hypoxic and inflammatory environment, potentiating local necrosis. Current treatments of AOI are mainly supportive. To improve patient outcomes, it is necessary to expand our understanding of the molecular mechanisms of AOI to further aid the development of novel highly targeted and efficacious drugs. This review will describe histone release, their extracellular pro-inflammatory interactions in organ injury and novel approaches for developing histone-targeting drugs.
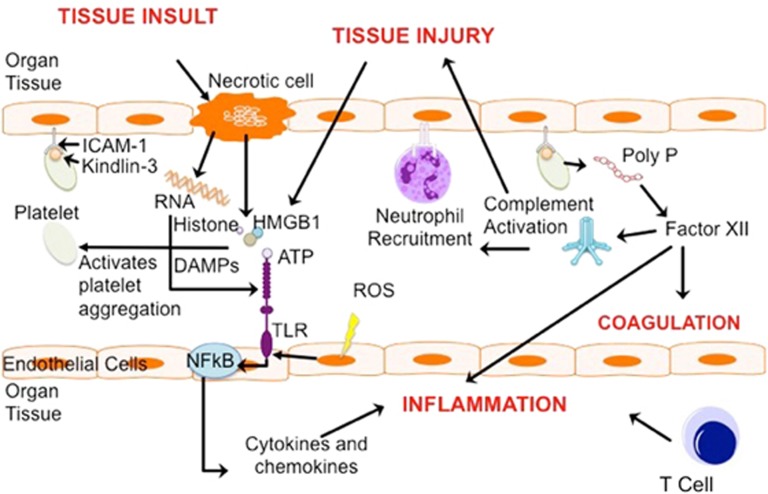
Figure 1.
Mechanisms of Sterile Organ Injury. Toxic insults, such as ischaemia or trauma, initiate both controlled and uncontrolled cell death in endothelial cells leading to apoptotic/necrotic tissue and release of intracellular cell components into the extracellular space. These include immunogenic compounds such as RNA and DAMPs (HMGB1, ATP and Histone) which bind to and activate specific TLRs, driving the NFkB-mediated transcription of pro-inflammatory cytokines. TLRs are upregulated by ROS as a result of hypoxic mitochondrial dysfunction. Reperfusion of the tissue and chemokine action results in leukocyte and platelet migration/extravasation. Platelets adhere to the endothelium via ICAM-1 and Kindlin-3. Activated platelets release Poly P, which activates Factor XII, and subsequently, complement. This results in activation of the coagulation pathways and further tissue injury, oedema and inflammation. Activated T cells release pro-inflammatory mediators and can cause direct cytotoxicity
Histone biology and function
Discovered in 1884 by Albrecht Kossel,6 histones are highly conserved, intra-nuclear, cationic proteins found to have a range of extensively characterised intracellular functions, such as enhancing the structure and stability of chromatin and the epigenetic regulation of DNA.7, 8, 9, 10, 11, 12 There are two functional subgroups of histone, ‘core’ (H2A, H2B, H3 and H4) and ‘linker’ (H1 and H5) proteins13 (Figure 2). A ‘nucleosome’ is formed of 147 base pairs of DNA wound around an octameric core histone complex. Linker histones join together adjacent nucleosomes and regulate DNA exposure to other intra-nuclear proteins, essential for transcription, replication and repair7, 8, 13, 14, 15 (Table 1).
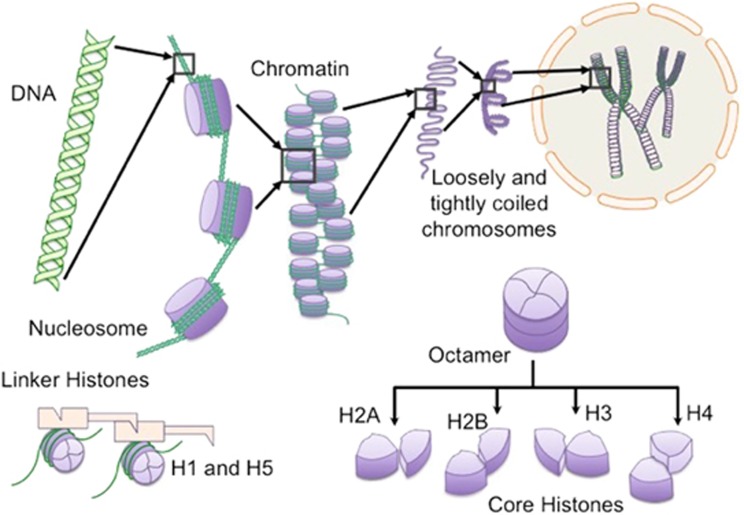
Figure 2.
Intracellular structure and function of histone. Histones are intra-nuclear alkaline proteins that contribute to the structural organisation and stability of chromatin. Individual core histone monomers (H2A, H2B, H3 and H4) combine to form octameric structures. Each octamer is made up of two H3-H4 and two H2A-H2B dimers. DNA strands (146 base pairs) wind around the octamers to form nucleosomes and are held together with linker histones (H1 and H5), forming chromatin. Chromatin coils and condenses to form chromosomes. This enables vast amounts of DNA to be compacted tightly within the nucleus of the cell
Table 1. Functions of histone.
| Intracellular functions | Extracellular functions |
|---|---|
| Nucleosome construction7 | DAMP signalling via TLR2 and TLR4 receptors16 |
| Chromatin stability7 | NLRP3 inflammasome activation17 |
| Epigenetic modifications of transcription, replication and repair of DNA15 | Cell mediated apoptosis18 |
| Neurogenesis, migration and endocytosis18 | |
| Direct cellular toxicity19 | |
| Regulation of inflammation, immunity, death, coagulation and thrombosis18 |
An extensive body of research now demonstrates that extracellular histone is pro-inflammatory.18 However, the complexity of this subject is amplified by the existence of two distinct extracellular forms of histone: ‘free histone’ and ‘DNA-bound histone (nucleosomes)’, both differing significantly in their mechanism of cellular release and extracellular interactions.20 Crudely illustrated, when intravenously administered to mice, free histone is lethal within minutes,21 whereas nucleosome infusions surprisingly produce no immediate cytotoxic effects.22 The discriminative discussion within the literature is invariably poor; with many authors failing to clearly differentiate between the two.20 It is therefore challenging to accurately interpret the evidence and calls into question the validity of such evidence. This is in part due to the lack of specific anti-histone mAbs that precisely distinguish between free histone and nucleosomic material in serum. To date, the most widely used antibodies, created by Xu et al.,21 detect H2A-H2B-DNA complexes and antibodies used to identify free histone also interact with histone in nucleosomes. Appraisal of the studies presented within this review is limited by these incongruences. With this in mind, it is vital for future research in this field to focus on accurately delineating the attributes of each distinct form of histone. We have taken care to refer to each form as ‘free histone’, ‘nucleosome’ or ‘NET’ as appropriate within this review.
