Abstract
Antiplatelet therapy is the cornerstone of the pharmacologic management of patients with acute coronary syndrome (ACS). Over the last years, several studies have evaluated old and new oral or intravenous antiplatelet agents in ACS patients. In particular, research was focused on assessing superiority of two novel platelet ADP P2Y12 receptor antagonists (i.e., prasugrel and ticagrelor) over clopidogrel. Several large randomized controlled trials have been undertaken in this setting and a wide variety of prespecified and post-hoc analyses are available that evaluated the potential benefits of novel antiplatelet therapies in different subsets of patients with ACS. The aim of this document is to review recent data on the use of current antiplatelet agents for in-hospital treatment of ACS patients. In addition, in order to overcome increasing clinical challenges and implement effective therapeutic interventions, this document identifies all potential specific care pathway for ACS patients and accordingly proposes individualized therapeutic options.
Keywords: Acute coronary syndromes, Non-ST elevation myocardial infarction, ST-elevation myocardial infarction, Ticagrelor, Prasugrel, Clopidogrel, Aspirin, Cangrelor, Bivalirudin, Heparins, Glycoprotein IIb/IIIa inhibitors, Oral anticoagulant agents, Vorapaxar
Document Revisors: Roberto Caporale, Giuseppe Patti, Roberta Rossini, Pasquale Caldarola, Giovanna Geraci, and Serafina Valente
Consensus Document Approval Faculty in appendix
Introduction
Practicing physicians may need systematic evidence-based guidance to facilitate decision making in delivering clinical care. One approach for the provision of such guidance is the use of clinical pathways as implementation tools. Clinical pathways represent structured intervention plans containing essential steps in the care of patients with specific clinical problems. They are usually developed by translating evidence-based guidelines into stepped care protocols for application in clinical practice. In accordance with such a concept, the main aim of this document is to provide clear, practical, and evidence-based indications for an effective management of antithrombotic therapy (anticoagulant and antiplatelet therapy) in the ever changing clinical scenario of acute coronary syndromes (ACSs).
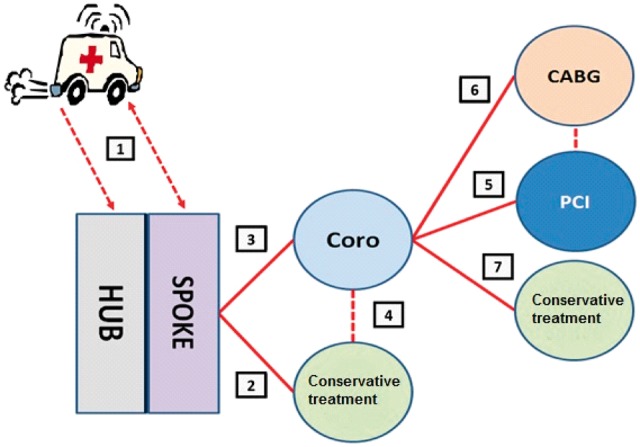
All therapeutic options outlined in this document are consistent with the recommendations of the European Society of Cardiology guidelines1,2 and are carefully considered for both indications and contraindications of each specific drug (Table 1). However, in order to overcome increasing clinical challenges and implement effective therapeutic interventions, this document identifies all potential specific care pathway for ACS patients and accordingly proposes individualized therapeutic options. In particular, as shown in Figure 1, the following care pathways have been preliminary identified:
Table 1.
Indications, contraindications, and precautions in using main antithrombotic drugs in ACSs
| Drug | Mode of action | Indications | Contraindications | Precautions |
|---|---|---|---|---|
| Clopidogrel | Irreversible inhibitor of the platelet P2Y12 receptor for ADP | (with ASA):
|
|
|
| Prasugrel | Irreversible inhibitor of the platelet P2Y12 receptor for ADP | (With ASA):
|
|
|
| Ticagrelor | Reversible inhibitor of the platelet P2Y12 receptor for ADP | (With ASA):
|
|
|
| Abciximab | Inhibitor of platelet glycoprotein IIb/IIIa receptors | (With UFH and ASA)
|
|
|
| Tirofiban | Inhibitor of platelet glycoprotein IIb/IIIa receptors | NSTE-ACS |
|
|
| Eptifibatide | Inhibitor of platelet glycoprotein IIb/IIIa receptors | (With UFH and ASA)
|
|
|
| UFH | Indirect thrombin inhibitor | Prophylaxis/therapy of venous and arterial thromboembolic disease |
|
Concomitant ASA |
| Enoxaparin | Indirect FXa and thrombin inhibitor |
|
|
|
| Fondaparinux | Indirect FXa inhibitor |
|
|
Acute hepatic impairment |
| Bivalirudin | Direct thrombin inhibitor | (With ASA and clopidogrel)
|
|
|
ADP, adenosine diphosphate; ASA, acetylsalicylic acid; NSTE-ACS, non-ST-segment elevation acute coronary syndrome; STEMI, ST-segment elevation myocardial infarction; PPI, proton pump inhibitor; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack; NSAID, non-steroidal anti-inflammatory drug; OAT, oral anticoagulant therapy; CABG, coronary artery bypass grafting; SAND, sinoatrial node disease; AVB, atrioventricular block; COPD, chronic obstructive pulmonary disease; UFH, unfractionated heparin; SBP, systolic blood pressure; DBP, diastolic blood pressure; eCrCl, estimated creatinine clearance; eGFR, estimated glomerular filtration rate.
Figure 1.
Patient care pathways proposed in the consensus paper. CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention.
Pathway 1: pre-hospital management of ACS;
Pathway 2: initially conservative management of ACS;
Pathway 3: management with immediate referral for coronary angiography;
Pathway 4: management with referral for coronary angiography after an initially conservative treatment;
Pathway 5: management in case of percutaneous coronary intervention (PCI);
Pathway 6: management in case of surgical myocardial revascularization procedure; and
Pathway 7: management with definite conservative treatment after coronary angiography.
Pathway 1: pre-hospital treatment of ACS
A pre-hospital pharmacological treatment is extremely important in ACS patients and becomes crucial in patients with confirmed diagnosis of ST-segment elevation myocardial infarction (STEMI), particularly within the framework of a well-organized emergency medical network.3 This pathway will therefore focus on the use of antithrombotic drugs for the pre-hospital management of STEMI patients.
Antithrombotic drugs, in association with coronary revascularization, are used in the management of STEMI to enhance myocardial reperfusion before a percutaneous coronary intervention (PCI) or to achieve an appropriate active drug level at the time of the mechanical reperfusion. The combination of PCI with early pharmacological reperfusion therapy for reducing the total ischaemic time and minimizing the detrimental effects of delayed revascularization has long been considered a fascinating pathophysiological approach and a useful practical solution to extend the benefits of primary PCI (pPCI) to the majority of STEMI patients.4 Recent randomized trials have shown that pre-hospital fibrinolysis in specific care settings and STEMI patient categories can be a reasonable therapeutic alternative to pPCI.5 On the other hand, certain drugs—based on their route of administration and relatively reduced bioavailability—are administered to reduce recurrent ischaemic events as soon as an STEMI diagnosis is confirmed. These drugs have no immediate effect on reperfusion, but when initiated early, they significantly reduce the incidence of recurrent ischaemic events compared with delayed administration.
Antiplatelet agents
To date, no trial has compared the timing of aspirin (ASA) administration with outcome in STEMI patients. However, the guidelines recommend the earliest possible administration of ASA at an initial dose of 150–325 mg.1
With regard to P2Y12 inhibitors, the latest guidelines on myocardial revascularization recommend the administration of a P2Y12 inhibitor upon first medical contact (Class 1 recommendation, level of evidence B). For many years, international guidelines have also recommended the pre-hospital administration of clopidogrel with a Class 1 recommendation, level of evidence C (consensus of expert opinion).1 No randomized clinical trials have been conducted on the upstream vs. downstream use of clopidogrel in STEMI patients. Indeed, the documented benefits of pre-treatment with clopidogrel in other patient groups with ACS have been applied tout court to STEMI patients. Data supporting the early use of clopidogrel are available from a meta-analysis6 of clinical studies that evaluated the association of 300 mg clopidogrel administered at a median time of about 2 h prior to pPCI with a decreased rate of mortality and reinfarction. Retrospective data from large registries or posthoc analyses of randomized clinical trials showed a beneficial effect of upstream treatment with 300 mg clopidogrel in STEMI patients on the composite endpoint of ischaemia and mortality.7 In a recent retrospective study,8 the administration of clopidogrel in the emergency department was associated with an improved long-term clinical outcome compared with the administration in the catheterization laboratory. Similarly, a posthoc analysis of the randomized HORIZONS-AMI trial suggested that upstream administration of a 600 mg loading dose of clopidogrel would boost such beneficial effect with no further increase in the rate of major bleeding.9 Of note, a 600 mg clopidogrel load has been associated with reduction of the infarct size when compared with 300 mg dose in patients undergoing pPCI and therefore the higher regimen merits recommendation in this setting.10
The recent European guidelines indicate the new platelet P2Y12 receptor inhibitors as first-choice drugs in the management of STEMI patients,1 as they provide for improved long-term clinical outcomes. However, a late antiplatelet effect seems to be associated also with this oral antiplatelet drug class. Thus, it is a reasonable assumption that upstream administration may result in improved beneficial clinical effect in terms of reduction of early, recurrent ischaemic events.
In the TRITON-TIMI 38 (TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38) trial randomization to 60 mg prasugrel vs. 300 mg clopidogrel before assessment of the coronary tree was allowed in STEMI patients who presented within 12 h of symptom onset, if pPCI was intended.11 Overall, in the STEMI cohort of the TRITON-TIMI 38 trial (n = 3534), an average time from symptom onset to drug administration of 228 min and an average time from symptom onset to pPCI of 252 min were registered; only 31% of these patients could benefit from prasugrel treatment before coronary angiography evaluation.12 Although there are no published data available on the patient population randomized to early treatment, it is assumed that the same clear benefits of prasugrel vs. clopidogrel observed in STEMI patients, with regard to the primary composite endpoint (death, myocardial infarction, and stroke) and stent thrombosis without associated increase in major bleeding, apply to that patient population too. On the other hand, there are no data from randomized clinical data comparing the efficacy and safety of pre-hospital treatment strategy with prasugrel vs. in-hospital prasugrel treatment of STEMI patients. The multinational, multicentre, prospective, MULTIPRAC (MULTInational non-interventional study of patients with ST-segment elevation myocardial infarction treated with PRimary Angioplasty and Concomitant use of upstream antiplatelet therapy with prasugrel or clopidogrel) registry was conducted to gain insights into the use patterns and outcomes of pre-hospital initiation of oral antiplatelet agents on top of ASA with prasugrel or clopidogrel.13 In this study, 2053 STEMI patients were enrolled. Pre-hospital use of prasugrel increased from 12.5% to 67.1% at study end. The major adverse cardiac events (MACE) rate was 1.6% in prasugrel-treated patients vs. 2.3% in clopidogrel-treated patients [adjusted odds ratio (OR) 0.749, 95% confidence interval (CI) [0.285–1.968]].13 Non-coronary artery bypass graft (non-CABG) bleeding occurred in 4.1% of prasugrel-treated patients vs. 6.1% of clopidogrel-treated patients (adjusted OR 0.686 [0.349–1.349]). Pre-percutaneous coronary intervention TIMI flow 2–3 was seen in 38.7% treated with prasugrel vs. 35.6% with clopidogrel (adjusted OR 1.170 [0.863–1.585]). Post-PCI ST-segment resolution ≥50%, was 71.6% with prasugrel vs. 65.0% with clopidogrel (adjusted OR 1.543 [1.138–2.093], P = 0.0052).13
Although there was no pre-hospital randomization to 180 mg ticagrelor vs. 300 mg or 600 mg clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) trial, the first dose of the study drug was administered as early as possible after patient recruitment and always before admission to the catheterization laboratory, with a median time of 25 min from first dose administration of the drug to PCI, for both treatment arms.14 Therefore, the clinical beneficial outcomes observed in the ticagrelor arm vs. clopidogrel can be reasonably extended to the subgroup of over 7000 STEMI patients randomized in the PLATO trial.14 The recent ATLANTIC (Administration of Ticagrelor in the cath Lab or in the Ambulance for New ST elevation myocardial Infarction to open the Coronary artery) study was the first randomized trial to compare the efficacy and safety of the pre-hospital administration of dual antiplatelet therapy (DAPT) with ticagrelor vs. in-hospital ticagrelor treatment.15 Approximately 1800 STEMI patients <6 h from symptom onset undergoing pPCI were randomized in this study. Although there was no difference in the primary outcome measures (i.e. a mechanical rather than clinical endpoints: achievement of TIMI flow 3 at initial angiography or ST-segment elevation resolution ≥70% at pre-PCI electrocardiogram) among the two treatment groups, the study provided further data on pre-hospital treatment with ticagrelor in STEMI patients,15 particularly showing that pre-treatment with ticagrelor is not associated with increased haemorrhagic risk (according to all applied definitions). It also showed that the drug significantly reduced (1.2% vs. 0.2%; P = 0.02) acute stent thrombosis (secondary endpoint of the study).15
The use of glycoprotein IIb/IIIa inhibitors (GPIs), particularly abciximab, in STEMI patients was associated with a reduction in the relative mortality risk and of composite endpoints of ischaemic events without increasing major bleeding episodes.16 However, the pre-hospital use of GPIs in STEMI, particularly when associated with high clopidogrel doses,17 is controversial; although the ADMIRAL (Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-term Follow-up)18 trial supports pre-hospital administration of abciximab, the recent large, randomized FINESSE trial (Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events)19 did not show any significantly improved clinical outcomes with a routine, early administration of GPIs. Conversely, the angiographic evaluation in the EUROTRANSFER Registry documented an improved incidence of TIMI flow 2 and 3 in the STEMI patients group treated with upstream abciximab, particularly in early onset patients20; this study also indicated improvement in long-term survival with abciximab pre-treatment, particularly in STEMI patients with early onset and low risk.21 Another GPI, tirofiban, when administered in the pre-hospital setting as double bolus in association with 600 mg clopidogrel, ASA, and heparin showed beneficial effects in terms of an average reduction of the ST-segment 1 h after pPCI compared with placebo in the ON-TIME 2 randomized trial (Ongoing Tirofiban In Myocardial infarction Evaluation 2).22 In a recent pre-specified angiography analysis of the ON-TIME 2 trial, the incidence of TIMI flow 2 and 3 and the reduction of intracoronary thrombus burden at initial coronary angiography were significantly higher in the group randomized to pre-hospital tirofiban compared with placebo.23
Anticoagulants
Current STEMI guidelines recommend the use of unfractionated heparin (UFH) with an intravenous bolus of 100 U/kg (to downgrade to 60 U/kg when associated with GPIs) in patients scheduled for pPCI,1 although there are no data from comparative trials of UFH vs. placebo to support such recommendation.