Free histone and nucleosomes are released from dying cells, particularly during necrosis, which occurs extensively in pathologies precipitating acute organ injury.23 ‘Necrosis’ results in an uncontrolled rupture of the plasma membrane, releasing the intracellular contents of a cell including intra-nuclear proteins4, 24 Histones are present in the surrounding extracellular milieu after other forms of regulated necrosis including necroptosis, pyroptosis and ferroptosis10 and are also expressed at the surface of apoptotic cells.25, 26, 27
Neutrophil extracellular traps (NETs) containing histone are released by innate immune cells during a process called NETosis.28 This unique form of immune cell death is mediated by protein arginine deaminase 4 (PAD4), an enzyme that facilitates citrullination of H3 and subsequent chromatin decondensation.29 Composed of a complex arrangement of antimicrobials, myeloperoxidase (MPO)-bound DNA and histones, NETs have protective and pathogenic functions.30, 31 When released from neutrophils during infection, the granule proteins and chromatin form a protective mesh that filters and destroys pathogenic organisms.30, 31, 32 However, NETosis also occurs inappropriately during sterile inflammation resulting in thrombosis,33 autoimmunity34 and NET-mediated cytotoxicity.35 It is still unclear what initiates pathological NETosis; however, there is growing evidence that TLR2, TLR4 and complement may have a significant role in initiating this unique process of immune cell death.36, 37, 38
In the extracellular space, histone acts as a DAMP protein (an endogenous danger signal) alerting the body to cellular death, by activating the immune system and repair processes.39 In human observational studies, the normal serum value of histone is reported to be ~0.06 ng/ml. However, serum levels as high as 3 ng/ml have been reported in multiple trauma patients40 and correlate with coagulopathy, endothelial damage and inflammation, all of which are hallmark features of AOI.41, 42 Dynamic serum changes after inflammatory insults could indicate the use of serum histone, nucleosome or NETs a novel biomarker of cell death/NETosis and, hence, disease severity, partly due to the brevity of inflammatory diseases that demonstrate DAMP involvement. DAMPs have been implicated in cancer,43, 44 autoimmune disease,45, 46, 47, 48, 49, 50 neurodegenerative disease,51 sterile inflammation and sepsis.21, 52 In 2009, Xu et al.21 published data demonstrating a significant role for eHistone in driving endothelial dysfunction and organ failure in sepsis. There now exists a vast body of literature that demonstrates histone-mediated pathogenesis in AOI, through activation of TLRs, immunomodulatory effects and the cytotoxic disruption of plasma membrane function (Table 2 and Figure 3).
Table 2. Organ-specific effects of extracellular histone in various models of sterile organ injury.
| Organ | Animal model of AOI | Effects of histone/anti-histone therapy |
|---|---|---|
| Brain | I/R53 | Chromatin released post I/R injury |
| Exogenous histone infusion | ||
| Increases infarct volume | ||
| Worsens neurological scores. | ||
| Improve neurological scores | ||
| Anti-H2A/H4 antibodies | ||
| Reduce infarct volume | ||
| Histone-induced toxicity54 | Dose-dependent, H1 neurotoxicity | |
| H1-mediated microglial | ||
| Survival | ||
| Increased reactivity | ||
| MHC class II receptor expression | ||
| Chemoattractant activity | ||
| Heart | I/R55, 56 | Accumulation of eHistones within myocardium |
| eHistone-mediated myocytoxicity | ||
| PAD4 KO mice are protected from MI injury | ||
| DNase1 treatment | ||
| Improves ventricular remodelling | ||
| Prolongs local cardiomyocyte survival | ||
| Reduces MI volume | ||
| Improves cardiac function | ||
| Reduces nucleosome release and neutrophil infiltration | ||
| No effect on mortality, infarct size or inflammation | ||
| Lungs | TRALI57 | Activated platelets promote NET formation in TRALI |
| NETs increase permeability of LPS primed endothelial cells | ||
| Anti-H2A/H4 antibodies attenuate | ||
| Histone-mediated lung oedema | ||
| Vascular permeability | ||
| Mortality | ||
| Prevent further NET formation | ||
| NET induced35 | NETs and eHistones induce cell death | |
| Epithelial | ||
| HUVECs | ||
| DNase | ||
| Does not decrease NET-mediated cytotoxicity | ||
| Anti-H1/2A/2B/4 antibodies, PSA and APC are protective | ||
| IgG and Complement induced36 | eHistone | |
| Released into BALF of ALI patients | ||
| Dependent on complement (C5aR and C5L2) activation | ||
| Exhibits alveolar epithelial cytotoxicity | ||
| C5aR and C5L2 activation induces neutrophil-dependent ALI (?NETs) | ||
| Anti-H4 IgG antibody attenuates ALI severity | ||
| Trauma19 | Serum histone reaches toxic levels post-trauma | |
| Release correlates with | ||
| Lung injury severity | ||
| Endothelial damage | ||
| Coagulation | ||
| eHistone actions | ||
| Direct toxicity to endothelial cells | ||
| Stimulate cytokine and NET release | ||
| Phospholipid-histone complexes result in direct cellular toxicity through membrane disruption and calcium influx | ||
| Liver | ConA and APAP induced2 | eHistones stimulate a “cytokine storm” |
| Potentiate TLR2 and TLR4 signal transduction | ||
| No activity at TLR3/5/7/8/9 | ||
| Cytokine release is abolished in TLR4 KO mice | ||
| H3 histone is released in ConA and APAP induced liver injury | ||
| Anti-H3 antibody | ||
| Reduces mortality and cytokine release | ||
| Does not prevent histone release or improve liver injury markers | ||
| I/R17, 58, 59, 60 | Histones released from hepatocytes post-I/R injury | |
| Histone infusion | ||
| Worsens markers of acute liver injury | ||
| Activates non-parenchymal KC TLR9-MyD88 pathways | ||
| Enhances DNA-TLR9 signalling | ||
| TLR9-mediates mitochondrial ROS production | ||
| ROS activates NLRP3 Inflammasome | ||
| Effects attenuated in TLR9 and NLRP3 KO mice | ||
| DAMPs (eHistone and HMGB1) stimulate NET formation post I/R injury by activating TLR4 and TLR9 | ||
| NETs | ||
| Hepatocytotoxic | ||
| Stimulate KC-cytokine release | ||
| Formation is inhibited by PAD4i and DNase1 | ||
| Anti-H3/H4 histone antibody | ||
| Attenuates TLR9 signalling | ||
| Improves markers of acute liver injury | ||
| Kidney | I/R16 | Necrotic TECs release histone |
| eHistone actions | ||
| Direct toxicity to renal endothelial and TECs | ||
| Leukocyte recruitment | ||
| Microvascular vascular leakage | ||
| Renal inflammation | ||
| Activates TLR2/TLR4 and potentiates NFkB, MyD88, MAPK signalling | ||
| Anti-histone IgG is protective | ||
| Pancreas | Gallstone and CCK61 | Histone released from necrotic acinar cells. |
| eHistone concentration correlates well with severity of tissue injury |
Abbreviations: APC, activated Protein C; ALF, acute liver failure; ALI, acute lung injury; APAP, Paracetemol/Acetominophen; BALF, bronchoalveolar lavage fluid; C5aR, component 5a receptor; C5L2, anaphylatoxin chemotactic receptor; citH3, citrullinated H3; ConA, Concanavalin A; DNA, deoxyribonucleic acid; DNase, deoxyribonuclease; EC, endothelial cells; eHistones, extracellular histones; ELISA, enzyme linked immunosorbent assay; H1/H2A/H2AX/H2B/H3/H4/H5, histone subtypes; HMGB1, high-mobility group box 1; HUVEC, human vascular endothelial cells; IgG, immunogloblin G; KC, kupffer cells; KO, knockout; I/R, ischaemia reperfusion; LPS, lipopolysaccharide; mAb, monoclonal antibody; MAPK, mitogen-activated protein kinases; MCA, middle cerebral artery; MHC, major histocompatibility complex; MI, myocardial Infarction; MPO, myeloperoxidase; MyD88, myeloid differentiation primary response gene 88; NET, neutrophil extracellular traps; NFkB, nuclear factor kappa B; NLRP3, nucleotide-binding domain leucine-rich repeat containing protein 3; NS, non-significant result; PAD4i, peptidyl-arginine-deiminase-4; PSA, polysialic acid; ROS, reactive oxygen species; S, significant result; TECs, tubular epithelial cells; TLR, Toll-like receptor; TRALI, transfusion-associated lung injury
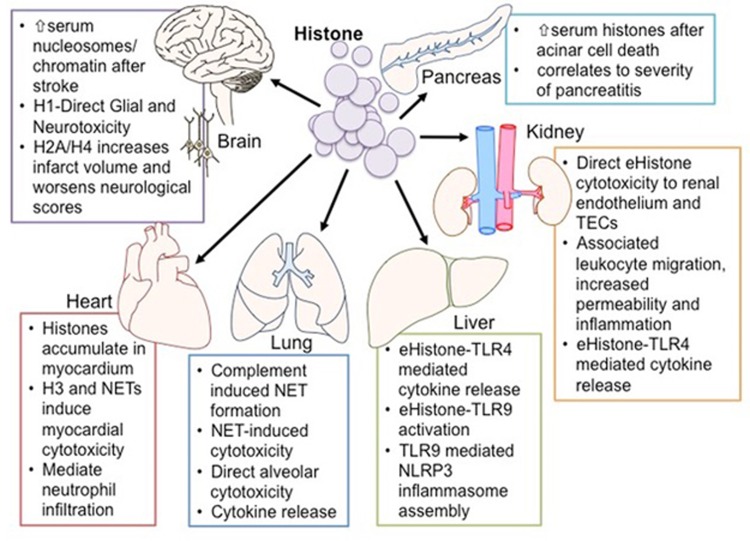
Figure 3.
Organ-specific extracellular histone effects. Histone (H) action is diverse, affecting a wide range of cells and tissue types. Common mechanisms include direct cytotoxicity, immune cell TLR stimulation and further immune activation (NLRP3 inflammasome and complement). Intravenous (IV) infusion of histone primarily causes death via alveolar cytotoxicity, before affecting other distal organs. Histone release is correlated with severity of disease and tissue damage
Mechanisms of histone-mediated inflammation
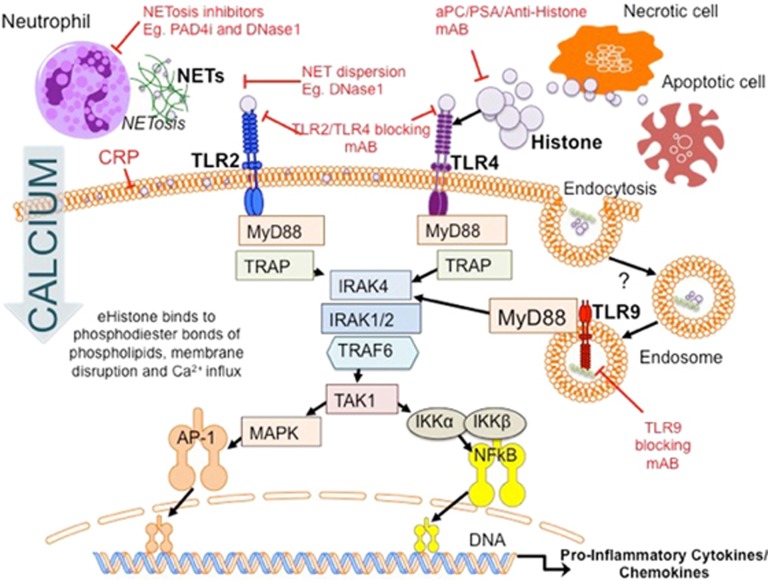
TLRs facilitate the recognition of invading pathogens by responding to pathogen-associated molecular pattern (PAMP) proteins such as bacterial CpG DNA and lipopolysaccharide.38, 62, 63, 64 It is now widely accepted that TLRs also mediate DAMP signalling.40, 65 Activation of TLR2 and TLR4 in particular, is likely to be responsible for the release of pro-inflammatory cytokines (IL-6, TNF-α) via MyD88-dependent pathways and the activation of platelets, which drive the augmented immune response in sterile AOI2, 65, 66, 67, 68 (Figure 4). Extensive evidence for TLR2 and TLR4 signalling, but not TLR3/5/7/8/9, has been reported. TLR2 and TLR4 KO mouse models are protected from lethal doses of histones;2, 58 and TLR2/4 blocking mAbs significantly protect wild-type animals.