Low-molecular-weight heparins (LMWHs) present less biological variability compared with UFH and seem to offer a better clinical efficacy in STEMI patients when administered intravenously.24,25 In the recent ATOLL (Acute STEMI Treated with primary angioplasty and intravenous enoxaparin Or UFH to Lower ischaemic and bleeding events at short and Long-term follow-up) trial, ∼900 STEMI patients treated with pPCI were randomized—over 70% in a pre-hospital setting—to receive an intravenous bolus of enoxaparin (0.5 mg/kg) or UFH.26 In the study, enoxaparin was not superior to UFH in reducing the primary composite endpoint (death, complication of myocardial infarction, procedural failure, and major bleeding). However, enoxaparin was associated with a significant reduction in the secondary endpoint (a composite of death, recurrent ACS, or urgent revascularization) and a significant reduction in individual endpoints, including mortality, major haemorrhage, and urgent revascularization.26
There are no studies specifically designed to assess the efficacy of the pre-hospital administration of fondaparinux, an activated factor X inhibitor. In the OASIS-6 (Organisation to Assess Strategies in Ischaemic Syndromes) trial,27 the impact of fondaparinux on mortality and reinfarction was assessed in over 12 000 STEMI patients, stratified in two groups based on their eligibility to receive UFH treatment. Fondaparinux was associated with a significant reduction in the incidence of the primary endpoint compared with UFH/placebo (9.7% vs. 11.2%; P = 0.008).27 Regarding the treatment, fondaparinux proved to be superior to placebo/UFH in patients who received thrombolysis (mainly streptokinase) or conservative treatment, whereas it proved to be inferior to UFH in pPCI patients.27
The efficacy of bivalirudin in STEMI patients was assessed in the HORIZONS AMI (Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction) trial28; the treatment with this direct thrombin inhibitor resulted in better net clinical outcomes (including reduction in mortality and bleeding) compared with UFH in association with the GPIs (see Pathway 6). The EUROMAX (European Ambulance Acute Coronary Syndrome Angiography) study29 recently evaluated the efficacy of a pre-hospital administration of a prolonged bivalirudin infusion (initiated 50 min prior to pPCI and continued for at least 4 h thereafter) in comparison with the use of heparin and routine or bailout GPIs (∼70% of the cases) in ∼2200 patients with STEMI undergoing pPCI. The results showed that bivalirudin was superior at 30 days in the composite endpoint of death and major bleeding not associated with CABG (8.4% vs. 5.1%, P = 0.03), mainly due to a decrease in the rates of major bleeding events (6.1% vs. 2.7%, P < 0.001).29 Conversely, bivalirudin showed a significant increase in the frequency of stent thrombosis (1.6% vs. 0.5%, P < 0.02) compared with the usual heparin regimen. Moreover, a recent aggregated data analysis of the HORIZONS-AMI and EUROMAX trials also confirmed a net benefit with bivalirudin in STEMI management with a documented significant increase in stent thrombosis vs. a combination of UFH and GPIs.30 The incidence of acute thrombosis is apparently not reduced by the upstream use of the newer oral antiplatelet agents (employed in about 50% of the cases). However, the prolongation of the bivalirudin infusion (1.75 mg/kg/h) seems to be associated with a lower incidence of this alarming complication.31 These data are yet to be confirmed through large randomized clinical trials. Of note, in a recent meta-analysis on overall 22 controlled randomized trials and 22 434 patients undergoing pPCI use of LMWH plus GPI provided the best protection from adverse events compared with UFH, fondaparinux, and bivalirudin, apparently without increase in bleeding complications; among the single-drug strategies, a similar degree of prevention from ischaemic events was found with UFH, fondaparinux, and bivalirudin; the latter, however, was associated with a 42% relative reduction in the risk of major bleeding vs. UFH alone.32
Executive summary
| In patients with a confirmed diagnosis of ACS (particularly STEMI) admitted in a structured and efficient network of emergency transport, it is reasonable to start antithrombotic therapies (DAPT + an anticoagulant agent) in the pre-hospital setting. |
| The bleeding risk beside the ischaemic risk should be carefully evaluated in all patients with ACS. |
| Pre-hospital thrombolysis is a valid alternative to pPCI in patients with a confirmed diagnosis of STEMI in whom pPCI cannot be performed within 1 h, a short time interval between symptom onset and diagnosis (<3 h), as long as followed by PCI at least 3 h after successful thrombolysis. |
| In patients treated with pre-hospital thrombolysis, it is recommended the use of clopidogrel (300 mg loading dose (LD)) and enoxaparin (30 mg intravenous bolus, followed by 1 mg/kg subcutaneous injection every 12 h in patients <75 years or only 0.75 mg/kg subcutaneous injection every 12 h in patients ≥75 years), with fondaparinux or UFH as second choice. |
In patients scheduled for pPCI:
|
Pathway 2: conservative treatment (unknown coronary anatomy)
International registries showed that about 30–50% of patients presented with ACS diagnosis are essentially subjected to conservative medical management.33–37 Recent data from the Italian EYESHOT Registry (EmploYEd antithrombotic therapies in patients with acute coronary Syndromes HOspitalised in iTalian cardiac care units) showed that about 42% of the study patients with confirmed non-ST-elevation ACS diagnosis did not receive revascularization at the time of admission.38 The risk associated with this patient group is believed to be too high to allow them to undergo angiography and possible revascularization. However, these patients have a much higher rate of in-hospital and long-term mortality.33–38 Besides a higher risk profile and lack of revascularization, this patient group does not apparently receive an optimal pharmacological treatment, as lower administration of antithrombotic drugs compared with guideline recommendations has been observed.33–38 These findings may at least in part explain the high frequency of long-term fatal events.
Antiplatelet agents
The use of ASA represents the basis for antiplatelet therapy in ACSs. ASA administration is based on the results of almost 30-year-old studies in patients with unstable angina. In this clinical setting, ASA significantly reduced recurrent infarction and mortality rates compared with placebo.39–41 Over the last decades, several studies focused on the association of ASA with thienopyridines in the acute and long-term treatment of ACS patients.
Ticlopidine was the first thienopyridine associated with ASA. This molecule was subsequently replaced with clopidogrel due to its higher bioavailability, easier use, and reduced incidence of adverse events incidence (mainly neutropenia, rush, and diarrhoea). ACS treatment with clopidogrel was validated in the CURE (Clopidogrel in Unstable angina to prevent Recurrent Events) trial, which was specifically designed to evaluate a conservative approach, and included primarily centres in which there was no routine policy of early use of angiography and revascularization.42 The CURE trial randomized over 12 500 patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) to receive clopidogrel (a 300 mg loading dose, followed by a 75 mg maintenance dose) or placebo in addition to ASA for up to 12 months. The incidence of both primary study endpoints (death from cardiovascular causes, myocardial infarction, or stroke and death from cardiovascular causes, myocardial infarction, stroke, or refractory ischaemia) was significantly reduced with clopidogrel administration, even though there was a significant increase of non-fatal major bleeding complications.42
Regarding the use of new inhibitors of the platelet P2Y12 receptor in the conservative strategy, relevant data on ticagrelor are available from a pre-specified analysis of the PLATO trial,43 which assessed patients (28% of the total study population) who were first initiated to a conservative management (although about 25% of this population later received percutaneous or surgical revascularization). In this analysis, the incidence of the primary endpoint (cardiovascular death, myocardial infarction, and stroke) was lower with ticagrelor than with clopidogrel (12.0% vs. 14.3%; HR: 0.85, 95% CI: 0.73–1.00; P = 0.04), and overall mortality was also reduced (6.1% vs. 8.2%; HR: 0.75, 95% CI: 0.61–0.93; P = 0.01).43
There are available data on the efficacy and safety of prasugrel in the conservative treatment from the recent TRILOGY ACS (Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes) trial.44 In this study, prasugrel was not associated with any beneficial effect in comparison with clopidogrel: at 30 months, the primary endpoint of death from cardiovascular causes, myocardial infarction, or stroke occurred in 13.9% of the prasugrel patient group and in 16.0% of the clopidogrel cohort (HR: 0.91; 95% CI: 0.79–1.05; P = 0.21). The study provided a particularly interesting efficacy outcome: a posthoc analysis on the drug effect showed similar occurrence of ischaemic events on the two arms within the first 12 months of the study and a trend towards a superiority of prasugrel in the following time frame up to 30 months: 0.99 (0.84–1.16) vs. 0.72 (0.54–0.97) (P = 0.07).44 Such potential long-term effect was identified in previous analyses on ACS patients treated conservatively45–47; this has, however, not yet been explained and may be the objective of further studies or trials with longer follow-up phases. With regard to safety in TRILOGY, major bleeding (according to TIMI criteria) in the two study groups occurred with similar frequency among patients aged <75 years: 2.1% with prasugrel vs. 1.5% with clopidogrel (HR: 1.31, 95% CI: 0.81–2.11, P = 0.27). Conversely, the rates of major or minor bleeding were higher in the prasugrel group: 3.3% vs. 2.1% (HR: 1.54; 95% CI: 1.06–2.23; P = 0.02).44 Considering the posthoc nature of the above-mentioned TRILOGY analysis on the long-term outcome, the results should be cautiously interpreted, as confirmed by the fact that, according to the product information sheet, the use of prasugrel is contraindicated in ACS patients receiving a conservative strategy.
Anticoagulants
UFH and LMWHs have been the most frequently used anticoagulants in the clinical practice for many decades. Among these, enoxaparin is certainly the most widely studied agent in randomized clinical trials.48,49 The ESSENCE (Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events) and TIMI 11B (Thrombolysis in Myocardial Infarction 11B) trials were performed in a period when conservative treatment was predominant; they were the first studies that compared UFH with enoxaparin in ACS: both trials showed a significant reduction of the primary composite endpoint of death and myocardial infarction in patients treated with LMWHs, without increase in major bleeding complications.50–52 The subsequent trials were conducted in a setting of more invasive strategies and with a more frequent usage of combined platelet aggregation inhibitors, including the A to Z (Aggrastat to Zocor)53 and SYNERGY (Superior Yield of the New Strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa Inhibitors) trials.54 However, these studies showed no significant reduction of the composite endpoint of death and myocardial infarction in the enoxaparin arm and revealed a trend towards increased bleeding events; however, in both investigations, a reduction of ischaemic adverse events was observed in ACS patients naive to antithrombotic treatment prior to randomization (∼25% of recruited patients). For the first time, this result highlighted the importance of keeping different anticoagulants during hospitalization separate instead of combining them. A subsequent meta-analysis also including the ACUTE II (Antithrombotic Combination Using Tirofiban and Enoxaparin II) and INTERACT (Integrilin and Enoxaparin Randomized Assessment of Acute Coronary Syndrome Treatment) trials confirmed the superiority of enoxaparin compared with UFH in terms of death and infarction at 30 days (10.1% vs. 11.0%; OR: 0.91; 95% CI: 0.83–0.99) in ∼22 000 patients with ACS, particularly in those who did not receive antithrombotic treatment prior to randomization (8.0% vs. 9.4%; OR: 0.81; 95% CI: 0.70–0.94), without any increase in major bleeding risk.48
Fondaparinux proved its non-inferiority compared with enoxaparin with respect to the primary efficacy endpoint (death, myocardial infarction, or refractory ischaemia) at 9 days (5.8% vs. 5.7%) in the OASIS-5 clinical trial.55 In this study, over 20 000 NSTE-ACS patients were randomized to receive either fondaparinux, at a dose of 2.5 mg once daily for 8 days by subcutaneous injection, or enoxaparin (1 mg/kg body weight twice daily by subcutaneous injection, with a dose reduction to 1 mg/kg once daily in cases of severe renal impairment) for 2–8 days. The incidence of major bleeding at 9 days was markedly lower with fondaparinux compared with enoxaparin (2.2% vs. 4.1%; HR: 0.52; P < 0.001) and was constant throughout the study and consistent among all the analysed subgroups. At the same time, a significant reduction in mortality with fondaparinux compared with LMWHs was observed at 30 days (2.9% vs. 3.5%; HR: 0.83; P = 0.02) and 6 months (5.8% vs. 6.5%; HR: 0.89; P = 0.05).55 A similar safety and efficacy beneficial outcome compared with treatment with heparin was also observed in STEMI patients undergoing pharmacological treatment from the OASIS-6 trial.56,57
Executive summary
| Antiplatelet agents |
[A] STEMI
|
[B] NSTEMI
|
| Anticoagulant agents |
[A] STEMI
|
[B] NSTEMI
|
Pathways 3 and 4: patient referral for coronary angiography
Over the last decades, the percentage of ACS patients referred for coronary angiography markedly increased.58–60 In Italy, the frequency of coronary angiography in ACS patients apparently depends more on the availability of a catheterization laboratory in the hospital of admission rather than on the patient’s ischaemic risk profile.38 In addition to this difference in strategic approach, which clearly differs according to the various hospital facilities, the time between diagnosis and invasive treatment initiation also varies considerably depending on the availability of a catheterization laboratory. In this context, therefore, an adequate antithrombotic pre-treatment and the selection of the appropriate pharmaceutical product become increasingly important in order to reduce the incidence of recurrent ischaemic events.