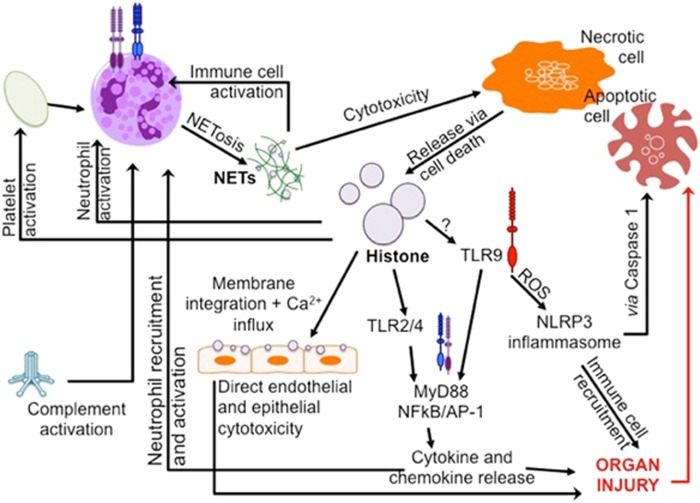
Figure 4.
Mechanisms of extracellular histone-induced organ injury. Histone is released from tissue cells through necrotic and apoptotic cell death caused by toxic stimuli, such as ischaemia. Cell membranes degrade, allowing intra-nuclear material and other DAMPs to be released into the extracellular space. Immune cell death (termed NETosis) may also release significant amounts of histone in the form of NETs. Thought to be an antimicrobial component of the innate immune system, NETs also exhibit significant cytotoxicity to tissues and further stimulate immune cell activation. Histone action is independent of its origin, causing damage in distal tissues and organs. Histones cause organ injury via direct endothelial/epithelial cytotoxicity, TLR and complement activation. Histone integrates into the phospholipid bilayer of cell membranes, altering their permeability, resulting in an influx of calcium ions and cell death. Histone-mediated complement activation recruits immune cells and results in further histone release. Histone-activation of TLRs stimulates MyD88-dependent signalling pathways and pro-inflammatory cytokine/chemokine release. Activation of TLR9 causes the release of ROS, activating the NLRP3 inflammasome, Caspase 1 and further inflammatory cell recruitment. These processes converge to cause significant organ injury
However, an additional role has been proposed for the endosomal TLR9 in animal models of hepatic IR injury.69 Quantitatively, eHistone-mediated TLR9 activation induces significant pro-inflammatory cytokine (TNFα and IL-6) release and increases necrotic tissue size fivefold.59 Anti-H3/H4 neutralising and TLR9 blocking mAbs significantly reduce these pathological changes, which are also attenuated in TLR9 and myd88 KO mice.
TLR9 activation may further contribute to NLRP3 inflammasome assembly in sterile inflammation.17 Classically, the NLRP3 inflammasome responds to microbial stimuli by activating the downstream caspase 1 pathway, generating pro-inflammatory cytokine production and leukocyte recruitment.70, 71 Histone-TLR9 activation mediates mitochondrial reactive oxygen species (ROS) production and subsequently activates the NLRP3 Inflammasome in non-parenchymal pro-inflammatory kupffer cells (KCs).17 This may be partially attenuated by anti-H3/H4 histone antibody and is absent in TLR9 and nlrp3 KO mice.
This research is surprising as TLR9 is typically activated by bacterial CpG-DNA. It is possible that remnant murine DNA bound to histone within the sample is exerting DNA-TLR9-dependent DAMP effects. eHistone may also enhance DNA-TLR9 signalling, as production of IL-6 significantly increases when co-administered.59 Further research should be undertaken to further validate the mechanistic relationship between eHistone and TLR9.
Extracellular histone also causes ‘direct cytotoxicity to epithelial and endothelial tissue’. Administration of high-dose exogenous histone ubiquitously results in a significant reduction in cell viability. Pereira et al.72 first characterised the ionic binding between cationic histone and anionic phospholipids in 1994. Current theories propose that eHistone binds to phospholipid–phosphodiester bonds, similar to their DNA-binding sites, altering membrane permeability,19, 21 and initiating calcium ion influx. Intracellular stores are released, disrupting processes necessary for survival. Interestingly, C-reactive protein (CRP), an endogenous acute-phase protein released during inflammatory diseases and typically used as a biomarker for disease regression, was found to protect against histone-mediated toxicity by binding to phospholipids, blocking histone integration into cell membranes and preventing calcium influx.73
Extracellular histone in acute organ injury
Brain
Cerebral vascular events are the most common cause of mortality and long-term morbidity across the world. Thrombo-embolism of the cerebral arteries results in acutely reduced cerebral perfusion, and if prolonged, irreversible inflammatory neuronal injury.74
In vivo animal studies conducted to characterise the release and functional role of extracellular nucleosomes53 show that animals exposed to moderate hypoxia (6% over 24 h), demonstrate a threefold rise in circulating levels of nucleosomes compared with control animals. Furthermore, models of ischaemic stroke show a sevenfold serum nucleosome increase. Human observational trials75, 76 measuring serum nucleosome after stroke corroborate this evidence, suggesting that poor oxygen perfusion stimulates the release of nucleosomes from cells into the systemic circulation.
Administering exogenous histone or anti-H2A/H4 antibody respectively increases or decreases the infarct size by ~30% when compared with control animals.53 The exacerbating effect of histone in these models of IR injury may be due to a combination of factors, including direct endothelial and blood–brain-barrier toxicity,21, 35, 77 which increases permeability, leukocyte migration and immune stimulation. In addition, histones activate platelets,33, 66 increasing the risk of further ischaemia during in the reperfusion phase.
Patients with neuro-inflammatory conditions, including Alzheimer’s and Parkinson’s, have been reported to have high serum concentrations of histones.78 Histone that has leaked into the extracellular space is able to damage glial cells, which support normal neuronal function. It is likely that the immune-derived microglial cells have a key role in the pathogenesis of these pathologies. H1 histone, but, interestingly, not core histones, show dose-dependent neurotoxicity,54 by promoting microglial survival. Low doses upregulate major histocompatibility complex class II receptor expression in microglia and demonstrate potent dose-dependent chemoattractant properties. In addition, H1 histone is neuro-immunomodulatory inducing astrocytes to take an activated stellate morphology, increasing their reactivity. These novel findings highlight the mechanistic specificity of histones, whereby the activity status of different cell types is directly affected by their interaction with histone molecules.
Heart
Cardiac injury is typically caused by I/R disease where a lack of oxygen delivery to cardiomyocytes results in necrosis and release of immunogenic intracellular components.79 Repeated ischaemic insults result in irreversible damage and cardiac failure. The prognosis for these patients remains poor despite the recent introduction of disease modifying therapies.