Antiplatelet agents
The risk/benefit profile of an early administration of a loading dose of thienopyridine is still debated (whereas early administration of ASA is generally approved, regardless of the strategy adopted initially). Based on the results of the EYESHOT Registry, which evidenced an 8-day median time between hospital admission and bypass intervention (when it was needed)—compatible with the programmed discontinuation of any P2Y12 receptor inhibitors38—the potential bleeding risk associated with pre-treatment in patients referred for CABG after coronary angiography appears unsupported.
With regard to pre-treatment with oral antiplatelet agents in STEMI patients, reference should be made to the paragraph on pre-hospital management in Pathway 1. In the CREDO (Clopidogrel for the Reduction of Events During Observation) trial, clopidogrel was associated with a clinical benefit in NSTE-ACS patients with a >6 h interval between diagnosis and coronary angiography.61 However, a meta-analysis of the PCI-CURE (PCI-Clopidogrel in Unstable angina to prevent Recurrent Events), CREDO, and PCI-CLARITY (PCI-Clopidogrel as Adjunctive Reperfusion Therapy) trials showed that clopidogrel pre-treatment was beneficial compared with the administration of the loading dose in the catheterization laboratory in terms of both infarction prior to PCI and mortality and infarction following PCI.62 Another meta-analysis of six randomized clinical trials and nine observational studies on close to 38 000 patients with ACS or stable chronic angina recently showed that clopidogrel pre-treatment has a beneficial effect on major cardiac events (9.8% vs. 12.3%; OR: 0.77; 95% CI: 0.66–0.89; P < 0.001), although no effects on overall mortality was observed.63
The PLATO trial was not a study aimed to evaluate upstream vs. downstream initiation of antiplatelet therapy in patients with ACS; it explored the benefit of ticagrelor compared with clopidogrel pre-treatment as patients were treated before coronary assessment and independently from the adopted strategy.64 This trial showed the superiority of ticagrelor when compared with clopidogrel in reducing clinical adverse events (death, myocardial infarction, or stroke) at 1 year (HR: 0.84; 95% CI: 0.77–0.92; P < 0.001), associated with a significant reduction in mortality (4.5%, vs. 5.9%; P < 0.001), also probably due to the pleiotropic effects related to the similarities with the adenosine mechanism.65 These beneficial effects were also particularly evident in patients with impairment of renal function (estimated glomerular filtration rate from 30 to 60 mL/min)66; treatment with ticagrelor was not associated with increased rates of overall major bleeding events (1.5% vs. 1.3%; P = 0.22), but higher incidence of non-CABG–related major bleeding (4.5% vs. 3.8%; P = 0.03) and intracranial fatal bleeding, even if the occurrence of such complication was very rare in both arms (0.1% vs. 0.01%).64 In addition, ticagrelor is not a prodrug and does not require metabolic conversion before activating: it is therefore an elective drug to achieve a faster platelet inhibition compared with other oral antiplatelet agents, as showed in recent studies on pharmacodynamics.67
The ACCOAST (A Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pre-treatment At the Time of Diagnosis in Patients with Non-ST-Elevation Myocardial Infarction) trial evaluated the possible benefit of prasugrel pre-treatment in NSTEMI patients who were scheduled to undergo coronary angiography and possible PCI; it compared two different loading doses of prasugrel: a 30 mg loading dose of prasugrel at the time of diagnosis and an additional 30 mg dose at the time of PCI (pre-treatment group) vs. a 60 mg loading dose at the time of angiography.68 The incidence of the primary endpoint, a composite of death from cardiovascular causes, myocardial infarction, stroke, urgent revascularization, or GPI bailout through Day 7, did not differ significantly between the two groups (HR with pre-treatment: 1.02; 95% CI: 0.84–1.25; P = 0.81).68 Conversely, the rate of TIMI major bleeding episodes, whether related or non-related to CABG, was significantly increased through Day 7 in the pre-treatment group (HR: 1.90; 95% CI: 1.19–3.02; P = 0.006), which led to the early interruption of the trial.68 A following meta-analysis, which included ACCOAST and studies on clopidogrel, further confirmed the increased risk of bleeding with no apparent improved ischaemic outcomes.69
Against this background, GPIs or the novel antiplatelet agents with intravenous administration (e.g. cangrelor70) can be useful to achieve a rapid and an effective platelet inhibition in ACS patients with high ischaemic risk and allow to wait for the initiation of oral thienopyridine treatment until the coronary tree has been assessed. Recently, the FABOLUS PRO (Facilitation through Aggrastat By drOpping or shortening Infusion Line in patients with ST-segment elevation myocardial infarction compared with or on top of PRasugrel given at loading dOse) study demonstrated that a standard dose of intravenous bolus-only tirofiban achieves a higher and earlier platelet aggregation inhibition compared with an oral 60 mg loading dose of prasugrel by 6 h.71
Various studies have evaluated the effects of GPIs in early treatment of ACS patients scheduled for coronary angiography. However, this treatment approach was associated with the administration of clopidogrel or other novel P2Y12 receptor inhibitors72 in only a few of these trials. A meta-analysis on ∼30 000 NSTE-ACS patients treated with a conservative approach or undergoing PCI showed a 9% reduction of the relative risk of death and non-fatal myocardial infarction with the use of GPIs when compared with placebo.73 This beneficial effect was associated to PCI performance, whereas no reduction in the death or infarction rates was observed in patients who received only medical therapy. Recent clinical trials assessed whether benefits from GPI treatment could be increased with pre-treatment instead of usage in the catheterization laboratory. The ACUITY Timing (Acute Catheterization and Urgent Intervention Triage strategY) trial evaluated deferred treatment (during PCI only) vs. upstream administration of a GPI in 9207 ACS patients pre-treated with thienopyridine in 64% of the cases.74 Deferred vs. upstream therapy was identified with a lower incidence of non-CABG–related major bleeding episodes at 30 days (4.9% vs. 6.1%; RR: 0.80; 95% CI: 0.67–0.95; P = 0.009), without any difference in the ischaemic adverse event rates (7.9% vs. 7.1%; RR: 1.12; 95% CI: 0.97–1.29; P = 0.13). In the EARLY-ACS (The Early Glycoprotein IIb/IIIa Inhibition in Non-ST-Segment Elevation Acute Coronary Syndrome) trial, 9500 ACS patients who were assigned to an invasive strategy were randomized to early vs. delayed treatment with eptifibatide75; the primary endpoint (a composite of death, myocardial infarction, urgent revascularization, or the occurrence of a thrombotic complication during PCI at 96 h) was similar between two groups (9.3% vs. 10.0%; OR: 0.92; 95% CI: 0.80–1.06; P = 0.23), without significant interaction among the various tested subgroups, including diabetic or troponin-positive patients. Conversely, major bleeding rate was higher in patients receiving early treatment with eptifibatide (2.6% vs. 1.8%; OR: 1.42; 95% CI: 1.97–1.89; P = 0.015).75 Following these studies, the upstream use of GPIs decreased drastically, especially in the era of the newer P2Y12 antagonists, and GPI treatment is now only applied within the catheterization laboratory based on the coronary angiography results (e.g. in the presence of intracoronary thrombi or based on the extent of the coronary artery disease) and after a careful evaluation of the haemorrhage risk for each patient. The guidelines suggest considering upstream treatment with GPIs in the presence of a large ischaemic area in high-risk patients.1,2
Anticoagulants
As in the initially conservative treatment, UFH and LMWHs represent the most commonly used anticoagulants also in patients diagnosed with ACS and scheduled for coronary angiography. The EXTRACT-TIMI 25 trial investigated patients diagnosed with STEMI treated with successful thrombolysis followed by PCI: the study showed the superiority of enoxaparin vs. UFH in reducing the incidence of major adverse events at 30 days in patients treated with TNK-tPA and clopidogrel, although a higher rate of minor and major bleeding events was observed.76
International guidelines strongly recommend consistency with the initially selected antithrombotic treatment and the avoidance of any crossover,1,2 as switches in the heparin treatment are associated with increased occurrence of fatal adverse events and short- and long-term major bleeding episodes.77,78
When treatment with fondaparinux is initially selected, it should be noted that this drug had been previously associated with higher incidence of catheter thrombosis in the OASIS-5 trial (see Pathway 2) when coronary angiography and subsequent PCI were performed.55 This problem was basically resolved by adding a UFH dose in the catheterization setting. In the FUTURA/OASIS-8 (Fondaparinux with UnfracTionated heparin dUring Revascularization in Acute coronary syndromes) trial, the addition of a standard dose of UFH (85 mg/kg or 60 mg/kg with concomitant use of GPIs) resulted in the decrease of catheter thrombosis rate to 0.1%79 (see Pathway 4, paragraph on PCI). On the other hand, if switching of anticoagulant treatment from UFH/LMWH therapy to bivalirudin in the catheterization laboratory setting (and related timing) is considered, such approach is apparently safe and still maintains the efficacy level of bivalirudin in terms of net clinical benefit at 30 days and 2 years.80,81
Executive summary
| Antiplatelet agents |
[A] STEMI
|
[B] NSTEMI
|
| Anticoagulant agents |
[A] STEMI
|
[B] NSTEMI
|
Pathway 5: percutaneous coronary revascularization (percutaneous coronary intervention)
Percutaneous coronary intervention is the most widely used revascularization procedure in the framework of ACSs, which led to a net improvement in the prognosis of these patients.82,83 Based on the assumption that ACS patients already present a high thrombin generation level and a substantial platelet aggregation activity, and that PCI with intracoronary stent implantation is per se associated with thrombotic activation, achieving a maximum antithrombotic effect is crucial. Over the past years, several randomized clinical trials have focused on peri-procedural antithrombotic therapy, a topic that requires a constant update of international guidelines on ACSs.
Antiplatelet agents
The CURRENT OASIS-7 (Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Optimal Antiplatelet Strategy for Interventions) trial was specifically aimed at investigating the optimal clopidogrel and aspirin doses for ACS patients undergoing PCI within 72 h from admission.84 Results indicated that a double dose of clopidogrel (600 mg loading dose, followed by 150 mg once daily for 1 week) could achieve a faster and stronger effect compared with a routine dose (300 mg loading dose, followed by 75 mg once daily), resulting in a more significant clinical benefit. The study, carried out in 597 coronary care units in 39 countries, included 25 000 patients, 17 000 of which underwent PCI. Among these, a significant 15% reduction of the primary endpoint (cardiovascular death, myocardial infarction, and stroke) was observed in the group treated with the clopidogrel double dose.84 Such reduction mainly concerned the infarction rate with a 22% decrease. In addition, a 42% reduction in the risk of stent thrombosis was registered. The double dose of the drug did not result in increased brain or fatal haemorrhages, but overall major bleeding was significantly increased. The study also showed no difference between low (75–100 mg once daily) and high dosage (300–325 mg once daily) of ASA in terms of both efficacy and safety.84
Data from the PLATO trial are based on the patient population for whom an invasive strategy was planned (PLATO INVASIVE). In the study, however, there are no specific data on the patient group undergoing PCI.85 At randomization, an invasive strategy was planned for 72% of the ACS patients enrolled. The primary endpoint occurred in 9% of the ticagrelor patient group vs. 10.7% of the clopidogrel group (HR: 0.84; 95% CI: 0.75–0.94; P = 0.0025), and no difference was observed between the two treatment groups in the rates of total major and severe bleedings at 1 year follow-up.85 The PEGASUS TIMI-54 trial (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54) recently also opened to long-term treatment in specific patient populations.86 The trial randomized 21 162 high-risk patients with previous infarction (83% of whom had already undergone PCI) to receive ticagrelor (90 or 60 mg) vs. placebo on top of low-dose ASA for a median duration of 33 months. Both dosages of ticagrelor significantly reduced the incidence of the primary endpoint (cardiovascular death, myocardial infarction, and stroke) compared with placebo (HR of 90 mg ticagrelor vs. placebo: 0.85; 95% CI: 0.75–0.96; P = 0.008; HR of 60 mg ticagrelor vs. placebo: 0.84; 95% CI: 0.74–0.95; P = 0.004). Both ticagrelor dosages increased the rate of major TIMI bleeding episodes (2.60% at 90 mg and 2.30% at 60 mg) compared with placebo (1.1%; P < 0.001 for each ticagrelor vs. placebo dose). However, the risk of intracranial haemorrhages and fatal bleedings was comparable between ticagrelor and placebo.86
Efficacy data with prasugrel in patients who underwent PCI are available from the TRITON TIMI 38 trial.87 In the study, 13 608 patients with moderate-to-high-risk ACS with scheduled PCI were randomized to receive clopidogrel (300 mg loading dose, followed by 75 mg once daily) or prasugrel (60 mg loading dose, followed by 10 mg once daily) in the catheterization laboratory following coronary angiography (with the exception of the STEMI population,21 for whom randomization before assessment of the coronary tree was allowed).87 Patients under chronic clopidogrel treatment, with bleeding diatheses or presenting any other features of high risk of bleeding, were not eligible to participate in the study. The primary endpoint was death from cardiovascular causes, non-fatal myocardial infarction or non-fatal stroke. The primary efficacy endpoint occurred in 12.1% of patients receiving clopidogrel and 9.9% of patients receiving prasugrel (HR: 0.81; 95% CI: 0.73–0.90; P < 0.001). The primary endpoint outcomes were mostly characterized by a 24% reduction in the prasugrel group in the rates of myocardial infarction.87 The benefit tended to be greater among the patient subgroup with diabetes, with a 30% reduction of the primary endpoint (HR: 0.70; 95% CI: 0.58–0.85) and a statistically significant interaction with respect to the reduction of myocardial infarction rate.87 It should be noted that a marked reduction in the stent thrombosis rate was registered in the TRITON-TIMI 38 trial in the patients randomized to receive prasugrel: possible or probable thrombosis (as per the Academic Research Consortium definition) was reduced by 52% (1.1% vs. 2.4%; P < 0.001), while the documented thrombosis rate (by coronary angiography or autopsy) decreased by 58% (0.9% vs. 2.0%; P < 0.001). Data were consistent among patients who received conventional or medicated stents.88 With regard to safety, the rate of TIMI major bleeding episodes not CABG-related was higher in the prasugrel treatment group (2.4% vs. 1.8%; HR: 1.32; 95% CI: 1.03–1.68; P = 0.03), with an increase in the rate of life-threatening and—although infrequent—fatal bleeding events. A multivariate analysis showed that the highest risk of major bleeding was registered among patients with 75 years of age or older, patients weighing <60 kg (relative contraindication for the use of prasugrel) and patients who had a history of cerebrovascular events (absolute contraindication for the use of prasugrel).87 In the other patients, the higher prasugrel efficacy was not associated with any increase of bleeding events.