Histones released from necrotic cells accumulate within the myocardium early after myocardial infarction (MI),55 inducing further dose-dependent myocardiocyte toxicity. NET-derived nucleosomes are also implicated in driving inflammatory signalling after cardiac ischaemia and are released via NETosis.56 Mice lacking a functional PAD4 enzyme produce lower circulating concentrations of nucleosomes post MI. Furthermore, acute treatment with DNase1, an endogenous endonuclease enzyme that disrupts chromatin stability by degrading linker DNA, significantly improves ventricular remodelling, local cardiomyocyte survival, cardiac function and reduces neutrophil infiltration. The observed protective effect of DNase1 treatment is attributed to dispersal of toxic histone molecules, preventing further direct cardiac toxicity. Despite this, no improvements in mortality, infarct size or inflammatory parameters have been observed, and although local cytotoxicity is reduced, immunostimulatory histone is free to disseminate hematogenously, with the potential to damage distal organs.35 Despite a relatively small benefit, DNase1 may provide a useful approach to the acute treatment of MI, whereby small amounts of the myocardium may be preserved and prolong cardiac function for high-risk patients.
Lung
The mortality rate associated with acute lung injury (ALI) is ~40%.80 ALI may arise secondary to transfusion, trauma or ischaemia, and affects normal alveolar and pulmonary endothelial cell functioning, resulting in inefficient gas exchange, increased protein permeability, cell death, inflammation and eventually, permanent lung dysfunction.81, 82
Animal models of transfusion-related lung injury (TRALI) demonstrate significant NET-mediated inflammation.57 Activated platelets induce NET formation from neutrophils and, once in the extracellular space, NETs increase pulmonary oedema and vascular endothelial permeability.35 It is unclear whether the histone, DNA or granular proteins within NETs, or indeed a combination of components, are responsible for mediating pulmonary cellular death and inflammation. It is likely that all three components have a part to play; however, Caudrillier et al.57 noted a significant reduction in alveolar cell viability upon administration of pure histones in vitro. Furthermore, pre-incubation with anti-histone (H1-DNA, H2A, H2B and H4) antibody significantly decreases NET-mediated cytotoxicity in TRALI-mice. The endogenous anionic glycan, polysialic acid (PSA) is also protective in this model and works by binding cationic histone directly, an interaction, which was previously linked to neural regeneration in cerebral tissue.35, 83 Interestingly, the seemingly inert properties of free H3 in alveolar tissue is also described by Saffarzedeh et al.35 While infusion of H1 and H4 induce alveolar cell death, H3 does not.36 In addition, the use of anti-H3/citH3 mAb confers no further protection in models of ALI,35 whereas anti-H4 IgG antibody significantly attenuates pro-inflammatory signalling, reducing ALI severity by 50%.36 These differences highlight the importance of conducting similar experiments in other tissues in order to elucidate the specific cytotoxic activities of H1-5.
Acute lung pathology may also frequently occur secondary to major traumatic events.84 Pathologically, major trauma results in pulmonary leukocyte infiltrates, tissue oedema, microvascular haemorrhage and thrombosis.85 In patients with an acute history of severe blunt trauma, circulating histones increase to toxic levels within 4 h and correlate with the severity of lung injury, endothelial damage and coagulation. Pathological changes observed in mouse models of trauma-associated lung injury include neutrophil-obstructed alveolar capillaries, pulmonary haemorrhage and tissue oedema.19 Immunohistochemical staining for histone in vitro and in vivo shows accumulation around endothelial cell membranes where they interact with phosphodiester bonds on phospholipids, which are similar to DNA-histone-binding sites.72, 86 The histone–phospholipid interaction alters membrane permeability and results in calcium influx-dependent cell death.19 Similar findings are also reported in other tissue types, including platelets, breast cancer cells, urinary bladder epithelium and thymocytes.65, 66, 87, 88, 89, 90 This discovery is fundamental to the understanding of the nonspecific, TLR independent, cytotoxic effects of free histone.
Liver
Hepatic ischaemia and drug toxicity are forms of sterile hepatitis that can result in liver enzyme derangement and synthetic function disturbance presenting as encephalopathy, coagulopathy (reduced clotting factors) and oedema (low albumin),91 all of which are hallmark attributes of acute liver failure (ALF).
Histone is released after hepatic sterile inflammation and activates TLRs on KCs, initiating a cytokine storm.2, 58 However, in contrast to the aforementioned models of ALI, the release of H3 into the ‘hepatic circulation’ is associated with significant hepatic damage that is attenuated in the presence of anti-H3 antibody, which reduces the risk of mortality and serum measurements of TNF-α and IL-6 in animal models of ALF. In addition there is controversy surrounding the role of TLR2, TLR4 and TLR9 in mediating inflammation in I/R animal models. This is discussed in detail in the previous section entitled ‘Mechanisms of histone-mediated inflammation’.
NETs also mediate hepatocytotoxicity.60 Initially, NETosis is stimulated by histone activation of TLR4 and TLR9 expressed on neutrophils (Figure 4). NETs stimulate immune-derived KCs to release pro-inflammatory mediators, which is abolished upon co-administration of NETosis inhibitors, PAD4i or DNase1. A reduction in both the histone-mediated release of NETs and the markers of hepatic injury is observed.
Kidney
Acute ischaemic kidney injury is a common clinical complication and carries a high rate of long-term morbidity and mortality.92 Ischaemic renal damage is characterised by apoptotic and necrotic cell death, and perpetuated by a rise in local pro-inflammatory cytokines and invading neutrophils.93 Repeated insults to the renal parenchyma can result in permanent fibrosis and renal dysfunction, which, if left untreated, may progress to chronic kidney disease.94
During acute renal ischaemia, histone is released into the extracellular space by necrotic tubular epithelial cells.16 eHistone induces dose-dependent toxicity to renal endothelium and tubular epithelium, leukocyte adhesion, increased vascular permeability and transendothelial migration within the renal arteries. Neutralisation of histone activity using anti-H4 mAb reduces cytokine and chemokine expression.95 It is highly likely that TLR2 and TLR4 mediate and activate pro-inflammatory MyD88-NFkB and MAPK pathways (Figure 5) and corroborating studies have shown that these pro-stimulatory effects are attenuated in MyD88 and TLR double-knockout animals.68, 96
Figure 5.
eHistone signalling via TLRs and potential therapeutic approaches. Histones are released via NETosis and cellular death mechanisms during organ injury and bind with TLRs. This activates the MyD88-dependent signalling pathways and results in the transcription and translation of pro-inflammatory cytokines and chemokines, including TNFα, IL-6. Several therapeutic approaches are proposed, including those that target eHistone directly such as activated protein C, polysialic acid and anti-histone antibody; those that block TLR receptor signal transduction and those that prevent eHistone release by reducing apoptotic and necrotic cell death
Pancreas
Ischaemic damage to the pancreas can occur after very brief episodes of reduced pancreatic perfusion and is characterised by rapid release of pancreatic enzymes and severe tissue inflammation.97 Commonly occurring after cardiac surgery, the pathology can progress to acute pancreatitis when acinar cell death occurs.98
During gallstone and cholecystokinin-induced necrotising pancreatitis,61 necrotic acinar cell death predominates with large volumes of histone released into the extracellular space. Oedema and inflammation alone does not result in a serum histone rise and it is likely that release of histone from acinar cells in pancreatic injury is a necrosis-dependent mechanism. However, histone may also be released during pancreatitis due to a lack of HMGB-1, which is protective against oxidative stress and DNA disruption.99 Once in the extracellular space, histone promotes HMGB-1 secretion from innate immune cells, further accelerating DNA damage in these cells by reducing the intra-nuclear concentration.100 Research showing the benefit of anti-H3 mAbs is promising in these models;99 however, the discussion of histone action in pancreatic injury is limited within the literature and further research is required to fully confirm the suspected involvement of TLR pathways involved in sterile pancreatic injury.
Novel approaches for targeting histone
Extracellular histone and nucleosome levels are correlated with poorer outcomes in human observational studies. There exists a theoretical potential to reduce the burden of AOI by blocking their extracellular actions. We propose three therapeutic strategies for attenuating the deleterious effects of histone (Table 3). They include blocking the release of histone, neutralising circulating histone and blocking eHistone signal transduction.
Table 3. Therapeutic approaches and potential anti-histone therapies.
| Blocking release | Neutralisation | Blocking signalling |
|---|---|---|
| PAD4i60 | Anti-H mAb2, 16, 19, 35, 36, 53, 57, 58, 59 | TLR blocking mAb |
| Blocks NETosis | Neutralises histone in circulation | PreventsTLR2/4/9 signal transduction |
| Inhibits citrullination of H3 | DNase155 | Reduces cytokine release |
| DNase157 | Degrades NET linker-DNA | Significant immunosuppressive side effects likely |
| Disperses NET-derived histone within circulation | Disperses histone | CRP73 |
| Prevents NET-mediated NETosis | aPC35 | Endogenous anionic acute phase protein |
| Serum protease | Prevents cationic histone from binding to phosphodiester bonds on phospholipids | |
| Degrades eHistone | ||
| PSA35 | ||
| Endogenous Anionic protein | ||
| Ionic interaction with H |
Abbreviations: aPC, activated Protein C; BWA3, H4 histone neutralising antibody; CRP, C-reactive protein; H, histone; NET, neutrophil extracellular trap; NETosis, NET release; PAD4i, protein arginine deiminase 4; PSA, polysialic acid; TLR, Toll-like receptor
Targeting the release of histone complexes and, in particular, inhibiting NETosis has demonstrated considerable specificity and efficacy when used in animal models of AOI55, 56, 60 and sepsis.101 Specific PAD4 inhibitors prevent the citrullination of H3, a key step in releasing nucleosomic material for NET formation,102 and are more effective than DNase1 in preventing tissue damage.103 DNase1 theoretically disperses DNA-bound-histone, and hence, reduced NET-mediated cytotoxicity.55 but are unlikely to be able to effectively access DNase1 binding sites within multifarious NET complexes.
Drugs targeting histone directly must only act in the extracellular space. Histone neutralising drugs that obtain intracellular access could potentially disrupt DNA structure or function, and result in catastrophic side effects. Interestingly, plasma concentrations of endogenous proteins such as activated protein C (aPC) and CRP inversely correlate with serum histone40 suggesting that histone action may be partially attenuated by the action of these molecules.104 APC is a serum protease, which degrades histone in the circulation and although a systematic review of five randomised control trials (RCTs; n=5101) demonstrated no benefit of using aPC in patients with severe sepsis,105 studies using recombinant aPC have shown significant protection in animal models of sterile inflammation.106 Similarly, the endogenous, acute-phase protein CRP protects against histone toxicity by preventing coagulation activation, inhibiting endothelial damage and reducing vascular permeability in murine models infused with histone.73 In addition, histone-CRP complexes are observed in the serum of patients and CRP is able to compete for phospholipid-binding sites, preventing histone integration into plasma membranes, calcium influx and cell lysis. Enhancing the activity of these proteins may provide a promising avenue for the future treatment of inflammatory diseases.
Despite their use in laboratory experiments, TLR blocking mAbs are unlikely to provide a promising direction for anti-histone drugs. Their intrinsic action of eliciting innate antigen-specific acquired immunity after host infection64 would mean that blocking their activity might cause substantial immunodeficiency. However, there is considerable evidence for the protective effects of histone neutralising mAbs in animal models of AOI.2, 16, 17, 19, 21, 35, 36, 53, 57, 58, 59 The data show an improvement in inflammation, functional scores and overall survival. This is encouraging and paves the way for future development of drugs with similar pharmacodynamic properties for human use in inflammatory pathology.
Future perspective
Future research in this field should focus on developing an accurate means of detecting free histone. This would significantly improve the validity of similar work and further elucidate the specific molecular interactions of histone in vivo compared to those currently attributed to NETs and nucleosomes. In addition to this misnomer, there is limited discussion within the literature describing the differences in cytotoxic activity of H1-5. Although Saffarzedeh et al. attempts this by presenting H1-H5 effects individually,36 these experiments only represent a single cell type and are not representative of their action in other models of AOI.
Conclusion
It is clear that both free and DNA-complexed histone have important roles in mediating pro-inflammatory signalling in sterile AOI. Released during periods of cell death and immune activation, histone, nucleosomes and NETs induce cytotoxicity by altering cell membrane permeability to calcium ions, activating TLRs on innate immune cells, stimulating NLRP3 inflammasome and complement systems, resulting in a sterile pro-inflammatory environment.
There are three distinct pharmacodynamic approaches to target histone-mediated inflammation, by reducing the release, neutralising or blocking histone signal transduction. Although these approaches have proven to provide significant protection from mortality in animal models of acute organ injury, further research is necessary to warrant their safe application in a clinical setting.
Ethical considerations
The authors declare that no ethical considerations were highlighted during the writing of this report.
Acknowledgments
The work was supported by BJA Fellowship and RCoA BOC chair grant.
Footnotes
Edited by Eleanor Silk
The authors declare no conflict of interest.