Despite biological evidence89 and the availability of recently published data from observational studies showing the relative safety of switching from clopidogrel to prasugrel in patients undergoing PCI,90–92 further data from larger studies are needed to investigate the risk/benefit profile of such therapeutic strategy.
The efficacy of cangrelor (an intravenous adenosine diphosphate (ADP)-receptor antagonist) during PCI performance was mostly assessed in patients with stable coronary artery diseases and in the CHAMPION PHOENIX (Clinical Trial Comparing Cangrelor to Clopidogrel Standard of Care Therapy in Subjects Who Require Percutaneous Coronary Intervention) trial: in the study population, which included over 11 000 stable and unstable patients naive to oral antiplatelet therapy, cangrelor significantly reduced the incidence of the primary endpoint, a composite of death, myocardial infarction, stent thrombosis, and revascularization, at 48 h compared with placebo (4.7% vs. 5.9%; P = 0.005), and reduced the rate of stent thrombosis at 48 h (0.8% vs. 1.4%; P = 0.01), with no increase in severe bleeding rate at 48 h (0.16% vs. 0.11%; P = 0.44).93,94
Vorapaxar is an orally active selective inhibitor of the platelet thrombin receptor PAR-1. The TRACER (Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome) trial enrolled 12 944 NSTE-ACS patients randomized to vorapaxar 40 mg loading and 2.5 mg daily maintenance or placebo in addition to ASA and clopidogrel. After a median follow-up of 502 days, the primary endpoint of cardiovascular death, myocardial infarction, stroke, recurrent ischaemia, and urgent revascularization did not differ significantly among the groups [vorapaxar 18.5% vs. placebo 19.9%; HR 0.92 (95% CI 0.85–1.01), P = 0.07], while severe bleeding events were more frequent in the study drug group [vorapaxar 7.2% vs. placebo 5.2%; HR 1.35 (95% CI 1.16–1.58), P < 0.001], with a marked increase in intracranial haemorrhage [HR 3.39 (95% CI 1.78–6.45), P < 0.001].95 In the TRA 2P-TIMI 50 (Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events) trial, including 26 449 patients with prior myocardial infarction, stroke, or peripheral vascular disease, randomization to vorapaxar was associated with a modest reduction in cardiovascular death, myocardial infarction, and stroke over 3 years [vorapaxar 9.3% vs. placebo 10.5%; HR 0.87 (95% CI 0.80–0.94), P < 0.001].96 Administration of the study drug was associated with an increase in intracranial haemorrhage, and the absolute increase in TIMI clinically significant bleeds [vorapaxar 15.8% vs. placebo 11.1%; HR 1.46 (95% CI 1.36–1.57), P < 0.001] was greater than the absolute reduction in ischaemic events. In the subgroup of 17 779 patients with prior myocardial infarction, rates of the primary endpoint over 3 years were 8.1% in the vorapaxar group vs. 9.7% in the placebo group [HR 0.80 (95% CI 0.72–0.89), P < 0.0001]. TIMI clinically significant bleeding occurred in 15.1% and 10.4% of patients, respectively [HR 1.49 (95% CI 1.36–1.63), P < 0.0001].96
Notably, around 5–8% of ACS patients undergoing PCI have an indication for long-term oral anticoagulation therapy (OAC) with vitamin K antagonists or novel OAC (NOAC) due to various conditions such as atrial fibrillation, mechanical heart valves, or venous thromboembolism. In the peri-procedural phase, it should be considered to perform coronary angiography on OAC, because interruption of OAC and bridging with parenteral anticoagulants may lead to an increase in both thromboembolic episodes and bleeds.1 Regarding long-term antithrombotic treatment, evidence to guide the management of ACS patients undergoing PCI and requiring long-term OAC is limited. Duration of triple therapy, defined as the combination of ASA, clopidogrel, and OAC, should be as limited as possible, depending on careful assessment of both thromboembolic and bleeding risks.1
Anticoagulants
The use of UFH during PCI is restricted because of the unpredictability of its anticoagulant effects, the narrow therapeutic windows it provides, and the related need for close monitoring.97 Despite those restrictions and the lack of supporting, randomized, placebo-controlled clinical trials of relevance, the use of anticoagulants during urgent or primary elective PCI has always focused on the application of UFH, based on personal experiences and empirical data. Currently, enoxaparin is the most scientifically supported LMWH in the PCI setting. It offers a predictable anticoagulation effect and no required anticoagulation monitoring.98,99 The drug can be subcutaneously or intravenously administered to achieve immediate anticoagulation. A recent meta-analysis of 23 trials, including approximately 31 000 patients with stable coronary artery disease, non-ST-elevation ACSs, and STEMI undergoing PCI, demonstrated the clinical benefits of enoxaparin vs. UFH with regard to reduction in mortality (relative risk: 0.66; 95% CI: 0.57–0.76; P < 0.001), the composite endpoint of death or myocardial infarction (0.68, 0.57–0.81; P < 0.001), complications of myocardial infarction (0.75, 0.6–0.85; P < 0.001) and major bleeding episodes (0.80, 0.68–0.95; P = 0.009).7
Bivalirudin apparently overcomes the limits of conventional anticoagulation agents, and thanks to its high efficacy and reduced plasma half-life, appeared to be an ideal drug for patients referred for PCI.100 The ACUITY trial101 enrolled over 13 800 moderate-to-high-risk patients with non-ST-elevation ACSs. The subjects were randomized to receive UFH or enoxaparin plus a GPI, bivalirudin plus a GPI, or bivalirudin alone and underwent coronary angiography with eventual PCI within 72 h after randomization; the study tested the hypothesis that bivalirudin would be non-inferior compared with the standard treatment with UFH + GPI. In the study, bivalirudin alone reduced the rates of major bleeding (48%) and the net clinical outcome endpoint (14%).101 In the recent ISAR REACT 4 trial, over 1700 patients with non-ST-segment elevation myocardial infarction were assigned to receive abciximab plus UFH or bivalirudin.102 This study’s specific objective was to assess the superiority of bivalirudin compared with the UFH + abciximab group in reducing the composite endpoint of death, recurrent myocardial infarction, target vessel revascularization, or major bleeding within 30 days. The primary endpoint occurred in 10.9% of the patients randomized to abciximab and in 11.0% of the bivalirudin group (relative risk with abciximab: 0.99; 95% CI: 0.74–1.32; P = 0.94). Major bleeding occurred in 4.6% of the patients in the abciximab group, compared with 2.6% in the bivalirudin group (relative risk: 1.84; 95% CI: 1.10–3.07; P = 0.02).102 As stated above, the HORIZONS-AMI trial tested bivalirudin in STEMI patients.10 Anticoagulation with bivalirudin compared with heparin plus GPIs consistently reduced the rate of major bleeding (4.9% vs. 8.3%; P < 0.001) and resulted in a reduced 30-day rate of net adverse clinical events, defined as major bleeding, mortality, urgent revascularization, myocardial infarction, and stroke (9.2% vs. 12.1%; P = 0.005). Interestingly, patients randomized to bivalirudin also showed reduced rates of death from all causes (2.1% vs. 3.1%; P = 0.047) and death from cardiac causes (1.8% vs. 2.9%; P = 0.03).10 In a landmark analysis at 3 years, which excluded 30-day adverse events, bivalirudin was still associated with a significantly reduced cardiac mortality compared with the population treated with UFH + GPI (1.1% vs. 2.2%; P = 0.01), even excluding patients with a history of major bleeding (3.8% vs. 2.6%; P = 0.048).103 Recent randomized clinical trials questioned the efficacy of bivalirudin in STEMI patients compared with UFH alone (without systemic use of GPIs). The multicentre, randomized BRAVE-4 (The Bavarian Reperfusion Alternatives Evaluation 4) trial, which was prematurely stopped due to issues with patient enrolment, randomized 548 STEMI patients (compared with a planned population of 1240 patients) to prasugrel plus bivalirudin regimen vs. treatment with clopidogrel plus UFH.104 At 30 days, the primary composite endpoint of death, myocardial infarction, urgent revascularization, stent thrombosis, stroke, or bleeding was comparable in the two treatment groups (15.6% vs. 14.5%; P = 0.68) and neither the composite of ischaemic complications nor bleeding presented major differences; results were consistent across the pre-specified subgroups of patients.104 The HEAT PPCI (Unfractionated heparin vs bivalirudin in primary percutaneous coronary intervention) trial enrolled ∼1800 STEMI patients from a single centre and randomized them to bivalirudin or UFH (GPIs were used in a little over 15% of the patient population).105 The incidence of the primary efficacy outcome, a composite of mortality, cerebrovascular accident, myocardial infarction, or urgent revascularization at 30 days, was lower in the UFH group (5.7% vs. 8.7%; P = 0.01), and no difference was observed in the rate of major bleeding events between the two groups (3.5% vs. 3.1%; P = 0.59). A significantly higher rate of stent thrombosis was registered in the bivalirudin group (3.4% vs. 0.9%; P = 0.001).105 Finally, in the recent MATRIX (Minimising Adverse haemorrhagic events by TRansradial access site and systemic Implementation of angioX) trial,106 which enrolled 6800 ACS patients undergoing invasive management, bivalirudin failed to significantly reduce the 30-day risk of MACE or net adverse clinical events, a co-primary endpoint including MACE and major bleeding, when compared with patients who received UFH. Treatment with bivalirudin did significantly reduce all-cause mortality, including a statistically significant 30% relative reduction in the risk of cardiovascular death and a 32% reduction in the risk of cardiac death (even if not powered for both endpoints). In addition, the use of bivalirudin did result in a significant reduction in non-access site bleeding, bleeding requiring a blood transfusion, fatal bleeding, and other bleeding measures.106
In the OASIS-5 (see Pathway 2) trial, fondaparinux was associated with a higher rate of catheter thrombosis when used in combination with invasive procedures compared with enoxaparin.55 This led to an amendment during the trial: the introduction of UFH in case of PCI prevented this dangerous thrombotic event.107 The subsequent FUTURA/OASIS-8 trial.79 was designed and conducted to assess the correct dose of UFH to be added to fondaparinux in case of PCI in high-risk ACS patients. The study randomly compared two different UFH dose regimens in addition to fondaparinux in 2000 patients undergoing PCI within 72 h: a low (50 U/kg, regardless of concomitant use of GPIs) or standard dose (85 U/kg and 60 U/kg in association with GPIs). Incidence of minor or major bleeding, or major vascular complications up to 48 h after PCI was similar in both dose regimens (OR: 0.80; 95% CI: 0.54–1.19; P = 0.27), with a lower rate of minor bleeding events in the low-dose group (OR: 0.40; 95% CI: 0.16–0.97; P = 0.04).79 For the clinical secondary outcome, defined as a composite of major bleeding at 48 h and death, myocardial infarction, or target vessel revascularization within Day 30, the rates in the UFH low dose arm lower (OR: 1.51; 95% CI: 1.00–2.28; P = 0.05) than in the standard dose group.79
Regarding long-term anticoagulation therapy, it is worth to quote the Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Aspirin with or without Thienopyridine Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction (ATLAS ACS 2-TIMI 51) study that compared rivaroxaban 2.5 mg or 5 mg twice daily with placebo in 15 526 patients following ACS, on top of ASA and clopidogrel.108 At a mean follow-up of 13 months, the primary efficacy endpoint of cardiovascular death, myocardial infarction, or stroke was 10.7% with placebo, 9.1% with rivaroxaban 2.5 mg [HR 0.84 (95% CI 0.72–0.97), P =0.02] and 8.8% with rivaroxaban 5 mg [HR 0.85 (95% CI 0.73–0.98), P = 0.03], with no interaction by ACS subtype. Rates of definite, probable, or possible stent thrombosis were 2.2% and 2.3% with 2.5 and 5 mg rivaroxaban, respectively, vs. 2.9% with placebo (P = 0.02 and P = 0.04, respectively).108 Rates of cardiovascular death were significantly lower only in the rivaroxaban 2.5 mg arm compared with placebo [2.7% vs. 4.1%; HR 0.66 (95% CI 0.51–0.86), P = 0.002]. On the other hand, intracranial haemorrhage rates were 0.4% with 2.5 mg and 0.7% with 5 mg rivaroxaban vs. 0.2% with placebo [HR 2.83 (95% CI 1.02–7.86), P= 0.04 for 2.5 mg; HR 3.74 (95% CI 1.39–10.07), P = 0.005 for 5 mg].108 Therefore, the use of rivaroxaban 2.5 mg twice daily, while not recommended in patients treated with ticagrelor or prasugrel, might be considered in combination with ASA and clopidogrel for ACS patients who have high ischaemic and low bleeding risks. It is contraindicated in patients with a prior history of ischaemic stroke/transient ischaemic attack and its use is cautioned in patients >75 years of age or <60 kg body weight.
Executive summary
| Antiplatelet agents |
|
[A] STEMIIn patients treated with thrombolysis before PCI:
|
In patients treated with pPCI:
|
[B] NSTEMI
|
| Anticoagulant agents |
[A] STEMI
|
[B] NSTEMI
|
Pathway 6: surgical myocardial revascularization (coronary artery bypass graft)
Today’s therapeutic and strategic choices are still influenced by the fear that ACS patients could present a coronary artery disease of such significant extent and severity as to require CABG. This fear led to under-treatment in early ACS stages in the attempt to avoid surgical revascularization, a procedure that is becoming increasingly rarer, particularly in an emergency setting, due to advanced cardiac interventional technology and the increased experience of operators.109 Moreover, recent surgical technologies, such as the off-pump surgery and emerging new methods for blood salvage and platelet transfusion markedly reduced the risk of major bleeding in ACS patients managed with single or dual antiplatelet therapy.110 The upstream antithrombotic treatment was gradually abandoned in consequence of this technological progress, as well as of recently designed studies, combined with a decreased time of diagnosis and coronary angiography in centres equipped with 24-h operational catheterization laboratories, which resulted in a worrisome increased risk of antithrombotic under-treatment in ACS patients.