References
- Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med 2011; 17: 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol 2011; 187: 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol 2010; 28: 321–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Jaffer T, Eguchi S, Wang Z, Linkermann A, Ma D. Role of necroptosis in the pathogenesis of solid organ injury 2015; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury 2007; 38: 1336–1345. [DOI] [PubMed] [Google Scholar]
- Doenecke D, Karlson P, Kossel A, Miescher F, Schulze E, Steiger E et al. Albrecht Kossel and the discovery of histones. Trends Biochem Sci 1984; 9: 404–405. [Google Scholar]
- Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet 2009; 43: 559–599. [DOI] [PubMed] [Google Scholar]
- Zentner GE, Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nat Struct Mol Biol 2013; 20: 259–266. [DOI] [PubMed] [Google Scholar]
- Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol 2007; 14: 1008–1016. [DOI] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature 2007; 447: 396–398. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G, Groudine M. Controlling the double helix. Nature 2003; 421: 448–453. [DOI] [PubMed] [Google Scholar]
- Venkatesh S, Workman JL. Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol 2015; 16: 178–189. [DOI] [PubMed] [Google Scholar]
- Chen R, Kang R, Fan X-G, Tang D. Release and activity of histone in diseases. Cell Death Dis 2014; 5: e1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews AJ, Luger K. Nucleosome structure(s) and stability: variations on a theme. Annu Rev Biophys 2011; 40: 99–117. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature 2000; 403: 41–45. [DOI] [PubMed] [Google Scholar]
- Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hägele H, Lichtnekert J et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol 2012; 23: 1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Chen H-W, Evankovich J, Yan W, Rosborough BR, Nace GW et al. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J Immunol 2013; 191: 2665–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allam R, Kumar SVR, Darisipudi MN, Anders H-J. Extracellular histones in tissue injury and inflammation. J Mol Med (Berl) 2014; 92: 465–472. [DOI] [PubMed] [Google Scholar]
- Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 2013; 187: 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsman G, Zeerleder S, Luken BM. Extracellular histones, cell-free DNA, or nucleosomes: differences in immunostimulation. Cell Death Dis 2016; 7: e2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F et al. Extracellular histones are major mediators of death in sepsis. Nat Med 2009; 15: 1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier VJ, Tyler LN, Mannik M. Blood clearance kinetics and liver uptake of mononucleosomes in mice. J Immunol 1996; 156: 1151–1156. [PubMed] [Google Scholar]
- Holdenrieder S, Stieber P, Bodenmüller H, Busch M, Von Pawel J, Schalhorn A et al. Circulating nucleosomes in serum. Ann N Y Acad Sci 2001; 945: 93–102. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med 2009; 361: 1570–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B, Lütz-Meindl U, Kerschbaum HH. From the nucleus to the plasma membrane: translocation of the nuclear proteins histone H3 and lamin B1 in apoptotic microglia. Apoptosis 2014; 19: 759–775. [DOI] [PubMed] [Google Scholar]
- Wu D, Ingram A, Lahti JH, Mazza B, Grenet J, Kapoor A et al. Apoptotic release of histones from nucleosomes. J Biol Chem 2002; 277: 12001–12008. [DOI] [PubMed] [Google Scholar]
- Wickman GR, Julian L, Mardilovich K, Schumacher S, Munro J, Rath N et al. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ 2013; 20: 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R et al. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res 2011; 21: 290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li M, Stadler S, Correll S, Li P, Wang D et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009; 184: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol 2009; 30: 513–521. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- von Köckritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008; 111: 3070–3080. [DOI] [PubMed] [Google Scholar]
- Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 2010; 107: 15880–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009; 15: 623–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 2012; 7: e32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann M, Grailer JJ, Ruemmler R, Russkamp NF, Zetoune FS, Sarma JV et al. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J 2013; 27: 5010–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 2012; 18: 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007; 13: 463–469. [DOI] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 2008; 8: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekaney ML, Otto GP, Sossdorf M, Sponholz C, Boehringer M, Loesche W et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit Care 2014; 18: 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson PI, Windeløv NA, Rasmussen LS, Sørensen AM, Ostrowski SR. Blood levels of histone-complexed DNA fragments are associated with coagulopathy, inflammation and endothelial damage early after trauma. J Emerg Trauma Shock 2013; 6: 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai C, Kotani H, Miyao M, Ishida T, Jemail L, Abiru H et al. Circulating extracellular histones are clinically relevant mediators of multiple organ injury. Am J Pathol 2016; 186: 829–843. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, Nagel D, Schalhorn A, Heinemann V, Wilkowski R, von Pawel J et al. Clinical relevance of circulating nucleosomes in cancer. Ann N Y Acad Sci 2008; 1137: 180–189. [DOI] [PubMed] [Google Scholar]
- Hou W, Zhang Q, Yan Z, Chen R, Zeh Iii HJ, Kang R et al. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis 2013; 4: e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi N, Radic M. Citrullination of autoantigens implicates NETosis in the induction of autoimmunity. Ann Rheum Dis 2014; 73: 483–491. [DOI] [PubMed] [Google Scholar]
- Monach PA, Hueber W, Kessler B, Tomooka BH, BenBarak M, Simmons BP et al. A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci USA 2009; 106: 15867–15872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratesi F, Dioni I, Tommasi C, Alcaro MC, Paolini I, Barbetti F et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann Rheum Dis 2014; 73: 1414–1422. [DOI] [PubMed] [Google Scholar]
- Bosch X. Systemic lupus erythematosus and the neutrophil. N Engl J Med 2011; 365: 758–760. [DOI] [PubMed] [Google Scholar]
- Leffler J, Martin M, Gullstrand B, Tydén H, Lood C, Truedsson L et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 2012; 188: 3522–3531. [DOI] [PubMed] [Google Scholar]
- Migliorini A, Anders H-J. A novel pathogenetic concept-antiviral immunity in lupus nephritis. Nat Rev Nephrol 2012; 8: 183–189. [DOI] [PubMed] [Google Scholar]
- Duce JA, Smith DP, Blake RE, Crouch PJ, Li Q-X, Masters CL et al. Linker histone H1 binds to disease associated amyloid-like fibrils. J Mol Biol 2006; 361: 493–505. [DOI] [PubMed] [Google Scholar]
- Chaput C, Zychlinsky A. Sepsis: the dark side of histones. Nat Med 2009; 15: 1245–1246. [DOI] [PubMed] [Google Scholar]
- De Meyer SF, Suidan GL, Fuchs TA, Monestier M, Wagner DD. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol 2012; 32: 1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilthorpe JD, Oozeer F, Nash J, Calvo M, Bennett DL, Lumsden A et al. Extracellular histone H1 is neurotoxic and drives a pro-inflammatory response in microglia. F1000Res 2013; 2: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel B, Shinagawa H, Hofmann U, Ertl G, Frantz S. Acute DNase1 treatment improves left ventricular remodeling after myocardial infarction by disruption of free chromatin. Basic Res Cardiol 2015; 110: 15. [DOI] [PubMed] [Google Scholar]
- Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L et al. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 2014; 123: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest 2012; 122: 2661–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Liu Y, Li F, Ren F, Chen D, Li X et al. Circulating histones exacerbate inflammation in mice with acute liver failure. J Cell Biochem 2013; 114: 2384–2391. [DOI] [PubMed] [Google Scholar]
- Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology 2011; 54: 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 2015; 62: 600–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X, Cheng Z, Liu T, Tang Z, Huang W, Szatmary P et al. Circulating histone levels reflect disease severity in animal models of acute pancreatitis. Pancreas 2015; 44: 1089–1095. [DOI] [PubMed] [Google Scholar]
- O’Neill LAJ, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol 2013; 13: 453–460. [DOI] [PubMed] [Google Scholar]
- Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev 2012; 249: 158–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004; 4: 499–511. [DOI] [PubMed] [Google Scholar]
- Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 2011; 118: 1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood 2011; 118: 3708–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Li L, Liu J, Lv B, Chen F. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-κB and AP-1. Thromb Res 2016; 137: 211–218. [DOI] [PubMed] [Google Scholar]
- Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJD, Kirschning CJ et al. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 2005; 115: 2894–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology 2010; 51: 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature 2012; 481: 278–286. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell 2010; 140: 821–832. [DOI] [PubMed] [Google Scholar]
- Pereira LF, Marco FM, Boimorto R, Caturla A, Bustos A, De la Concha EG et al. Histones interact with anionic phospholipids with high avidity; its relevance for the binding of histone-antihistone immune complexes. Clin Exp Immunol 1994; 97: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams ST, Zhang N, Dart C, Wang SS, Thachil J, Guan Y et al. Human CRP defends against the toxicity of circulating histones. J Immunol 2013; 191: 2495–2502. [DOI] [PubMed] [Google Scholar]
- Deb P, Sharma S, Hassan KM, Bakhai A, Heuschmann PU, Berger K et al. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology 2010; 17: 197–218. [DOI] [PubMed] [Google Scholar]
- Geiger S, Holdenrieder S, Stieber P, Hamann GF, Bruening R, Ma J et al. Nucleosomes in serum of patients with early cerebral stroke. Cerebrovasc Dis 2006; 21: 32–37. [DOI] [PubMed] [Google Scholar]
- Geiger S, Holdenrieder S, Stieber P, Hamann GF, Bruening R, Ma J et al. Nucleosomes as a new prognostic marker in early cerebral stroke. J Neurol 2007; 254: 617–623. [DOI] [PubMed] [Google Scholar]
- Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 2015; 35: 888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton SJ, Russelakis-Carneiro M, Betmouni S, Perry VH. Non-nuclear histone H1 is upregulated in neurones and astrocytes in prion and Alzheimer’s diseases but not in acute neurodegeneration. Neuropathol Appl Neurobiol 1999; 25: 425–432. [DOI] [PubMed] [Google Scholar]
- Burke AP, Virmani R. Pathophysiology of acute myocardial infarction. Med Clin North Am 2007; 91: 553–572 ix. [DOI] [PubMed] [Google Scholar]
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353: 1685–1693. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012; 122: 2731–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TR, Matute-Bello G. Experimental models and emerging hypotheses for acute lung injury. Crit Care Clin 2011; 27: 735–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B, von der Ohe M, Schulze C, Bian S, Makhina T, Loers G et al. Functional role of the interaction between polysialic acid and extracellular histone H1. J Neurosci 2010; 30: 12400–12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. The role of the lung in postinjury multiple organ failure. Surgery 2005; 138: 749–57–8. [DOI] [PubMed] [Google Scholar]
- Martin AM, Soloway HB, Simmons RL. Pathologic anatomy of the lungs following shock and trauma. J Trauma 1968; 8: 687–699. [DOI] [PubMed] [Google Scholar]
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997; 389: 251–260. [DOI] [PubMed] [Google Scholar]
- Abakushin DN, Zamulaeva IA, Poverenny AM. Histones evoke thymocyte death in vitro; histone-binding immunoglobulins decrease their cytotoxicity. Biochemistry (Mosc) 1999; 64: 693–698. [PubMed] [Google Scholar]
- Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost 2011; 9: 1795–1803. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Shyamala Devi CS. Effect of histone H1 on the cytosolic calcium levels in human breast cancer MCF 7 cells. Life Sci 2005; 76: 2631–2641. [DOI] [PubMed] [Google Scholar]
- Kleine TJ, Lewis PN, Lewis SA. Histone-induced damage of a mammalian epithelium: the role of protein and membrane structure. Am J Physiol Cell Physiol 1997; 273: C1925–C1936. [DOI] [PubMed] [Google Scholar]
- Akamatsu N, Sugawara Y, Kokudo N. Acute liver failure and liver transplantation. Intractable Rare Dis Res 2013; 2: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 2011; 7: 189–200. [DOI] [PubMed] [Google Scholar]
- Kanagasundaram NS. Pathophysiology of ischaemic acute kidney injury. Ann Clin Biochem An Int J Biochem Lab Med 2015; 52: 193–205. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 2011; 121: 4210–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Okusa MD. Dying cells and extracellular histones in AKI: beyond a NET effect? J Am Soc Nephrol 2012; 23: 1275–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 2007; 117: 2847–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-del Castillo C, Harringer W, Warshaw AL, Vlahakes GJ, Koski G, Zaslavsky AM et al. Risk factors for pancreatic cellular injury after cardiopulmonary bypass. N Engl J Med 1991; 325: 382–387. [DOI] [PubMed] [Google Scholar]
- Gullo L, Cavicchi L, Tomassetti P, Spagnolo C, Freyrie A, D’Addato M. Effects of ischemia on the human pancreas. Gastroenterology 1996; 111: 1033–1038. [DOI] [PubMed] [Google Scholar]
- Kang R, Zhang Q, Hou W, Yan Z, Chen R, Bonaroti J et al. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology 2014; 146: 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Lotze MT, Zeh HJ, Billiar TR, Tang D. Cell death and DAMPs in acute pancreatitis. Mol Med 2014; 20: 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu Z, Liu B, Zhao T, Chong W, Wang Y et al. Citrullinated histone H3: a novel target for the treatment of sepsis. Surgery 2014; 156: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol 2015; 11: 189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Jenne CN, Surewaard BGJ, Thanabalasuriar A, Lee W-Y, Sanz M-J et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun 2015; 6: 6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutcher ME, Xu J, Vilardi RF, Ho C, Esmon CT, Cohen MJ. Extracellular histone release in response to traumatic injury: implications for a compensatory role of activated protein C. J Trauma Acute Care Surg 2012; 73: 1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí-Carvajal AJ, Solà I, Lathyris D, Cardona AF. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst Rev 2012; (3): CD004388. [DOI] [PubMed]
- Fernández JA, Xu X, Liu D, Zlokovic BV, Griffin JH. Recombinant murine-activated protein C is neuroprotective in a murine ischemic stroke model. Blood Cells Mol Dis 2003; 30: 271–276. [DOI] [PubMed] [Google Scholar]