Antiplatelet agents
Several studies showed that perioperative use of ASA and clopidogrel is associated with a higher risk of bleeding, transfusion, and reoperation for bleeding.111–115 In a study carried out in 14 centres on 350 patients undergoing CABG, a three-fold increase in the risk of reoperation for bleeding was observed in patients who received clopidogrel within 5 days from surgery.116 This is consistent with the data from a sub-analysis of the CURE trial117 and led to the recommendation that treatment with clopidogrel in patients scheduled for CABG should be withheld 5–7 days before surgery.
There are relatively few data on clopidogrel safety in CABG patients, as the only available information can be inferred from the TRITON-TIMI 38 trial population, a study basically designed to include patients with scheduled PCI, where only 1% of the enrolled population underwent surgical revascularization.87 In this small patient subgroup, prasugrel was associated with reduced all-cause mortality (adjusted odds ratio: 0.26; P = 0.025), registering, however, a significant 4.5-fold increase in major bleeding vs. clopidogrel (13% vs. 3%; P < 0.001).118 Therefore, the indicated practical approach in case of early prasugrel treatment in patients then scheduled for CABG is comparable to clopidogrel (drug-free period of 5–7 days before surgery).
In the PLATO trial, about 2000 patients underwent CABG post-randomization. Based on the study protocol, the study drug was withheld for 5 days in the clopidogrel group and for 24 to 72 h in the ticagrelor group.64 Overall, approximately 1200 patients underwent CABG within 7 days after interruption of the study drug. In the PLATO CABG sub-study, ticagrelor showed a better outcome in the primary ischaemic endpoint, which was consistent with the overall results of the trial, without difference in the rates of CABG-related bleeding events. However, the treatment with ticagrelor instead of clopidogrel resulted in lower total mortality (4.7% vs. 9.7%; HR: 0.49; 95% CI: 0.32–0.77; P < 0.01) and cardiovascular mortality (4.1% vs 7.9%; HR: 0.52; 95% CI: 0.32–0.85; P < 0.01).119 This impressive mortality decline is likely to be due to the drug’s favourable pharmacokinetics and on/off effect, which in this setting resulted in a drastic decrease in major bleeding and perioperative infections compared with clopidogrel.120
Due to their reduced plasma half-life, GPIs can be relatively safely interrupted shortly before surgery: eptifibatide and tirofiban have a short half-life of approximately 2 h; abciximab has an even shorter plasma half-life (10 min), but dissociates slowly from the platelet so that full recovery of platelet aggregation responses takes ∼48 h after the infusion has been terminated. Patients undergoing urgent CABG surgery while receiving oral antiplatelet agents or GPI IIb/IIIa receptors require appropriate measures to ensure adequate haemostasis, such as platelet transfusion,1,2 to begin with. In any event, antiplatelet therapy should be resumed 24 h after the resolution of the major bleeding event.
Anticoagulants
As for PCI, the use of UFH has become widespread in CABG procedures. Treatment can be continued until a few hours before CABG, it is easily manageable in the perioperative time frame and is not associated with significant bleeding. LMWHs can also be conveniently administered up to 12 h before surgical revascularization with no associated major bleeding.121–123
Executive summary
| Antiplatelet agents |
In all ACS patients (STEMI and NSTEMI):
|
| Anticoagulant agents |
In all ACS patients (STEMI and NSTEMI):
|
Pathway 7: conservative treatment (after coronary angiography evaluation)
This patient subgroup presents similarities with the patient category described in Pathway 2 (initially conservative treatment). The difference between the two groups is that in Pathway 2 coronary angiography is seldom performed because of the estimated high clinical risk, whereas conservative treatment for this patient subgroup is selected in view of the high anatomical/procedural risk or because there is minimal/no evidence of coronary disease at the angiography evaluation. A further difference with patients receiving an initially conservative treatment lies in the fact that knowledge of the coronary anatomy leads to a more aggressive use of pharmacological treatment.124 However, this population still presents a high risk of long-term events compared with patients benefiting from revascularization procedures. An analysis of the EARLY-ACS trial showed that patients with greater extent of coronary disease (patients with three-vessel coronary disease and critical involvement of the left main) treated with a conservative strategy present a significantly higher risk of both short-term and long-term mortality compared with patients with the same extent of coronary disease who have undergone some kind of revascularization.36
Antiplatelet agents
What was described in Pathway 2 with regard to antiplatelet agents also applies to antiplatelet therapy within this pathway: clopidogrel and ticagrelor are beneficial in a conservative setting even after coronary angiography, performed in 35% of the patients enrolled in the CURE trial42 and in 42% of the PLATO patients undergoing conservative treatment.43 The PLATO study also confirmed the superiority of ticagrelor compared with clopidogrel in the population that was treated conservatively. ESC guidelines recommend the use of ticagrelor, and only when ticagrelor is unavailable or contraindicated of clopidogrel. Treatment with prasugrel is covered in the TRILOGY-ACS trial44; it should be noted that although there was no beneficial outcome in the overall population associated with acute-phase and long-term treatment with prasugrel compared with clopidogrel, a reduction of the primary long-term endpoint was registered in the prasugrel group within the 3000 patients treated with a conservative approach after coronary angiography (10.7% vs. 14.9%; HR: 0.77; 95% CI: 0.61–0.98; P = 0.031). This was observed even though the P value for interaction between the patients who underwent coronary angiography and those who did not was not significant (P = 0.08).44 However, it should be noted that, as mentioned above, this is a posthoc analysis of a study that failed to meet its primary endpoint.
Anticoagulants
Recommendations on the use of anticoagulant agents are also similar to those described in Pathway 2. As recommended in the guidelines, anticoagulation treatment should be selected based on the assessment of the ischaemic and haemorrhagic risk profile of each ACS patient and after an evaluation of the efficacy/risk profile of the selected agent (Class 1A recommendation).1,2 In the setting of a conservative treatment, the guidelines also recommend the use of an anticoagulant agent throughout hospitalization (Class 1A recommendation) and discourage the crossing over from one heparin to another.1,2
Executive summary
| Antiplatelet agents |
In all ACS patients (STEMI and NSTEMI):
|
| Anticoagulant agents |
In all ACS patients (STEMI and NSTEMI):
|
Consensus Document Approval Faculty
Abrignani Maurizio Giuseppe, Alunni Gianfranco, Amico Antonio Francesco, Amodeo Vincenzo, Angeli Fabio, Aspromonte Nadia, Audo Andrea, Azzarito Michele, Battistoni Ilaria, Bianca Innocenzo, Bisceglia Irma, Bongarzoni Amedeo, Bonvicini Marco, Cacciavillani Luisa, Calculli Giacinto, Canzone Giuseppe, Capecchi Alessandro, Caretta Giorgio, Carmina Maria Gabriella, Casazza Franco, Casolo Giancarlo, Cassin Matteo, Casu Gavino, Cemin Roberto, Chiarandà Giacomo, Chiarella Francesco, Chiatto Mario, Ciccone Marco Matteo, Cicini Maria Paola, Clerico Aldo, Comoglio Francesca Maria, D'Agostino Carlo, De Luca Giovanni, De Maria Renata, Del Sindaco Donatella, Di Fusco Stefania Angela, Di Lenarda Andrea, Di Tano Giuseppe, Egidy Assenza Gabriele, Egman Sabrina, Enea Iolanda, Fattirolli Francesco, Francese Giuseppina Maura, Gabrielli Domenico, Giardina Achille, Greco Cesare, Gregorio Giovanni, Iacoviello Massimo, Khoury Georgette, Ledda Antonietta, Lucà Fabiana, Macera Francesca, Marini Marco, Mascia Franco, Masson Serge, Maurea Nicola, Mazzanti Marco, Mennuni Mauro, Menotti Alberto, Menozzi Alberto, Mininni Nicola, Molon Giulio, Moreo Antonella, Moretti Luciano, Mortara Andrea, Mureddu Gian Francesco, Murrone Adriano, Navazio Alessandro, Nicolosi Gian Luigi, Oliva Fabrizio, Oreglia Jacopo, Parato Vito Maurizio, Parrini Iris, Patanè Leonardo, Pini Daniela, Pino Paolo Giuseppe, Pirelli Salvatore, Procaccini Vincenza, Pulignano Giovanni, Radini Donatella, Rao Carmelo Massimiliano, Rasetti Gerardo, Riccio Carmine, Roncon Loris, Rugolotto Matteo, Sanna Fabiola, Sauro Rosario, Scalvini Simonetta, Severi Silva, Sicuro Marco, Silvestri Paolo, Sisto Francesco, Tarantini Luigi, Themistoclakis Sakis, Uguccioni Massimo, Urbinati Stefano, Vatrano Marco, Vianello Gabriele, Vinci Eugenio, and Zuin Guerrino
Conflict of Interest: L.D.L. reports personal fees from Abbott Vascular, Astra Zeneca, Bayer, Boehringer-Ingelheim, Eli Lilly, Daiichi Sankyo, Pharmevo, Menarini and The Medicines Company, outside the submitted work. G.M. reports personal fees from Abbott Vascular, Astra Zeneca, Daiichi Sankyo, Menarini, MSD, St. Jude Medical and The Medicine Company, outside the submitted work. The other authors have no conflicts of interest to declare.
References
- 1. Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D.. for the Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–619. [DOI] [PubMed] [Google Scholar]
- 2. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S.; ESC Committee for Practice Guidelines. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 3. Beygui F, Castren M, Brunetti ND, osell-Ortiz F, Christ M, Zeymer U, Huber K, Folke F, Svensson L, Bueno H, Van't Hof A, Nikolaou N, Nibbe L, Charpentier S, Swahn E, Tubaro M, Goldstein P; ACCA study group on pre-hospital care. Pre-hospital management of patients with chest pain and/or dyspnoea of cardiac origin. A position paper of the Acute Cardiovascular Care Association (ACCA) of the ESC. Eur Heart J Acute Cardiovasc Care 2015 Aug 27. pii: 2048872615604119. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4. Leopold JA. Does Thrombolytic Therapy Facilitate or Foil Primary PCI? N Engl J Med 2008;358:2257–79. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong PW, Gershlick AH, Goldstein P, Wilcox R, Danays T, Lambert Y, Sulimov V, Rosell Ortiz F, Ostojic M, Welsh RC, Carvalho AC, Nanas J, Arntz HR, Halvorsen S, Huber K, Grajek S, Fresco C, Bluhmki E, Regelin A, Vandenberghe K, Bogaerts K, Van de Werf F; STREAM Investigative Team. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med 2013;368:1379–87. [DOI] [PubMed] [Google Scholar]
- 6. Vlaar PJ, Svilaas T, Damman K, de Smet BJ, Tijssen JG, Hillege HL, Zijlstra F.. Impact of pretreatment with clopidogrel on initial patency and outcome in patients treatd with primary percutaneous intervention for ST-segment elevation myocardial infarction. A systematic review. Circulation 2008;118:1828–36. [DOI] [PubMed] [Google Scholar]
- 7. Koul S, Smith JG, Scherstén F, James S, Lagerqvist B, Erlinge D.. Effect of upstream clopidogrel treatment in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J 2011;32:2989–97. [DOI] [PubMed] [Google Scholar]
- 8. Fefer P, Hod H, Hammermann H, Segev A, Segev A, Beinart R, Boyko V, Behar S, Matetzky S.. Usefulness of pretreatment with high-dose clopidogrel in patients undergoing primary angioplasty for ST-elevation myocardial infarction. Am J Cardiol 2009;104:514–518. [DOI] [PubMed] [Google Scholar]
- 9. Dangas G, Mehran R, Guagliumi G, Caixeta A, Witzenbichler B, Aoki J, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Rabbani LE, Parise H, Stone GW; HORIZONS-AMI Trial Investigators. Role of clopidogrel loading dose in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: results from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol 2009;54:1438–46. [DOI] [PubMed] [Google Scholar]
- 10. Patti G, Barczi G, Orlic D, Mangiacapra F, Colonna G, Pasceri V, Barbato E, Merkely B, Edes I, Ostojic M, Wijns W, Di Sciascio G.. Outcome comparison of 600- and 300-mg loading doses of clopidogrel in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: results from the ARMYDA-6 MI (Antiplatelet therapy for Reduction of Myocardial Damage during Angioplasty-Myocardial Infarction) randomized study. J Am Coll Cardiol 2011;58:1592–9. [DOI] [PubMed] [Google Scholar]
- 11. Wiviott SD, Antman EM, Gibson CM, Montalescot G, Riesmeyer J, Weerakkody G, Winters KJ, Warmke JW, McCabe CH, Braunwald E; TRITON-TIMI 38 Investigators. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006;152:627–35. [DOI] [PubMed] [Google Scholar]
- 12. Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM; TRITON-TIMI 38 investigators. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomized controlled trial. Lancet 2009;373:723–31. [DOI] [PubMed] [Google Scholar]
- 13. Clemmensen P, Grieco N, Ince H, Danchin N, Goedlcke J, Ramos Y, Schmitt J, Goldstein P.. MULTInational non-interventional study of patients with ST-segment elevation myocardial infarction treated with PRimary Angioplasty and Concomitant use of upstream antiplatelet therapy with prasugrel or clopidogrel–the European MULTIPRAC Registry. Eur Heart J Acute Cardiovasc Care 2015;4:220–9. [DOI] [PubMed] [Google Scholar]
- 14. Steg PG, James S, Harrington RA, Ardissino D, Becker RC, Cannon CP, Emanuelsson H, Finkelstein A, Husted S, Katus H, Kilhamn J, Olofsson S, Storey RF, Weaver WD, Wallentin L; PLATO Study Group. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: A Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation 2010;122:2131–2141. [DOI] [PubMed] [Google Scholar]
- 15. Montalescot G, van 't Hof AW, Lapostolle F, Silvain J, Lassen JF, Bolognese L, Cantor WJ, Cequier A, Chettibi M, Goodman SG, Hammett CJ, Huber K, Janzon M, Merkely B, Storey RF, Zeymer U, Stibbe O, Ecollan P, Heutz WM, Swahn E, Collet JP, Willems FF, Baradat C, Licour M, Tsatsaris A, Vicaut E, Hamm CW; ATLANTIC Investigators Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med 2014;371:1016–27. [DOI] [PubMed] [Google Scholar]
- 16. Montalescot G, Antoniucci D, Kastrati A, Neumann FJ, Borentain M, Migliorini A, Boutron C, Collet JP, Vicaut E.. Abciximab in primary coronary stenting of ST-elevation myocardial infarction: a European meta-analysis on individual patients' data with long-term follow-up. Eur Heart J 2007; 28:443–449. [DOI] [PubMed] [Google Scholar]
- 17. Mehilli J, Kastrati A, Schulz S, Früngel S, Nekolla SG, Moshage W, Dotzer F, Huber K, Pache J, Dirschinger J, Seyfarth M, Martinoff S, Schwaiger M, Schömig A. ; Bavarian Reperfusion Alternatives Evaluation-3 (BRAVE-3) Study Investigators. Abciximab in patients with acute ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention after clopidogrel loading: a randomized double-blind trial. Circulation 2009;119:1933–40. [DOI] [PubMed] [Google Scholar]
- 18. Montalescot G, Barragan P, Wittenberg O, Ecollan P, Elhadad S, Villain P, Boulenc JM, Morice MC, Maillard L, Pansiéri M, Choussat R, Pinton P; ADMIRAL Investigators. Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-Term Follow-up. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med 2001; 344:1895–903. [DOI] [PubMed] [Google Scholar]
- 19. Ellis SG, Tendera M, de Belder MA, van Boven AJ, Widimsky P, Janssens L, Andersen HR, Betriu A, Savonitto S, Adamus J, Peruga JZ, Kosmider M, Katz O, Neunteufl T, Jorgova J, Dorobantu M, Grinfeld L, Armstrong P, Brodie BR, Herrmann HC, Montalescot G, Neumann FJ, Effron MB, Barnathan ES, Topol EJ; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008;358:2205–17. [DOI] [PubMed] [Google Scholar]
- 20. Dudek D, Siudak Z, Janzon M, Birkemeyer R, Aldama-Lopez G, Lettieri C, Janus B, Wisniewski A, Berti S, Olivari Z, Rakowski T, Partyka L, Goedicke J, Zmudka K; EUROTRANSFER Registry Investigators. European registry on patients with ST-elevation myocardial infarction transferred for mechanical reperfusion with a special focus on early administration of abciximab – EUROTRANSFER Registry. Am Heart J 2008;156:1147–54. [DOI] [PubMed] [Google Scholar]
- 21. Rakowski T, Siudak Z, Dziewierz A, Birkemeyer R, Legutko J, Mielecki W, Depukat R, Janzon M, Stefaniak J, Zmudka K, Dubiel JS, Partyka L, Dudek D.. Early abciximab administration before transfer for primary percutaneous coronary interventions for ST-elevation myocardial infarction reduces 1-year mortality in patients with high-risk profile. Results from EUROTRANSFER registry. Am Heart J 2009;158:569–75. [DOI] [PubMed] [Google Scholar]
- 22. Van't Hof AW, Ten Berg J, Heestermans T, Dill T, Funck RC, van Werkum W, Dambrink JH, Suryapranata H, van Houwelingen G, Ottervanger JP, Stella P, Giannitsis E, Hamm C; Ongoing Tirofiban In Myocardial infarction Evaluation (On-TIME) 2 study group. Prehospital initiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (On-TIME 2): a multicentre, double-blind, randomized controlled trial. Lancet 2008;372:537–46. [DOI] [PubMed] [Google Scholar]
- 23. Hermanides RS, van Werkum JW, Ottervanger JP, Breet NJ, Gosselink AT, van Houwelingen KG, Dambrink JH, Hamm C, ten Berg JM, van't Hof AW; Ongoing Tirofiban In Myocardial infarction Evaluation (On-TIME) 2 study group. The effect of pre-hospital glycoprotein IIb-IIIa inhibitors on angiographic outcome in STEMI patients who are candidates for primary PCI. Catheter Cardiovasc Interv 2012;79:956–64. [DOI] [PubMed] [Google Scholar]
- 24. Murphy SA, Gibson CM, Morrow DA, Van de Werf F, Menown IB, Goodman SG, Mahaffey KW, Cohen M, McCabe CH, Antman EM, Braunwald E.. Efficacy and safety of the low-molecular-weight heparin enoxaparin compared with unfractionated heparin across the acute coronary syndrome spectrum: a meta-analysis. Eur Heart J 2007;28:2077–86. [DOI] [PubMed] [Google Scholar]
- 25. Silvain J, Beygui F, Barthélémy O, Pollack C Jr, Cohen M, Zeymer U, Huber K, Goldstein P, Cayla G, Collet JP, Vicaut E, Montalescot G.. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ 2012;344:e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montalescot G, Zeymer U, Silvain J, Boulanger B, Cohen M, Goldstein P, Ecollan P, Combes X, Huber K, Pollack C Jr, Bénezet JF, Stibbe O, Filippi E, Teiger E, Cayla G, Elhadad S, Adnet F, Chouihed T, Gallula S, Greffet A, Aout M, Collet JP, Vicaut E; ATOLL Investigators. Intravenous enoxaparin or unfractionated heparin in primary percutaneous coronary intervention for ST-elevation myocardial infarction: the international randomized open-label ATOLL trial. Lancet 2011;378:693–703. [DOI] [PubMed] [Google Scholar]
- 27. Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA; OASIS-6 Trial Group. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA 2006;295:1519–30. [DOI] [PubMed] [Google Scholar]
- 28. Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R; HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2008;358:2218–2230. [DOI] [PubMed] [Google Scholar]
- 29. Steg PG, van 't Hof A, Hamm CW, Clemmensen P, Lapostolle F, Coste P, Ten Berg J, Van Grunsven P, Eggink GJ, Nibbe L, Zeymer U, Campo dell' Orto M, Nef H, Steinmetz J, Soulat L, Huber K, Deliargyris EN, Bernstein D, Schuette D, Prats J, Clayton T, Pocock S, Hamon M, Goldstein P; EUROMAX Investigators. Bivalirudin started during emergency transport for primary PCI. N Engl J Med 2013;369:2207–17. [DOI] [PubMed] [Google Scholar]
- 30. Stone GW, Mehran R, Goldstein P, Witzenbichler B, Van't Hof A, Guagliumi G, Hamm CW, Généreux P, Clemmensen P, Pocock SJ, Gersh BJ, Bernstein D, Deliargyris EN, Steg PG.. Bivalirudin Versus Heparin With or Without Glycoprotein IIb/IIIa Inhibitors in Patients With STEMI Undergoing Primary Percutaneous Coronary Intervention: Pooled Patient-Level Analysis From the HORIZONS-AMI and EUROMAX Trials. J Am Coll Cardiol 2015;65:27–38. [DOI] [PubMed] [Google Scholar]
- 31. Zeymer U, van 't Hof A, Adgey J, Nibbe L, Clemmensen P, Cavallini C, ten Berg J, Coste P, Huber K, Deliargyris EN, Day J, Bernstein D, Goldstein P, Hamm C, Steg PG.. Bivalirudin is superior to heparins alone with bailout GP IIb/IIIa inhibitors in patients with ST-segment elevation myocardial infarction transported emergently for primary percutaneous coronary intervention: a pre-specified analysis from the EUROMAX trial. Eur Heart J 2014;35:2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bangalore S, Toklu B, Kotwal A, Volodarskiy A, Sharma S, Kirtane AJ, Feit F.. Anticoagulant therapy during primary percutaneous coronary intervention for acute myocardial infarction: a meta-analysis of randomized trials in the era of stents and P2Y12 inhibitors. BMJ 2014;349:g6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanna EB, Chen AY, Roe MT, Wiviott SD, Fox CS, Saucedo JF.. Characteristics and in-hospital outcomes of patients with non-ST-segment elevation myocardial infarction and chronic kidney disease undergoing percutaneous coronary intervention. JACC Cardiovasc Interv 2011;4:1002–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kadakia MB, Desai NR, Alexander KP, Chen AY, Foody JM, Cannon CP, Wiviott SD, Scirica BM; National Cardiovascular Data Registry. Use of anticoagulant agents and risk of bleeding among patients admitted with myocardial infarction: a report from the NCDR ACTION Registry–GWTG (National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With the Guidelines). JACC Cardiovasc Interv 2010;3:1166–77. [DOI] [PubMed] [Google Scholar]
- 35. Amsterdam EA, Peterson ED, Ou FS, Newby LK, Pollack CV Jr, Gibler WB, Ohman EM, Roe MT.. Comparative trends in guidelines adherence among patients with non-ST-segment elevation acute coronary syndromes treated with invasive versus conservative management strategies: Results from the CRUSADE quality improvement initiative. Am Heart J 2009;158:748–754. [DOI] [PubMed] [Google Scholar]
- 36. Roe MT, White JA, Kaul P, Tricoci P, Lokhnygina Y, Miller CD, van't Hof AW, Montalescot G, James SK, Saucedo J, Ohman EM, Pollack CV Jr, Hochman JS, Armstrong PW, Giugliano RP, Harrington RA, Van de Werf F, Califf RM, Newby LK.. Regional patterns of use of a medical management strategy for patients with non-ST-segment elevation acute coronary syndromes: insights from the EARLY-ACS Trial. Circ Cardiovasc Qual Outcomes 2012;5:205–13. [DOI] [PubMed] [Google Scholar]
- 37. Chan MY, Mahaffey KW, Sun LJ, Pieper KS, White HD, Aylward PE, Ferguson JJ, Califf RM, Roe MT.. Prevalence, predictors, and impact of conservative medical management for patients with non-ST-segment elevation acute coronary syndromes who have angiographically documented significant coronary disease. JACC Cardiovasc Interv 2008;1:369–78. [DOI] [PubMed] [Google Scholar]
- 38. De Luca L, Leonardi S, Cavallini C, Lucci D, Musumeci G, Caporale R, Abrignani MG, Lupi A, Rakar S, Gulizia MM, Bovenzi FM, De Servi S; EYESHOT Investigators. Contemporary antithrombotic strategies in patients with acute coronary syndrome admitted to cardiac care units in Italy: The EYESHOT Study. Eur Heart J Acute Cardiovasc Care 2015;4:441–452. [DOI] [PubMed] [Google Scholar]
- 39. Theroux P, Ouimet H, McCans J, Latour JG, Joly P, Lévy G, Pelletier E, Juneau M, Stasiak J, deGuise P.. Aspirin, heparin, or both to treat acute unstable angina. N Engl J Med 1988;319:1105–1111. [DOI] [PubMed] [Google Scholar]
- 40. Theroux P, Waters D, Qiu S, McCans J, de Guise P, Juneau M.. Aspirin versus heparin to prevent myocardial infarction during the acute phase of unstable angina. Circulation 1993;88:2045–2048. [DOI] [PubMed] [Google Scholar]
- 41. Cairns JA, Gent M, Singer J, Finnie KJ, Froggatt GM, Holder DA, Jablonsky G, Kostuk WJ, Melendez LJ, Myers MG.. Aspirin, sulfinpyrazone, or both in unstable angina. Results of a Canadian multicenter trial. N Engl J Med 1985;313:1369–1375. [DOI] [PubMed] [Google Scholar]
- 42. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK.. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 43. James SK, Roe MT, Cannon CP, Cornel JH, Horrow J, Husted S, Katus H, Morais J, Steg PG, Storey RF, Stevens S, Wallentin L, Harrington RA; PLATO Study Group. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ 2011;342:d3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, Cornel JH, Bhatt DL, Clemmensen P, Martinez F, Ardissino D, Nicolau JC, Boden WE, Gurbel PA, Ruzyllo W, Dalby AJ, McGuire DK, Leiva-Pons JL, Parkhomenko A, Gottlieb S, Topacio GO, Hamm C, Pavlides G, Goudev AR, Oto A, Tseng CD, Merkely B, Gasparovic V, Corbalan R, Cinteză M, McLendon RC, Winters KJ, Brown EB, Lokhnygina Y, Aylward PE, Huber K, Hochman JS, Ohman EM; TRILOGY ACS Investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297–309. [DOI] [PubMed] [Google Scholar]
- 45. Fox KA, Clayton TC, Damman P, Pocock SJ, de Winter RJ, Tijssen JG, Lagerqvist B, Wallentin L; FIR Collaboration. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol 2010;55:2435–45. [DOI] [PubMed] [Google Scholar]
- 46. Damman P, Hirsch A, Windhausen F, Tijssen JG, de Winter RJ; ICTUS Investigators. 5-year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes) trial a randomized comparison of an early invasive versus selective invasive management in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2010;55:858–64. [DOI] [PubMed] [Google Scholar]
- 47. Hirsch A, Windhausen F, Tijssen JG, Oude Ophuis AJ, van der Giessen WJ, van der Zee PM, Cornel JH, Verheugt FW, de Winter RJ; Invasive versus Conservative Treatment in Unstable coronary Syndromes Investigators. Diverging associations of an intended early invasive strategy compared with actual revascularization, and outcome in patients with non-ST-segment elevation acute coronary syndrome: the problem of treatment selection bias. Eur Heart J 2009;30:645–54. [DOI] [PubMed] [Google Scholar]
- 48. Petersen JL, Mahaffey KW, Hasselblad V, Antman EM, Cohen M, Goodman SG, Langer A, Blazing MA, Le-Moigne-Amrani A, de Lemos JA, Nessel CC, Harrington RA, Ferguson JJ, Braunwald E, Califf RM.. Efficacy and bleeding complications among patients randomized to enoxaparin or unfractionated heparin for antithrombin therapy in non-ST-Segment elevation acute coronary syndromes: a systematic overview. JAMA 2004;292:89–96. [DOI] [PubMed] [Google Scholar]
- 49. Budaj A, Breiger D, Steg G, Goodman SG, Dabbous OH, Fox KA, Avezum A, Cannon CP, Mazurek T, Flather MD, Van De Werf F; GRACE Investigators. Global patterns of use of antithrombotic and antiplatelet therapies in patients with acute coronary syndromes: insights from the Global Registry of Acute Coronary Syndromes (GRACE). Am Heart J 2003;146:999–1006. [DOI] [PubMed] [Google Scholar]
- 50. Cohen M, Demers C, Gurfinkel EP, Turpie AG, Fromell GJ, Goodman S, Langer A, Califf RM, Fox KA, Premmereur J, Bigonzi F.. A comparison of low-molecular weight heparin with unfractionated heparin for unstable coronary artery disease. N Engl J Med 1997;337:447–452. [DOI] [PubMed] [Google Scholar]
- 51. Antman EM, McCabe CH, Gurfinkel EP, Turpie AG, Bernink PJ, Salein D, Bayes De Luna A, Fox K, Lablanche JM, Radley D, Premmereur J, Braunwald E.. Enoxaparin prevents death and cardiac ischemic events in unstable angina/non-Q-wave myocardial infarction: results of the TIMI 11B trial. Circulation 1999;100:1593–1601. [DOI] [PubMed] [Google Scholar]
- 52. Antman EM, Cohen M, Radley D, McCabe C, Rush J, Premmereur J, Braunwald E.. Assessment of the treatment effect of enoxaparin for unstable angina/non-Q-wave myocardial infarction. Circulation 1999;100:1602–1608. [DOI] [PubMed] [Google Scholar]
- 53. Blazing MA, de Lemos JA, White HD, Fox KA, Verheugt FW, Ardissino D, DiBattiste PM, Palmisano J, Bilheimer DW, Snapinn SM, Ramsey KE, Gardner LH, Hasselblad V, Pfeffer MA, Lewis EF, Braunwald E, Califf RM; ‘A to Z’ Investigators. Safety and efficacy of enoxaparin vs unfractionated heparin in patients with non–ST-segment elevation acute coronary syndromes who receive tirofiban and aspirin: a randomized controlled trial. JAMA 2004;292:55–64. [DOI] [PubMed] [Google Scholar]
- 54. The SYNERGY Trial Investigators. Enoxaparin vs unfractionated heparin in high-risk patients with non–ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 2004;292:45–54. [DOI] [PubMed] [Google Scholar]
- 55. Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA.. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006;354:1464–1476. [DOI] [PubMed] [Google Scholar]
- 56. Mehta SR, Boden WE, Eikelboom JW, Flather M, Steg PG, Avezum A, Afzal R, Piegas LS, Faxon DP, Widimsky P, Budaj A, Chrolavicius S, Rupprecht HJ, Jolly S, Granger CB, Fox KA, Bassand JP, Yusuf S; OASIS 5 and 6 Investigators. Antithrombotic therapy with fondaparinux in relation to interventional management strategy in patients with ST- and non-ST-segment elevation acute coronary syndromes: an individual patient-level combined analysis of the Fifth and Sixth Organization to Assess Strategies in Ischemic Syndromes (OASIS 5 and 6) randomized trials. Circulation 2008;118:2038–46 [DOI] [PubMed] [Google Scholar]
- 57. Oldgren J, Wallentin L, Afzal R, Bassand JP, Budaj A, Chrolavicius S, Fox KA, Granger CB, Mehta SR, Pais P, Peters RJ, Xavier D, Zhu J, Yusuf S; OASIS-6 Investigators. Effects of fondaparinux in patients with ST-segment elevation acute myocardial infarction not receiving reperfusion treatment. Eur Heart J 2008;29:315–23. [DOI] [PubMed] [Google Scholar]
- 58. Bagnall AJ, Goodman SG, Fox KA, Yan RT, Gore JM, Cheema AN, Huynh T, Chauret D, Fitchett DH, Langer A, Yan AT; Canadian Acute Coronary Syndrome Registry I and II Investigators.; Canadian Global Registry of Acute Coronary Events (GRACE/GRACE2) Investigators. Influence of age on use of cardiac catheterization and associated outcomes in patients with non-ST-elevation acute coronary syndromes. Am J Cardiol 2009;103:1530–6. [DOI] [PubMed] [Google Scholar]
- 59. Lee TC, Goodman SG, Yan RT, Grondin FR, Welsh RC, Rose B, Gyenes G, Zimmerman RH, Brossoit R, Saposnik G, Graham JJ, Yan AT; Canadian Acute Coronary Syndromes I and II, Canadian Global Registry of Acute Coronary Events (GRACE/GRACE2) and the Canadian Registry of Acute Coronary Events (CANRACE) Investigators. Disparities in management patterns and outcomes of patients with non-ST-elevation acute coronary syndrome with and without a history of cerebrovascular disease. Am J Cardiol 2010;105:1083–9. [DOI] [PubMed] [Google Scholar]
- 60. Gogo PB Jr, Dauerman HL, Mulgund J, Ohman EM, Patel MR, Cohen DJ, Saucedo JF, Harrington RA, Gibler WB, Smith SC Jr, Peterson ED, Roe MT; CRUSADE Investigators. Changes in patterns of coronary revascularization strategies for patients with acute coronary syndromes (from the CRUSADE Quality Improvement Initiative). Am J Cardiol 2007;99:1222–6. [DOI] [PubMed] [Google Scholar]
- 61. Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, DeLago A, Wilmer C, Topol EJ; CREDO Investigators. Clopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;288:2411–20. [DOI] [PubMed] [Google Scholar]
- 62. Sabatine MS, Cannon CP, Gibson CM, ópez-Sendón JL, Montalescot G, Theroux P, Lewis BS, Murphy SA, McCabe CH, Braunwald E; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005;294:1224–32. [DOI] [PubMed] [Google Scholar]
- 63. Bellemain-Appaix A, O'Connor SA, Silvain J, Cucherat M, Beygui F, Barthélémy O, Collet JP, Jacq L, Bernasconi F, Montalescot G; ACTION Group. Association of clopidogrel pretreatment with mortality, cardiovascular events, and major bleeding among patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. JAMA 2012;308:2507–16. [DOI] [PubMed] [Google Scholar]
- 64. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57. [DOI] [PubMed] [Google Scholar]
- 65. Wittfeldt A, Emanuelsson H, Brandrup-Wognsen G, van Giezen JJ, Jonasson J, Nylander S, Gan LM.. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J Am Coll Cardiol 2013;61:723–7. [DOI] [PubMed] [Google Scholar]
- 66. James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, Harrington RA, Horrow J, Katus H, Keltai M, Lewis BS, Parikh K, Storey RF, Szummer K, Wojdyla D, Wallentin L.. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 2010;122:1056–67. [DOI] [PubMed] [Google Scholar]
- 67. Alexopoulos D, Galati A, Xanthopoulou I, Mavronasiou E, Kassimis G, Theodoropoulos KC, Makris G, Damelou A, Tsigkas G, Hahalis G, Davlouros P.. Ticagrelor versus prasugrel in acute coronary syndrome patients with high on-clopidogrel platelet reactivity following percutaneous coronary intervention: a pharmacodynamic study. J Am Coll Cardiol 2012;60:193–9. [DOI] [PubMed] [Google Scholar]
- 68. Montalescot G, Bolognese L, Dudek D, Goldstein P, Hamm C, Tanguay JF, ten Berg JM, Miller DL, Costigan TM, Goedicke J, Silvain J, Angioli P, Legutko J, Niethammer M, Motovska Z, Jakubowski JA, Cayla G, Visconti LO, Vicaut E, Widimsky P; ACCOAST Investigators. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med 2013;369:999–1010. [DOI] [PubMed] [Google Scholar]
- 69. Bellemain-Appaix A, Kerneis M, O’Connor SA, Silvain J, Cucherat M, Beygui F, Barthélémy O, Collet JP, Jacq L, Bernasconi F, Montalescot G; ACTION Study Group. Reappraisal of thienopyridine pretreatment in patients with non-ST elevation acute coronary syndrome: a systematic review and meta-analysis. BMJ 2014;349:g6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lange RA, Hillis LD.. The duel between dual antiplatelet therapies. N Engl J Med 2013;368:1356–7. [DOI] [PubMed] [Google Scholar]
- 71. Valgimigli M, Tebaldi M, Campo G, Gambetti S, Bristot L, Monti M, Parrinello G, Ferrari R; FABOLUS PRO Investigators. Prasugrel versus tirofiban bolus with or without short post-bolus infusion with or without concomitant prasugrel administration in patients with myocardial infarction undergoing coronary stenting: the FABOLUS PRO (Facilitation through Aggrastat By drOpping or shortening Infusion Line in patients with ST-segment elevation myocardial infarction compared to or on top of PRasugrel given at loading dOse) trial. JACC Cardiovasc Interv 2012;5:268–77. [DOI] [PubMed] [Google Scholar]
- 72. De Luca G, Navarese EP, Cassetti E, Verdoia M, Suryapranata H.. Meta-analysis of randomized trials of glycoprotein IIb/IIIa inhibitors in high-risk acute coronary syndromes patients undergoing invasive strategy. Am J Cardiol 2011;107:198–203. [DOI] [PubMed] [Google Scholar]
- 73. Roffi M, Chew DP, Mukherjee D, Bhatt DL, White JA, Moliterno DJ, Heeschen C, Hamm CW, Robbins MA, Kleiman NS, Théroux P, White HD, Topol EJ.. Platelet glycoprotein IIb/IIIa inhibition in acute coronary syndromes. Gradaynt of benefit related to the revascularization strategy. Eur Heart J 2002;23:1441–1448. [DOI] [PubMed] [Google Scholar]
- 74. Stone GW, Bertrand ME, Moses JW, Ohman EM, Lincoff AM, Ware JH, Pocock SJ, McLaurin BT, Cox DA, Jafar MZ, Chandna H, Hartmann F, Leisch F, Strasser RH, Desaga M, Stuckey TD, Zelman RB, Lieber IH, Cohen DJ, Mehran R, White HD; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007;297:591–602. [DOI] [PubMed] [Google Scholar]
- 75. Giugliano RP, White JA, Bode C, Armstrong PW, Montalescot G, Lewis BS, van 't Hof A, Berdan LG, Lee KL, Strony JT, Hildemann S, Veltri E, Van de Werf F, Braunwald E, Harrington RA, Califf RM, Newby LK; EARLY ACS Investigators. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009;360:2176–2190. [DOI] [PubMed] [Google Scholar]
- 76. Antman EM, Morrow DA, McCabe CH, Murphy SA, Ruda M, Sadowski Z, Budaj A, López-Sendón JL, Guneri S, Jiang F, White HD, Fox KA, Braunwald E; ExTRACT-TIMI 25 Investigators. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med 2006;354:1477–88. [DOI] [PubMed] [Google Scholar]
- 77. Cohen M, Mahaffey KW, Pieper K, Pollack CV Jr, Antman EM, Hoekstra J, Goodman SG, Langer A, Col JJ, White HD, Califf RM, Ferguson JJ; SYNERGY Trial Investigators. A subgroup analysis of the impact of prerandomization antithrombin therapy on outcomes in the SYNERGY trial: enoxaparin versus unfractionated heparin in non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol 2006;48:1346–54. [DOI] [PubMed] [Google Scholar]
- 78. Mahaffey KW, Pieper KS, Lokhnygina Y, Califf RM, Antman EM, Kleiman NS, Goodman SG, White HD, Rao SV, Hochman JS, Cohen M, Col JJ, Roe MT, Ferguson JJ; SYNERGY Investigators. The impact of postrandomization crossover of therapy in acute coronary syndromes care. Circ Cardiovasc Qual Outcomes 2011;4:211–9. [DOI] [PubMed] [Google Scholar]
- 79. FUTURA/OASIS-8 Trial Group, Steg PG, Jolly SS, Mehta SR, Afzal R, Xavier D, Rupprecht HJ, López-Sendón JL, Budaj A, Diaz R, Avezum A, Widimsky P, Rao SV, Chrolavicius S, Meeks B, Joyner C, Pogue J, Yusuf S.. Low-dose vs standard-dose unfractionated heparin for percutaneous coronary intervention in acute coronary syndromes treated with fondaparinux: the FUTURA/OASIS-8 randomized trial. JAMA 2010;304:1339–49. [DOI] [PubMed] [Google Scholar]
- 80. White HD, Chew DP, Hoekstra JW, Miller CD, Pollack CV Jr, Feit F, Lincoff AM, Bertrand M, Pocock S, Ware J, Ohman EM, Mehran R, Stone GW.. Safety and efficacy of switching from either unfractionated heparin or enoxaparin to bivalirudin in patients with non-ST-segment elevation acute coronary syndromes managed with an invasive strategy: results from the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. J Am Coll Cardiol 2008;51:1734–41. [DOI] [PubMed] [Google Scholar]
- 81. Dangas GD, Mehran R, Nikolsky E, Claessen BE, Lansky AJ, Brodie BR, Witzenbichler B, Guagliumi G, Peruga JZ, Dudek D, Möckel M, Caixeta A, Parise H, White H, Stone GW; HORIZONS-AMI Trial Investigators. Effect of switching antithrombin agents for primary angioplasty in acute myocardial infarction: the HORIZONS-SWITCH analysis. J Am Col Cardiol 2011;57:2309–16. [DOI] [PubMed] [Google Scholar]
- 82. Puymirat E, Simon T, Steg PG, Schiele F, Guéret P, Blanchard D, Khalife K, Goldstein P, Cattan S, Vaur L, Cambou JP, Ferrières J, Danchin N; USIK USIC 2000 Investigators.; FAST MI Investigators. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA 2012;308:998–1006. [DOI] [PubMed] [Google Scholar]
- 83. Fokkema ML, James SK, Albertsson P, Akerblom A, Calais F, Eriksson P, Jensen J, Nilsson T, de Smet BJ, Sjögren I, Thorvinger B, Lagerqvist B.. Population Trends in Percutaneous Coronary Intervention: 20 Year Results from the Swedish Coronary Angiography and Angioplasty Registry. J Am Coll Cardiol 2013;61:1222–1230. [DOI] [PubMed] [Google Scholar]
- 84. Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S; CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomized factorial trial. Lancet 2010;376:1233–43. [DOI] [PubMed] [Google Scholar]
- 85. Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, Husted S, Katus H, Keltai M, Khurmi NS, Kontny F, Lewis BS, Steg PG, Storey RF, Wojdyla D, Wallentin L; PLATelet inhibition and patient Outcomes Investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomized double-blind study. Lancet 2010;375:283–93. [DOI] [PubMed] [Google Scholar]
- 86. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-Term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015. NEJMoa150085/NEJMoa1500857. [Google Scholar]
- 87. Wiviott SD, Braunwald E, McCabe CH, ontalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–15. [DOI] [PubMed] [Google Scholar]
- 88. Wiviott SD, Braunwald E, McCabe CH, Horvath I, Keltai M, Herrman JP, Van de Werf F, Downey WE, Scirica BM, Murphy SA, Antman EM; TRITON-TIMI 38 Investigators. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomized trial. Lancet 2008;371:1353–63. [DOI] [PubMed] [Google Scholar]
- 89. Salisbury AC, Wang K, Cohen DJ, Li Y, Jones PG, Spertus JA.. Selecting antiplatelet therapy at the time of percutaneous intervention for an acute coronary syndrome: weighing the benefits and risks of prasugrel versus clopidogrel. Circ Cardiovasc Qual Outcomes 2013;6:27–34. [DOI] [PubMed] [Google Scholar]
- 90. Rollini F, Franchi F, Angiolillo DJ.. Switching P2Y12-receptor inhibitors in patients with coronary artery disease. Nat Rev Cardiol 2016;13:11–27. [DOI] [PubMed] [Google Scholar]
- 91. Loh JP, Pendyala LK, Kitabata H, Torguson R, Chen F, Kent KM, Satler LF, Suddath WO, Pichard AD, Waksman R.. Safety of reloading prasugrel in addition to clopidogrel loading in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol 2013;111:841–5. [DOI] [PubMed] [Google Scholar]
- 92. Saucedo JF, Angiolillo DJ, DeRaad R, Frelinger AL 3rd, Gurbel PA, Costigan TM, Jakubowski JA, Ojeh CK, Duvvuru S, Effron MB; SWAP Investigators. Decrease in high on-treatment platelet reactivity (HPR) prevalence on switching from clopidogrel to prasugrel: insights from the switching anti-platelet (SWAP) study. Thromb Haemost 2013;109:347–55. [DOI] [PubMed] [Google Scholar]
- 93. Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, Price MJ, Leonardi S, Gallup D, Bramucci E, Radke PW, Widimský P, Tousek F, Tauth J, Spriggs D, McLaurin BT, Angiolillo DJ, Généreux P, Liu T, Prats J, Todd M, Skerjanec S, White HD, Harrington RA; CHAMPION PHOENIX Investigators. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med 2013;368:1303–13. [DOI] [PubMed] [Google Scholar]
- 94. Steg PG, Bhatt DL, Hamm CW, tone GW, Gibson CM, Mahaffey KW, Leonardi S, Liu T, Skerjanec S, Day JR, Iwaoka RS, Stuckey TD, Gogia HS, Gruberg L, French WJ, White HD, Harrington RA; CHAMPION Investigators. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet 2013;382:1981–92. [DOI] [PubMed] [Google Scholar]
- 95. Tricoci P, Huang Z, Held C, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, Lokhnygina Y, Pei J, Leonardi S, Rorick TL, Kilian AM, Jennings LH, Ambrosio G, Bode C, Cequier A, Cornel JH, Diaz R, Erkan A, Huber K, Hudson MP, Jiang L, Jukema JW, Lewis BS, Lincoff AM, Montalescot G, Nicolau JC, Ogawa H, Pfisterer M, Prieto JC, Ruzyllo W, Sinnaeve PR, Storey RF, Valgimigli M, Whellan DJ, Widimsky P, Strony J, Harrington RA, Mahaffey KW; TRACER Investigators. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 2012;366:20–33. [DOI] [PubMed] [Google Scholar]
- 96. Morrow DA, Braunwald E, Bonaca MP, meriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJ, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA; TRA 2P–TIMI 50 Steering Committee and Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012;366:1404–1413. [DOI] [PubMed] [Google Scholar]
- 97. Brener SJ, Moliterno DJ, Lincoff AM, Steinhubl SR, Wolski KE, Topol EJ.. Relationship between activated clotting time and ischemic or hemorrhagic complications: analysis of 4 recent randomized clinical trials of percutaneous coronary intervention. Circulation 2004;110:994–8. [DOI] [PubMed] [Google Scholar]
- 98. Silvain J, Beygui F, Ankri A, ellemain-Appaix A, Pena A, Barthelemy O, Cayla G, Gallois V, Galier S, Costagliola D, Collet JP, Montalescot G.. Enoxaparin anticoagulation monitoring in the catheterization laboratory using a new bedside test. J Am Coll Cardiol 2010;55:617–25. [DOI] [PubMed] [Google Scholar]
- 99. Montalescot G, Collet JP, Tanguy ML, nkri A, Payot L, Dumaine R, Choussat R, Beygui F, Gallois V, Thomas D.. Anti-Xa activity relates to survival and efficacy in unselected acute coronary syndrome patients treated with enoxaparin. Circulation 2004;110:392–8. [DOI] [PubMed] [Google Scholar]
- 100. Rao SV, Ohman EM.. Anticoagulant therapy for percutaneous coronary intervention. Circ Cardiovasc Interv 2010;3:80–88. [DOI] [PubMed] [Google Scholar]
- 101. Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006;355:2203–2216. [DOI] [PubMed] [Google Scholar]
- 102. Kastrati A, Neumann FJ, Schulz S, Massberg S, Byrne RA, Ferenc M, Laugwitz KL, Pache J, Ott I, Hausleiter J, Seyfarth M, Gick M, Antoniucci D, Schömig A, Berger PB, Mehilli J; ISAR-REACT 4 Trial Investigators. Abciximab and heparin versus bivalirudin for non-ST-elevation myocardial infarction. N Engl J Med 2011;365:1980–9. [DOI] [PubMed] [Google Scholar]
- 103. Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Fahy M, Parise H, Mehran R; HORIZONS-AMI Trial Investigators. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomized controlled trial. Lancet 2011;377:2193–204. [DOI] [PubMed] [Google Scholar]
- 104. Schulz S, Richardt G, Laugwitz KL, Morath T, Neudecker J, Hoppmann P, Mehran R, Gershlick AH, Tölg R, Anette Fiedler K, Abdel-Wahab M, Kufner S, Schneider S, Schunkert H, Ibrahim T, Mehilli J, Kastrati A; Bavarian Reperfusion Alternatives Evaluation (BRAVE) 4 Investigators. Prasugrel plus bivalirudin vs. clopidogrel plus heparin in patients with ST-segment elevation myocardial infarction. Eur Heart J 2014;35:2285–94. [DOI] [PubMed] [Google Scholar]
- 105. Shahzad A, Kemp I, Mars C, Wilson K, Roome C, Cooper R, Andron M, Appleby C, Fisher M, Khand A, Kunadian B, Mills JD, Morris JL, Morrison WL, Munir S, Palmer ND, Perry RA, Ramsdale DR, Velavan P, Stables RH; HEAT-PPCI trial investigators. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomized controlled trial. Lancet 2014;384:1849–58. [DOI] [PubMed] [Google Scholar]
- 106. Valgimigli M, Frigoli E, Leonardi S, othenbühler M, Gagnor A, Calabrò P, Garducci S, Rubartelli P, Briguori C, Andò G, Repetto A, Limbruno U, Garbo R, Sganzerla P, Russo F, Lupi A, Cortese B, Ausiello A, Ierna S, Esposito G, Presbitero P, Santarelli A, Sardella G, Varbella F, Tresoldi S, de Cesare N, Rigattieri S, Zingarelli A, Tosi P, van 't Hof A, Boccuzzi G, Omerovic E, Sabaté M, Heg D, Jüni P, Vranckx P; MATRIX Investigators. Bivalirudin or unfractionated heparin in acute coronary syndromes. N Engl J Med 2015;373:997–1009. [DOI] [PubMed] [Google Scholar]
- 107. Mehta SR, Granger CB, Eikelboom JW, Bassand JP, Wallentin L, Faxon DP, Peters RJ, Budaj A, Afzal R, Chrolavicius S, Fox KA, Yusuf S.. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007;50:1742–51. [DOI] [PubMed] [Google Scholar]
- 108. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM; ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 109. Hassan A, Newman A, Ko DT, Rinfret S, Hirsch G, Ghali WA, Tu JV.. Increasing rates of angioplasty versus bypass surgery in Canada, 1994-2005. Am Heart J 2010;160:958–65. [DOI] [PubMed] [Google Scholar]
- 110. Brown C, Joshi B, Faraday N, Shah A, Yuh D, Rade JJ, Hogue CW.. Emergency cardiac surgery in patients with acute coronary syndromes: a review of the evidence and perioperative implications of medical and mechanical therapeutics. Anesth Analg 2011;112:777–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hongo RH, Ley J, Dick SE, Yee RR.. The effect of clopidogrel in combination with aspirin when given before coronary artery bypass grafting. J Am Coll Cardiol 2002;40:231–7. [DOI] [PubMed] [Google Scholar]
- 112. Firanescu CE, Martens EJ, Schonberger JP, Soliman Hamad MA, van Straten AH.. Postoperative blood loss in patients undergoing coronary artery bypass surgery after preoperative treatment with clopidogrel. A prospective randomized controlled study. Eur J Cardiothorac Surg 2009; 36: 856–62. [DOI] [PubMed] [Google Scholar]
- 113. Herman CR, Buth KJ, Kent BA, Hirsch GM.. Clopidogrel increases blood transfusion and hemorrhagic complications in patients undergoing cardiac surgery. Ann Thorac Surg 2010;89:397–402. [DOI] [PubMed] [Google Scholar]
- 114. Kim JH, Newby LK, Clare RM, Shaw LK, Lodge AJ, Smith PK, Jolicoeur EM, Rao SV, Becker RC, Mark DB, Granger CB.. Clopidogrel use and bleeding after coronary artery bypass graft surgery. Am Heart J 2008;156:886–92. [DOI] [PubMed] [Google Scholar]
- 115. Vaccarino GN, Thierer J, Albertal M, Vrancic M, Piccinini F, Benzadón M, Raich H, Navia DO.. Impact of preoperative clopidogrel in off pump coronary artery bypass surgery: a propensity score analysis. J Thorac Cardiovasc Surg 2009;137:309–13. [DOI] [PubMed] [Google Scholar]
- 116. Berger JS, Frye CB, Harshaw Q, Edwards FH, Steinhubl SR, Becker RC.. Impact of clopidogrel in patients with acute coronary syndromes requiring coronary artery bypass surgery: a multicenter analysis. J Am Coll Cardiol 2008;52:1693–701. [DOI] [PubMed] [Google Scholar]
- 117. Fox KA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, Yusuf S; Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004;110:1202–8. [DOI] [PubMed] [Google Scholar]
- 118. Smith PK, Goodnough LT, Levy JH, Poston RS, Short MA, Weerakkody GJ, Lenarz LA.. Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis. J Am Coll Cardiol 2012;60:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Held C, Asenblad N, Bassand JP, Becker RC, Cannon CP, Claeys MJ, Harrington RA, Horrow J, Husted S, James SK, Mahaffey KW, Nicolau JC, Scirica BM, Storey RF, Vintila M, Ycas J, Wallentin L.. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol 2011;57:672–84. [DOI] [PubMed] [Google Scholar]
- 120. Varenhorst C, Alström U, Scirica BM, Hogue CW, Åsenblad N, Storey RF, Steg PG, Horrow J, Mahaffey KW, Becker RC, James S, Cannon CP, Brandrup-Wognsen G, Wallentin L, Held C.. Factors contributing to the lower mortality with ticagrelor compared with clopidogrel in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol 2012;60:1623–30. [DOI] [PubMed] [Google Scholar]
- 121. McDonald SB, Renna M, Spitznagel EL, Avidan M, Hogue CW Jr, Moon MR, Barzilai B, Saleem R, McDonald JM, Despotis GJ.. Preoperative use of enoxaparin increases the risk of postoperative bleeding and re-exploration in cardiac surgery patients. J Cardiothorac Vasc Anesth 2005;19:4–10. [DOI] [PubMed] [Google Scholar]
- 122. Jones HU, Muhlestein JB, Jones KW, Bair TL, Lavasani F, Sohrevardi M, Horne BD, Doty D, Lappe DL.. Preoperative use of enoxaparin compared with unfractionated heparin increases the incidence of re-exploration for postoperative bleeding after open-heart surgery in patients who present with an acute coronary syndrome: clinical investigation and reports. Circulation 2002;106:I19–22. [PubMed] [Google Scholar]
- 123. Kincaid EH, Monroe ML, Saliba DL, Kon ND, Byerly WG, Reichert MG.. Effects of preoperative enoxaparin versus unfractionated heparin on bleeding indices in patients undergoing coronary artery bypass grafting. Ann Thorac Surg 2003;76:124–8. [DOI] [PubMed] [Google Scholar]
- 124. Cannon CP, Brindis RG, Chaitman BR, Cohen DJ, Cross JT Jr, Drozda JP Jr, Fesmire FM, Fintel DJ, Fonarow GC, Fox KA, Gray DT, Harrington RA, Hicks KA, Hollander JE, Krumholz H, Labarthe DR, Long JB, Mascette AM, Meyer C, Peterson ED, Radford MJ, Roe MT, Richmann JB, Selker HP, Shahian DM, Shaw RE, Sprenger S, Swor R, Underberg JA, Van de Werf F, Weiner BH, Weintraub WS; American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards; American College of Emergency Physicians; Emergency Nurses Association; National Association of Emergency Medical Technicians; National Association of EMS Physicians; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Cardiovascular Patient Care; Society of Thoracic Surgeons. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Acute Coronary Syndromes and Coronary Artery Disease Clinical Data Standards). Circulation 2013;127:1052–89. [DOI] [PubMed] [Google Scholar]


